Bacterial Colonization within the First Six Weeks of Life and Pulmonary Outcome in Preterm Infants <1000 g
Abstract
:1. Introduction
2. Experimental Section
2.1. Patient Cohort, Exclusion Criteria, and Ethics Vote
2.2. Longitudinal Screening for Bacterial Colonization
2.3. Data Acquisition and Parameter Definition
2.4. Statistical Analysis
3. Results
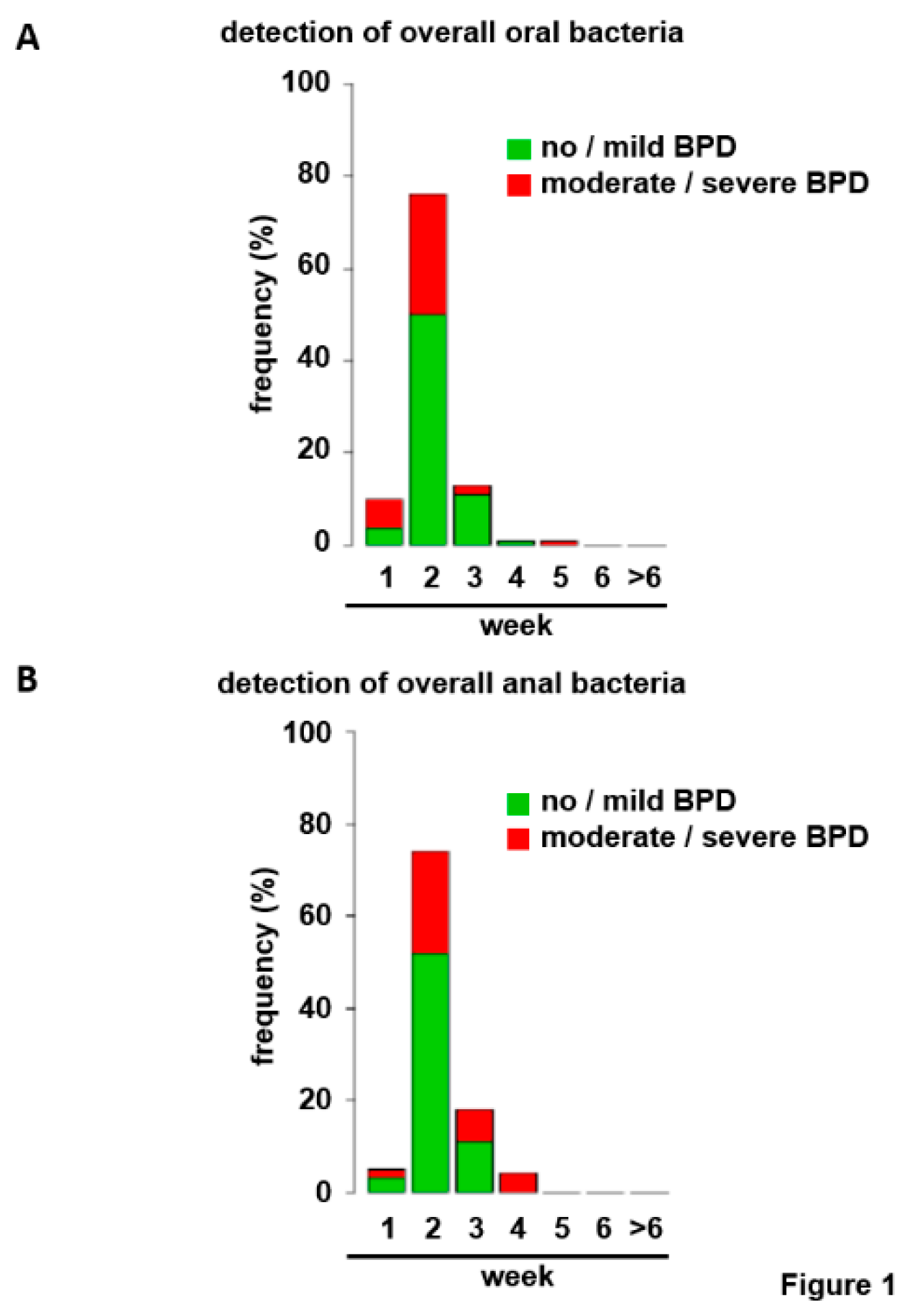
3.1. Bacterial Colonization of the Upper Respiratory Tract and the Gut within the First Six Weeks of Life
3.2. Upper Airway Colonization with Potential Pathogenic Bacteria Separates BPD Disease Severities
3.3. No Significant Impact of Varied Definition of Antibiotic Exposure and Breast Milk Supply
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Islam, J.Y.; Keller, R.L.; Aschner, J.L.; Hartert, T.V.; Moore, P.E. Understanding the Short- and Long-Term Respiratory Outcomes of Prematurity and Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2015, 192, 134–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, T.; Radajewski, S.; Chao, C.-M.; Bellusci, S.; Ehrhardt, H. Pathogenesis of bronchopulmonary dysplasia: When inflammation meets organ development. Mol. Cell. Pediatr. 2016, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Lignelli, E.; Palumbo, F.; Myti, D.; Morty, R.E. Recent advances in our understanding of the mechanisms of lung alveolarization and bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L832–L887. [Google Scholar] [CrossRef]
- Hartling, L.; Liang, Y.; Lacaze-Masmonteil, T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F8–F17. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.; Watkins, W.J.; Edwards, M.O.; Spiller, O.B.; Jacqz-Aigrain, E.; Kotecha, S.J.; Kotecha, S. Association between pulmonary ureaplasma colonization and bronchopulmonary dysplasia in preterm infants: Updated systematic review and meta-analysis. Pediatr. Infect. Dis. J. 2014, 33, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Kallapur, S.G.; Kramer, B.W.; Knox, C.L.; Berry, C.A.; Collins, J.J.P.; Kemp, M.W.; Nitsos, I.; Polglase, G.R.; Robinson, J.; Hillman, N.H.; et al. Chronic fetal exposure to Ureaplasma parvum suppresses innate immune responses in sheep. J. Immunol. 2011, 187, 2688–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuypers, E.; Collins, J.J.P.; Kramer, B.W.; Ofman, G.; Nitsos, I.; Pillow, J.J.; Polglase, G.R.; Kemp, M.W.; Newnham, J.P.; Gavilanes, A.W.D.; et al. Intra-amniotic LPS and antenatal betamethasone: Inflammation and maturation in preterm lamb lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L380–L389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizia, M.; Lloyd, J.; Allen, M.; Klein, N.; Peebles, D. Immune status in very preterm neonates. Pediatrics 2012, 129, e967–e974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staude, B.; Oehmke, F.; Lauer, T.; Behnke, J.; Göpel, W.; Schloter, M.; Schulz, H.; Krauss-Etschmann, S.; Ehrhardt, H. The Microbiome and Preterm Birth: A Change in Paradigm with Profound Implications for Pathophysiologic Concepts and Novel Therapeutic Strategies. Biomed Res. Int. 2018, 2018, 7218187. [Google Scholar] [CrossRef]
