Usefulness of 18F-FDG PET/CT in Patients with Cardiac Implantable Electronic Device Suspected of Late Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Patients
2.2. 18F-Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography (18F-FGD PET/CT) Technique
2.3. 18F-FGD PET/CT Imaging Interpretation
2.4. Assessment of Patients’ Outcome
2.5. Statistical Analysis
3. Results
3.1. Patients’ Baseline Characteristics
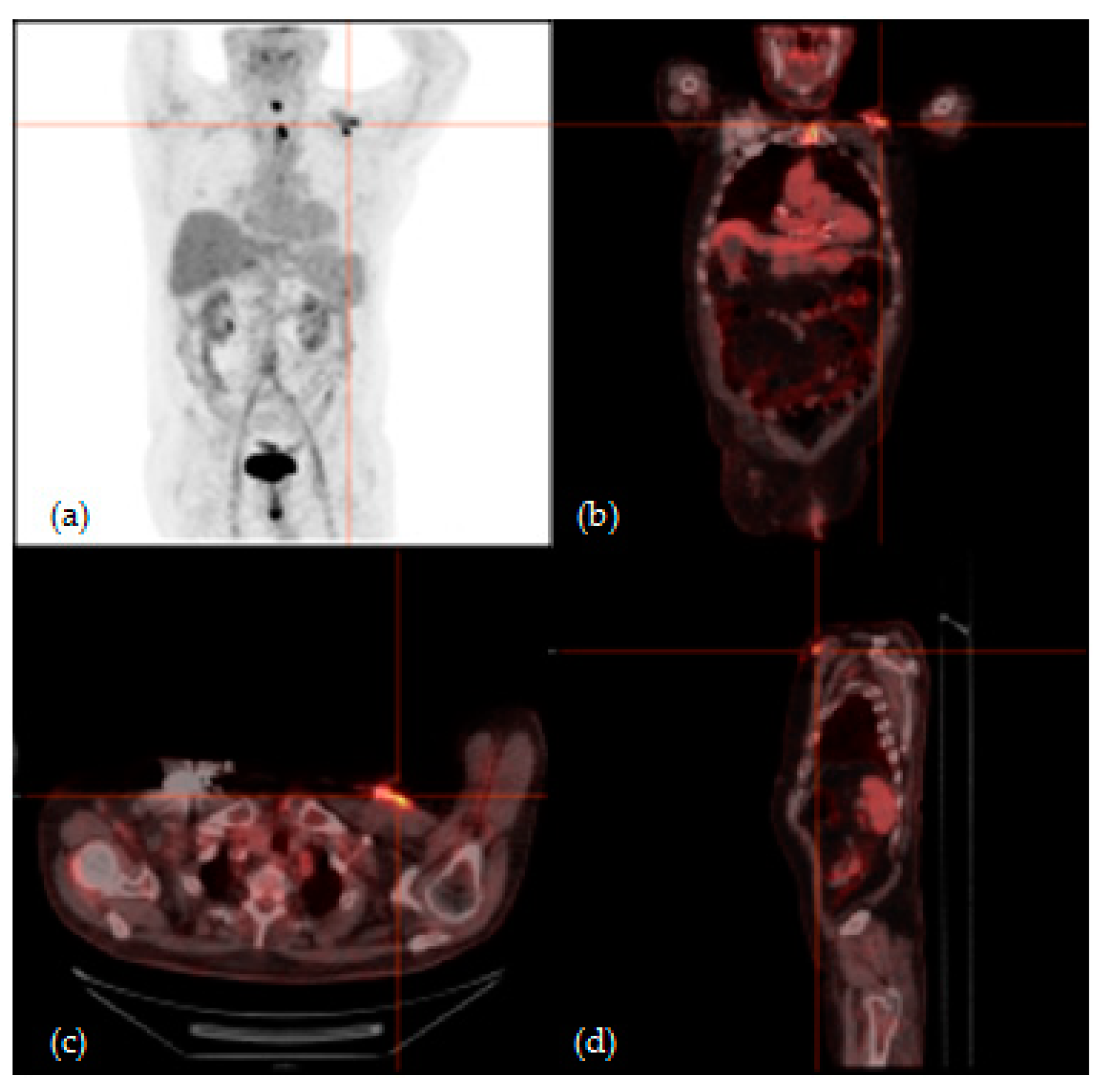
3.2. 18F-FGD PET/CT Analysis Results
3.3. Patients’ Outcome
3.4. Statistical Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Polewczyk, A.; Jacheć, W.; Polewczyk, A.M.; Tomasik, A.; Janion, M.; Kutarski, A. Infectious complications in patients with cardiac implantable electronic devices: Risk factors, prevention, and prognosis. Pol. Arch. Intern. Med. 2017, 127, 597–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrispin, J.; Love, C.J. Cardiac Implantable Electronic Device Infections and Lead Extraction: Are Patients with Renal Insufficiency Special? Circ. Arrhythmia Electrophysiol. 2018, 11, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bongiorni, M.G.; Burri, H.; Deharo, J.C.; Starck, C.; Kennergren, C.; Saghy, L.; Rao, A.; Tascini, C.; Lever, N.; Kutarski, A.; et al. 2018 EHRA expert consensus statement on lead extraction: Recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: Endorsed by APHRS/HRS/LAHRS. Europace 2018, 20, 1217. [Google Scholar] [CrossRef] [PubMed]
- Juneau, D.; Golfam, M.; Hazra, S.; Zuckier, L.S.; Garas, S.; Redpath, C.; Bernick, J.; Leung, E.; Chih, S.; Wells, G.; et al. Positron Emission Tomography and Single-Photon Emission Computed Tomography Imaging in the Diagnosis of Cardiac Implantable Electronic Device Infection. Circ. Cardiovasc. Imaging 2017, 10, e005772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voigt, A.; Shalaby, A.; Saba, S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: Temporal trends and causative insights. PACE-Pacing Clin. Electrophysiol. 2010, 33, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Cha, Y.M.; Wilson, W.R. Infections of cardiovascular implantable electronic devices. N. Engl. J. Med. 2012, 367, 842–849. [Google Scholar] [CrossRef] [Green Version]
- Nery, P.B.; Fernandes, R.; Nair, G.M.; Sumner, G.L.; Ribas, C.S.; Menon, S.M.D.; Wang, X.; Krahn, A.D.; Morillo, C.A.; Connolly, S.J.; et al. Device-related infection among patients with pacemakers and implantable defibrillators: Incidence, risk factors, and consequences. J. Cardiovasc. Electrophysiol. 2010, 21, 786–790. [Google Scholar] [CrossRef]
- Baddour, L.M.; Epstein, A.E.; Erickson, C.C.; Knight, B.P.; Levison, M.E.; Lockhart, P.B.; Masoudi, F.A.; Okum, E.J.; Wilson, W.R.; Beerman, L.B.; et al. Update on cardiovascular implantable electronic device infections and their management: A scientific statement from the american heart association. Circulation 2010, 121, 458–477. [Google Scholar] [CrossRef]