- Combs, C.A.; Gravett, M.; Garite, T.J.; Hickok, D.E.; Lapidus, J.; Porreco, R.; Rael, J.; Grove, T.; Morgan, T.K.; Clewell, W.; et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am. J. Obstet. Gynecol. 2014, 210, 125.e1–125.e15. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redline, R.W. Inflammatory responses in the placenta and umbilical cord. Semin. Fetal Neonatal Med. 2006, 11, 296–301. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef] [Green Version]
- Gentle, S.J.; Lal, C.V. Predicting BPD: Lessons Learned From the Airway Microbiome of Preterm Infants. Front. Pediatr. 2019, 7, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pammi, M.; Lal, C.V.; Wagner, B.D.; Mourani, P.M.; Lohmann, P.; Luna, R.A.; Sisson, A.; Shivanna, B.; Hollister, E.B.; Abman, S.H.; et al. Airway Microbiome and Development of Bronchopulmonary Dysplasia in Preterm Infants: A Systematic Review. J. Pediatr. 2019, 204, 126–133.e2. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.V.; Kandasamy, J.; Dolma, K.; Ramani, M.; Kumar, R.; Wilson, L.; Aghai, Z.; Barnes, S.; Blalock, J.E.; Gaggar, A.; et al. Early airway microbial metagenomic and metabolomic signatures are associated with development of severe bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L810–L815. [Google Scholar] [CrossRef] [PubMed]
- Rofael, S.A.D.; McHugh, T.D.; Troughton, R.; Beckmann, J.; Spratt, D.; Marlow, N.; Hurst, J.R. Airway microbiome in adult survivors of extremely preterm birth: The EPICure study. Eur. Respir. J. 2019, 53, 1801225. [Google Scholar] [CrossRef]
- Hoy, C.; Millar, M.R.; MacKay, P.; Godwin, P.G.; Langdale, V.; Levene, M.I. Quantitative changes in faecal microflora preceding necrotising enterocolitis in premature neonates. Arch. Dis. Child. 1990, 65, 1057–1059. [Google Scholar] [CrossRef] [Green Version]
- Millar, M.R.; MacKay, P.; Levene, M.; Langdale, V.; Martin, C. Enterobacteriaceae and neonatal necrotising enterocolitis. Arch. Dis. Child. 1992, 67, 53–56. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Tang, J.; Shi, J.; Qu, Y.; Xiong, T.; Mu, D. Human milk as a protective factor for bronchopulmonary dysplasia: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F128–F136. [Google Scholar] [CrossRef] [PubMed]
- Villamor-Martínez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Villamor, E. Mother’s Own Milk and Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Front. Pediatr. 2019, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Ting, J.Y.; Synnes, A.; Roberts, A.; Deshpandey, A.; Dow, K.; Yoon, E.W.; Lee, K.-S.; Dobson, S.; Lee, S.K.; Shah, P.S. Association Between Antibiotic Use and Neonatal Mortality and Morbidities in Very Low-Birth-Weight Infants Without Culture-Proven Sepsis or Necrotizing Enterocolitis. JAMA Pediatr. 2016, 170, 1181–1187. [Google Scholar] [CrossRef]
- Cantey, J.B.; Huffman, L.W.; Subramanian, A.; Marshall, A.S.; Ballard, A.R.; Lefevre, C.; Sagar, M.; Pruszynski, J.E.; Mallett, L.H. Antibiotic Exposure and Risk for Death or Bronchopulmonary Dysplasia in Very Low Birth Weight Infants. J. Pediatr. 2017, 181, 289–293.e1. [Google Scholar] [CrossRef]
- Fajardo, C.; Alshaikh, B.; Harabor, A. Prolonged use of antibiotics after birth is associated with increased morbidity in preterm infants with negative cultures. J. Matern. Fetal Neonatal Med. 2019, 32, 4060–4066. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Jefferies, A.L.; Yoon, E.W.; Lee, S.K.; Shah, P.S. Risk Factors and Outcomes of Late-Onset Bacterial Sepsis in Preterm Neonates Born at < 32 Weeks’ Gestation. Am. J. Perinatol. 2015, 32, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Lee, B.S. Late-Onset Sepsis as a Risk Factor for Bronchopulmonary Dysplasia in Extremely Low Birth Weight Infants: A Nationwide Cohort Study. Sci. Rep. 2019, 9, 15448. [Google Scholar] [CrossRef] [Green Version]
- Dermyshi, E.; Wang, Y.; Yan, C.; Hong, W.; Qiu, G.; Gong, X.; Zhang, T. The “Golden Age” of Probiotics: A Systematic Review and Meta-Analysis of Randomized and Observational Studies in Preterm Infants. Neonatology 2017, 112, 9–23. [Google Scholar] [CrossRef]
- Villamor-Martínez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Kramer, B.; Villamor, E. Probiotic Supplementation in Preterm Infants Does Not Affect the Risk of Bronchopulmonary Dysplasia: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, 1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surate Solaligue, D.E.; Rodríguez-Castillo, J.A.; Ahlbrecht, K.; Morty, R.E. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L1101–L1153. [Google Scholar] [CrossRef] [PubMed]
- Gortner, L.; Misselwitz, B.; Milligan, D.; Zeitlin, J.; Kollée, L.; Boerch, K.; Agostino, R.; van Reempts, P.; Chabernaud, J.-L.; Bréart, G.; et al. Rates of bronchopulmonary dysplasia in very preterm neonates in Europe: Results from the MOSAIC cohort. Neonatology 2011, 99, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Christoph, J.; Dame, C.; Eckmanns, T.; Gärtner, B.; Geffers, C.; Gille, C.; Haertel, C.; Haller, S.; Hartl, D.; Kraus-Haas, M.; et al. Risikocharakterisierung intensivmedizinisch behandelter Früh- und Neugeborener und Daten zur Ist-Situation in deutschen neonatologischen Intensivpflegestationen 2013—Fachliche Erläuterungen zu folgender Empfehlung: Praktische Umsetzung sowie krankenhaushygienische und infektionspräventive Konsequenzen des mikrobiellen Kolonisationsscreenings bei intensivmedizinisch behandelten Früh- und Neugeborenen Ergänzende Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut, Berlin zur Implementierung der Empfehlungen zur Prävention nosokomialer Infektionen bei neonatologischen Intensivpflegepatienten mit einem Geburtsgewicht unter 1.500 g aus dem Jahr 2007 und 2012. Epid. Bull. 2013, 42, 1–52. [Google Scholar]