- Maytin, M.; Jones, S.O.; Epstein, L.M. Long-term mortality after transvenous lead extraction. Circ. Arrhythmia Electrophysiol. 2012, 5, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Welch, M.; Uslan, D.Z.; Greenspon, A.J.; Sohail, M.R.; Baddour, L.M.; Blank, E.; Carrillo, R.G.; Danik, S.B.; Del Rio, A.; Hellinger, W.; et al. Variability in clinical features of early versus late cardiovascular implantable electronic device pocket infections. PACE-Pacing Clin. Electrophysiol. 2014, 37, 955–962. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA-J. Am. Med. Assoc. 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Sandoe, J.A.T.; Barlow, G.; Chambers, J.B.; Gammage, M.; Guleri, A.; Howard, P.; Olson, E.; Perry, J.D.; Prendergast, B.D.; Spry, M.J.; et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. report of a joint working party project on behalf of the british society for antimicrobial chemotherapy (BSAC, host organization), british heart rh. J. Antimicrob. Chemother. 2015, 70, 325–359. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, D.C.; Sohail, M.R. Approach to diagnosis of cardiovascular implantable-electronic-device infection. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Sajadi, M.M.; Dilsizian, V. Merits of FDG PET/CT and Functional Molecular Imaging Over Anatomic Imaging With Echocardiography and CT Angiography for the Diagnosis of Cardiac Device Infections. JACC Cardiovasc. Imaging 2018, 11, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Asabella, A.N.; Di Palo, A.; Altini, C.; Ferrari, C.; Rubini, G. Multimodality imaging in tumor angiogenesis: Present status and perspectives. Int. J. Mol. Sci. 2017, 18, 1864. [Google Scholar] [CrossRef] [Green Version]
- Niccoli Asabella, A.; Simone, M.; Ballini, A.; Altini, C.; Ferrari, C.; Lavelli, V.; Luca, R.D.E.; Inchingolo, F.; Rubini, G. Predictive value of 18F-FDG PET/CT on survival in locally advanced rectal cancer after neoadjuvant chemoradiation. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8227–8236. [Google Scholar] [CrossRef]
- Ferrari, C.; Niccoli Asabella, A.; Merenda, N.; Altini, C.; Fanelli, M.; Muggeo, P.; De Leonardis, F.; Perillo, T.; Santoro, N.; Rubini, G. Pediatric Hodgkin lymphoma: Predictive value of interim 18 F-FDG PET/CT in therapy response assessment. Medicine 2017, 96, e5973. [Google Scholar] [CrossRef]
- Alavi, A.; Hess, S.; Werner, T.J.; Høilund-Carlsen, P.F. An update on the unparalleled impact of FDG-PET imaging on the day-to-day practice of medicine with emphasis on management of infectious/inflammatory disorders. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Gormsen, L.C.; Hess, S. Challenging but Clinically Useful: Fluorodeoxyglucose PET/Computed Tomography in Inflammatory and Infectious Diseases. PET Clin. 2020, 15, xi–xii. [Google Scholar] [CrossRef] [Green Version]