- Parm, U.; Metsvaht, T.; Sepp, E.; Ilmoja, M.-L.; Pisarev, H.; Pauskar, M.; Lutsar, I. Risk factors associated with gut and nasopharyngeal colonization by common Gram-negative species and yeasts in neonatal intensive care units patients. Early Hum. Dev. 2011, 87, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Sgro, M.; Shah, P.S.; Campbell, D.; Tenuta, A.; Shivananda, S.; Lee, S.K. Early-onset neonatal sepsis: Rate and organism pattern between 2003 and 2008. J. Perinatol. 2011, 31, 794–798. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, D.; Fairchild, K.D. Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infants. Clin. Microbiol. Rev. 2004, 17, 638–680, table of contents. [Google Scholar] [CrossRef] [Green Version]
- Voigt, M.; Schneider, K.T.; Jährig, K. Analyse des Geburtengutes des Jahrgangs 1992 der Bundesrepublik Deutschland. Teil 1: Neue Perzentilwerte für die Körpermasse von Neugeborenen. Geburtshilfe Frauenheilkd. 1996, 56, 550–558. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Andersen, C.C.; Smith, K.; Holberton, J. Pharyngeal pressure with high-flow nasal cannulae in premature infants. J. Perinatol. 2008, 28, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Walsh, M.; Engle, W.; Laptook, A.; Kazzi, S.N.J.; Buchter, S.; Rasmussen, M.; Yao, Q. Oxygen delivery through nasal cannulae to preterm infants: Can practice be improved? Pediatrics 2005, 116, 857–861. [Google Scholar] [CrossRef] [Green Version]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; de Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef]
- Lapcharoensap, W.; Gage, S.C.; Kan, P.; Profit, J.; Shaw, G.M.; Gould, J.B.; Stevenson, D.K.; O’Brodovich, H.; Lee, H.C. Hospital variation and risk factors for bronchopulmonary dysplasia in a population-based cohort. JAMA Pediatr. 2015, 169, e143676. [Google Scholar] [CrossRef] [Green Version]
- Hartz, L.E.; Bradshaw, W.; Brandon, D.H. Potential NICU Environmental Influences on the Neonate’s Microbiome: A Systematic Review. Adv. Neonatal Care Off. J. Natl. Assoc. Neonatal Nurses 2015, 15, 324–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hufnagel, M.; Liese, C.; Loescher, C.; Kunze, M.; Proempeler, H.; Berner, R.; Krueger, M. Enterococcal colonization of infants in a neonatal intensive care unit: Associated predictors, risk factors and seasonal patterns. BMC Infect. Dis. 2007, 7, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, C.V.; Bhandari, V.; Ambalavanan, N. Genomics, microbiomics, proteomics, and metabolomics in bronchopulmonary dysplasia. Semin. Perinatol. 2018, 42, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Olin, A.; Henckel, E.; Chen, Y.; Lakshmikanth, T.; Pou, C.; Mikes, J.; Gustafsson, A.; Bernhardsson, A.K.; Zhang, C.; Bohlin, K.; et al. Stereotypic Immune System Development in Newborn Children. Cell 2018, 174, 1277–1292.e14. [Google Scholar] [CrossRef] [Green Version]
- Lal, C.V.; Travers, C.; Aghai, Z.H.; Eipers, P.; Jilling, T.; Halloran, B.; Carlo, W.A.; Keeley, J.; Rezonzew, G.; Kumar, R.; et al. The Airway Microbiome at Birth. Sci. Rep. 2016, 6, 31023. [Google Scholar] [CrossRef] [Green Version]
- Glaser, K.; Silwedel, C.; Fehrholz, M.; Waaga-Gasser, A.M.; Henrich, B.; Claus, H.; Speer, C.P. Ureaplasma Species Differentially Modulate Pro- and Anti-Inflammatory Cytokine Responses in Newborn and Adult Human Monocytes Pushing the State Toward Pro-Inflammation. Front. Cell. Infect. Microbiol. 2017, 7, 484. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Li, S.; Wang, N.; Tan, H.-Y.; Zhang, Z.; Feng, Y. The Cross-Talk Between Gut Microbiota and Lungs in Common Lung Diseases. Front. Microbiol. 2020, 11, 301. [Google Scholar] [CrossRef]
- Gallacher, D.; Mitchell, E.; Alber, D.; Wach, R.; Klein, N.; Marchesi, J.R.; Kotecha, S. Dissimilarity of the gut-lung axis and dysbiosis of the lower airways in ventilated preterm infants. Eur. Respir. J. 2020, 55, 1901909. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, M.; Husted, C.; Chen, C.; Thiagarajan, K.; Johns, J.L.; Rao, S.P.; Alvira, C.M. Activation of the nuclear factor-κB pathway during postnatal lung inflammation preserves alveolarization by suppressing macrophage inflammatory protein-2. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L593–L604. [Google Scholar] [CrossRef] [Green Version]
- McKenna, S.; Michaelis, K.A.; Agboke, F.; Liu, T.; Han, K.; Yang, G.; Dennery, P.A.; Wright, C.J. Sustained hyperoxia-induced NF-κB activation improves survival and preserves lung development in neonatal mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L1078–L1089. [Google Scholar] [CrossRef] [Green Version]
- Iosef, C.; Alastalo, T.-P.; Hou, Y.; Chen, C.; Adams, E.S.; Lyu, S.-C.; Cornfield, D.N.; Alvira, C.M. Inhibiting NF-κB in the developing lung disrupts angiogenesis and alveolarization. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L1023–L1036. [Google Scholar] [CrossRef] [Green Version]
- Ehrhardt, H.; Pritzke, T.; Oak, P.; Kossert, M.; Biebach, L.; Förster, K.; Koschlig, M.; Alvira, C.M.; Hilgendorff, A. Absence of TNF-α enhances inflammatory response in the newborn lung undergoing mechanical ventilation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L909–L918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wang, G.; Lin, S.; Wang, C.; Zha, J. Loss of interleukin-6 enhances the inflammatory response associated with hyperoxia-induced lung injury in neonatal mice. Exp. Ther. Med. 2019, 17, 3101–3107. [Google Scholar] [CrossRef] [Green Version]