- Sarrazin, J.-F.; Philippon, F.; Trottier, M.; Tessier, M. Role of radionuclide imaging for diagnosis of device and prosthetic valve infections. World J. Cardiol. 2016, 8, 534. [Google Scholar] [CrossRef]
- Ahmed, F.Z.; James, J.; Cunnington, C.; Motwani, M.; Fullwood, C.; Hooper, J.; Burns, P.; Qamruddin, A.; Al-Bahrani, G.; Armstrong, I.; et al. Early diagnosis of cardiac implantable electronic device generator pocket infection using 18F-FDG-PET/CT. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 521–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granados, U.; Fuster, D.; Pericas, J.M.; Llopis, J.L.; Ninot, S.; Quintana, E.; Almela, M.; Pari, C.; Tolosana, J.M.; Falces, C.; et al. Diagnostic accuracy of 18F-FDG PET/CT in infective endocarditis and implantable cardiac electronic device infection: A cross-sectional study. J. Nucl. Med. 2016, 57, 1726–1732. [Google Scholar] [CrossRef] [Green Version]
- Sarrazin, J.F.; Philippon, F.; Tessier, M.; Guimond, J.; Molin, F.; Champagne, J.; Nault, I.; Blier, L.; Nadeau, M.; Charbonneau, L.; et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J. Am. Coll. Cardiol. 2012, 59, 1616–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diemberger, I.; Bonfiglioli, R.; Martignani, C.; Graziosi, M.; Biffi, M.; Lorenzetti, S.; Ziacchi, M.; Nanni, C.; Fanti, S.; Boriani, G. Contribution of PET imaging to mortality risk stratification in candidates to lead extraction for pacemaker or defibrillator infection: A prospective single center study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Erba, P.A.; Lancellotti, P.; Vilacosta, I.; Gaemperli, O.; Rouzet, F.; Hacker, M.; Signore, A.; Slart, R.H.J.A.; Habib, G. Recommendations on nuclear and multimodality imaging in IE and CIED infections. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1795–1815. [Google Scholar] [CrossRef]
- Tlili, G.; Amroui, S.; Mesguich, C.; Rivière, A.; Bordachar, P.; Hindié, E.; Bordenave, L. High performances of 18F-fluorodeoxyglucose PET-CT in cardiac implantable device infections: A study of 40 patients. J. Nucl. Cardiol. 2015, 22, 787–798. [Google Scholar] [CrossRef]
- Cautela, J.; Alessandrini, S.; Cammilleri, S.; Giorgi, R.; Richet, H.; Casalta, J.P.; Habib, G.; Raoult, D.; Mundler, O.; Deharo, J.C. Diagnostic yield of FDG positron-emission tomography/computed tomography in patients with CEID infection: A pilot study. Europace 2013, 15, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Graziosi, M.; Nanni, C.; Lorenzini, M.; Diemberger, I.; Bonfiglioli, R.; Pasquale, F.; Ziacchi, M.; Biffi, M.; Martignani, C.; Bartoletti, M.; et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: A prospective study. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1617–1623. [Google Scholar] [CrossRef]
- Osborne, M.T.; Hulten, E.A.; Murthy, V.L.; Skali, H.; Taqueti, V.R.; Dorbala, S.; DiCarli, M.F.; Blankstein, R. Patient preparation for cardiac fluorine-18 fluorodeoxyglucose positron emission tomography imaging of inflammation. J. Nucl. Cardiol. 2017, 24, 86–99. [Google Scholar] [CrossRef] [Green Version]