- Lapcharoensap, W.; Kan, P.; Powers, R.J.; Shaw, G.M.; Stevenson, D.K.; Gould, J.B.; Wirtschafter, D.D.; Lee, H.C. The Relationship of Nosocomial Infection Reduction to Changes in Neonatal Intensive Care Unit Rates of Bronchopulmonary Dysplasia. J. Pediatr. 2017, 180, 105–109.e1. [Google Scholar] [CrossRef]
- Lu, L.; Yu, Y.; Guo, Y.; Wang, Y.; Chang, E.B.; Claud, E.C. Transcriptional modulation of intestinal innate defense/inflammation genes by preterm infant microbiota in a humanized gnotobiotic mouse model. PLoS ONE 2015, 10, e0124504. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.L.; Johnson, T.J.; Robin, B.; Bigger, H.R.; Buchanan, A.; Christian, E.; Nandhan, V.; Shroff, A.; Schoeny, M.; Engstrom, J.L.; et al. Influence of own mother’s milk on bronchopulmonary dysplasia and costs. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F256–F261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, D.; Hallberg, B.; Hansen-Pupp, I.; Dani, C.; Ramenghi, L.A.; Marlow, N.; Beardsall, K.; Bhatti, F.; Dunger, D.; Higginson, J.D.; et al. rhIGF-1/rhIGFBP-3 in Preterm Infants: A Phase 2 Randomized Controlled Trial. J. Pediatr. 2019, 206, 56–65.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronbach, J.; Shahzad, T.; Radajewski, S.; Chao, C.-M.; Bellusci, S.; Morty, R.E.; Reicherzer, T.; Ehrhardt, H. The Potentials and Caveats of Mesenchymal Stromal Cell-Based Therapies in the Preterm Infant. Stem Cells Int. 2018, 2018, 9652897. [Google Scholar] [CrossRef] [Green Version]
- Behnke, J.; Kremer, S.; Shahzad, T.; Chao, C.-M.; Böttcher-Friebertshäuser, E.; Morty, R.E.; Bellusci, S.; Ehrhardt, H. MSC Based Therapies—New Perspectives for the Injured Lung. J. Clin. Med. 2020, 9, 682. [Google Scholar] [CrossRef] [Green Version]

| Gram-Positive Strains | Gram-Negative Strains |
|---|---|
| Enterococcus species (n = 72) | Citrobacter species (n = 2) |
| Staphylococcus aureus (n = 44) | Enterobacter species (n = 28) |
| Streptococcus group A (n = 1) | Escherichia coli (n = 63) |
| Streptococcus group B (n = 2) | Haemophilus species (n = 2) |
| Klebsiella species (n = 17) | |
| Proteus species (n = 4) | |
| Pseudomonas species (n = 6) | |
| Serratia species (n = 0) | |
| Stenotrophomonas maltophilia (n = 0) |
| Total Cohort | No or Mild | Moderate or Severe | p-Value | |
|---|---|---|---|---|
| (n = 102) | BPD (n = 67) | BPD (n = 35) | ||
| gestational age (weeks) | 26 + 3 (23 + 4 − 30 + 5) | 26 + 6 (24 + 3 − 30 + 5) | 25 + 4 (23 + 4 − 29 + 1) | p = 0.001 ** |
| birth weight (g) | 795 (320–995) | 880 (490–995) | 690 (320–990) | p < 0.001 ** |
| male gender (%) | 45/102 (44.1%) | 24/67 (35.8%) | 21/35 (60.0%) | p = 0.020 * |
| small for gestational age (%) | 11/102 (10.8%) | 2/67 (3.0%) | 9/35 (25.7%) | p < 0.001 ** |
| multiple birth (%) | 47/102 (46.1%) | 32/67 (47.8%) | 15/35 (42.9%) | p = 0.660 |
| bronchopulmonary dysplasia no (%) | 18/102 (17.6%) | 18/67 (26.9%) | - | |
| mild (%) | 49/102 (48.0%) | 49/67 (73.1%) | ||
| moderate (%) | 5/102 (4.9%) | 5/35 (14.3%) | ||
| severe (%) | 30/102 (29.4%) | 30/35 (85.7%) | ||
| intraventricular hemorrhage | ||||
| total incidence (%) | 15/102 (14.7%) | 9/67 (13.4%) | 6/35 (17.1%) | p = 0.621 |
| grade III or IV (%) | 11/102 (10.8%) | 7/67 (10.4%) | 4/35 (11.4%) | p = 0.885 |
| periventricular leukomalacia (%) | 1/102 (1.0%) | 1/67 (1.5%) | 0/35 (0.0%) | p = 0.485 |
| retinopathy of prematurity | ||||
| any stage (%) | 66/102 (64.7%) | 36/67 (53.7%) | 30/35 (85.7%) | p = 0.001 ** |
| ≥stage 3 antenatal steroids | 23/102 (22.5%) | 7/67 (10.4%) | 16/35 (45.7%) | p < 0.001 ** |
| <24 h | 14/102/13.7%) | 8/67 (11.9%) | 6/35 (17.1%) | p = 0.026 * |
| 24 h—7 days | 48/102 (47.1%) | 27/67 (40.3%) | 21/35 (60.0%) | |
| >7 days | 40/102 (39.2%) | 32/67 (47.58%) | 8/35 (22.9%) | |
| provision of breast milk (%) | 93/102 (91.2%) | 62/67 (92.5%) | 31/35 (88.6%) | p = 0.509 |
| antibiotic therapy | ||||
| any (%) | 90/102 (88.2%) | 58/67 (86.6%) | 32/35 (91.4%) | p = 0.475 |
| started directly after birth (%) | 76/102 (74.5%) | 52/67 (77.6%) | 24/35 (68.6%) | p = 0.325 |
| duration antibiotic therapy (days) | 5 (0–41) | 5 (0–38) | 9 (0–41) | p = 0.002 ** |
| Estimate | Standard Error | z-Value | p-Value | |
|---|---|---|---|---|
| (intercept) | 8.8811 | 5.5682 | 1.595 | 0.1107 |
| gestational age (days) | −0.0288 | 0.0331 | −0.871 | 0.3838 |
| birth weight (g) | −0.0078 | 0.0026 | −3.023 | 0.0025 ** |
| small for gestational age | 2.1459 | 1.3547 | 1.584 | 0.1132 |
| male gender | 1.4725 | 0.6621 | 2.224 | 0.0262 * |
| multiple birth | 0.4519 | 0.3816 | 1.184 | 0.2363 |
| antenatal steroids | −0.0689 | 0.0396 | −1.740 | 0.0818 |
| (days before delivery) | ||||
| duration antibiotic therapy (days) | 0.0660 | 0.0495 | 1.334 | 0.1822 |
| Residual Degrees of Freedom | Residual Deviance | Degrees of Freedom | Deviance | p-Value | |
|---|---|---|---|---|---|
| upper airway pathogenic bacteria | 87 | 63.662 | 5 | 17.1102 | 0.0043 ** |
| upper airway gram-negative bacteria | 87 | 77.876 | 5 | 2.8959 | 0.7160 |
| anal pathogenic bacteria | 87 | 74.365 | 5 | 6.4067 | 0.2686 |
| anal gram-negative bacteria | 87 | 71.086 | 5 | 9.6856 | 0.0847 |
| Estimated | Regression Coefficient | Standard Error | z-Value | p-Value |
|---|---|---|---|---|
| (intercept) | 14.7012 | 6.9322 | 2.121 | 0.0339 * |
| gestational age (days) | −0.0434 | 0.0395 | −1.099 | 0.2716 |
| birth weight (g) | −0.0119 | 0.0034 | −3.552 | 0.0004 *** |
| small for gestational age | 2.6637 | 1.4345 | 1.857 | 0.0633 |
| gender | 2.1162 | 0.7970 | 2.655 | 0.0079 ** |
| multiple birth | 0.8535 | 0.4926 | 1.733 | 0.0832 |
| Antenatal steroids (days before delivery) | −0.0764 | 0.0474 | −1.610 | 0.1073 |
| duration antibiotic therapy (days) | 0.0727 | 0.0709 | 1.025 | 0.3052 |
| oral pathogenic bacteria/week 3 | −3.6769 | 1.2262 | −2.998 | 0.0027 ** |
| oral pathogenic bacteria/week 4 | −0.5549 | 0.9716 | −0.571 | 0.5679 |
| oral pathogenic bacteria/week 5 | −2.3071 | 1.1739 | −1.965 | 0.0494 * |
| oral pathogenic bacteria/week 6 | −0.0651 | 1.1884 | −0.055 | 0.9563 |
| oral pathogenic bacteria/week >6 | −0.1528 | 1.6931 | −0.090 | 0.9281 |
| Estimate | Standard Error | z-Value | p-Value | Regression Cofficient |
|---|---|---|---|---|
| (intercept) | 22.4478 | 7.8924 | 2.844 | 0.0045 ** |
| gestational age (days) | −0.1007 | 0.0456 | −2.208 | 0.0272 * |
| birth weight (g) | −0.0068 | 0.0027 | −2.524 | 0.0116 * |
| small for gestational age | 2.4235 | 1.4029 | 1.728 | 0.0841 |
| male gender | 1.3853 | 0.6866 | 2.018 | 0.0436 * |
| multiple birth | 0.5009 | 0.3889 | 1.288 | 0.1977 |
| antenatal steroids | −0.0667 | 0.0413 | −1.617 | 0.1059 |
| (days before delivery) | ||||
| antibiotic therapy started immediately after birth | −1.6629 | 0.8801 | −1.889 | 0.0588 |
| Estimated | Standard Error | z-Value | p-Value | Regression Coefficient |
|---|---|---|---|---|
| (intercept) | 10.5035 | 5.8628 | 1.792 | 0.0732 |
| gestational age (days) | −0.0361 | 0.0338 | −1.071 | 0.2841 |
| birth weight (g) | −0.0085 | 0.0027 | −3.110 | 0.0019 ** |
| small for gestational age | 2.1268 | 1.3412 | 1.586 | 0.0887 |
| male gender | 1.7519 | 0.6869 | 2.550 | 0.0108 * |
| multiple birth | 0.4434 | 0.3746 | 1.184 | 0.2366 |
| antenatal steroids | −0.0698 | 0.0400 | −1.746 | 0.0807 |
| (days before delivery) | ||||
| antibiotic therapy (yes/no) within the first 6 weeks of life | 0.6543 | 1.1178 | 0.585 | 0.5583 |
| Residual Degrees of Freedom | Residual Deviance | Degrees of Freedom | Deviance | p-Value | |
|---|---|---|---|---|---|
| duration antibiotic therapy (days) | 87 | 63.662 | 5 | 17.110 | 0.0043 ** |
| antibiotic therapy started | 87 | 61.739 | 5 | 17.199 | 0.0041 ** |
| immediately after birth | |||||
| antibiotic therapy (yes/no) | 87 | 64.477 | 5 | 17.912 | 0.0031 ** |
| within the first 6 weeks of life |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauer, T.; Behnke, J.; Oehmke, F.; Baecker, J.; Gentil, K.; Chakraborty, T.; Schloter, M.; Gertheiss, J.; Ehrhardt, H. Bacterial Colonization within the First Six Weeks of Life and Pulmonary Outcome in Preterm Infants <1000 g. J. Clin. Med. 2020, 9, 2240. https://doi.org/10.3390/jcm9072240
Lauer T, Behnke J, Oehmke F, Baecker J, Gentil K, Chakraborty T, Schloter M, Gertheiss J, Ehrhardt H. Bacterial Colonization within the First Six Weeks of Life and Pulmonary Outcome in Preterm Infants <1000 g. Journal of Clinical Medicine. 2020; 9(7):2240. https://doi.org/10.3390/jcm9072240
Chicago/Turabian StyleLauer, Tina, Judith Behnke, Frank Oehmke, Johanna Baecker, Katrin Gentil, Trinad Chakraborty, Michael Schloter, Jan Gertheiss, and Harald Ehrhardt. 2020. "Bacterial Colonization within the First Six Weeks of Life and Pulmonary Outcome in Preterm Infants <1000 g" Journal of Clinical Medicine 9, no. 7: 2240. https://doi.org/10.3390/jcm9072240