- Niccoli-Asabella, A.; Iuele, F.; Merenda, N.; Pisani, A.R.; Notaristefano, A.; Rubini, G. 18F-FDGPET/CT: Diabetes and hyperglycaemia. Nucl. Med. Rev. 2013, 16, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Grazia Bongiorni, M.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Anna Erba, P.; Iung, B.; et al. ESC GUIDELINES 2015 ESC Guidelines for the management of infective endocarditis The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC) Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef] [PubMed]
- Erba, P.A.; Pizzi, M.N.; Roque, A.; Salaun, E.; Lancellotti, P.; Tornos, P.; Habib, G. Multimodality Imaging in Infective Endocarditis: An Imaging Team Within the Endocarditis Team. Circulation 2019, 140, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Marciniak-Emmons, M.B.; Sterliński, M.; Syska, P.; Maciąg, A.; Farkowski, M.M.; Firek, B.; Dziuk, M.; Zając, D.; Pytkowski, M.; Szwed, H. New diagnostic pathways urgently needed. Protocol of PET Guidance i pilot study: Positron emission tomography in suspected cardiac implantable electronic device-related infection. Kardiol. Pol. 2016, 74, 47–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dy Chua, J.; Abdul-Karim, A.; Mawhorter, S.; Procop, G.W.; Tchou, P.; Niebauer, M.; Saliba, W.; Schweikert, R.; Wilkoff, B.L. The role of swab and tissue culture in the diagnosis of implantable cardiac device infection. PACE-Pacing Clin. Electrophysiol. 2005, 28, 1276–1281. [Google Scholar] [CrossRef]
- Saby, L.; Laas, O.; Habib, G.; Cammilleri, S.; Mancini, J.; Tessonnier, L.; Casalta, J.P.; Gouriet, F.; Riberi, A.; Avierinos, J.F.; et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: Increased valvular 18F- fluorodeoxyglucose uptake as a novel major criterion. J. Am. Coll. Cardiol. 2013, 61, 2374–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensimhon, L.; Lavergne, T.; Hugonnet, F.; Mainardi, J.L.; Latremouille, C.; Maunoury, C.; Lepillier, A.; Le Heuzey, J.Y.; Faraggi, M. Whole body [18F]fluorodeoxyglucose positron emission tomography imaging for the diagnosis of pacemaker or implantable cardioverter defibrillator infection: A preliminary prospective study. Clin. Microbiol. Infect. 2011, 17, 836–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertagna, F.; Bisleri, G.; Motta, F.; Merli, G.; Cossalter, E.; Lucchini, S.; Biasiotto, G.; Bosio, G.; Terzi, A.; Muneretto, C.; et al. Possible role of F18-FDG-PET/CT in the diagnosis of endocarditis: Preliminary evidence from a review of the literature. Int. J. Cardiovasc. Imaging 2012, 28, 1417–1425. [Google Scholar] [CrossRef]



| Patients | Clinical Signs | Laboratory Signs | Instrumental Signs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Age | Sex | Type of CIED Implanted | Fever | Local Signs of CIED Infection | ESR (v.n. <20 mm/h) | CRP (v.n. <2.9 mg/L) | PCT (v.n. <0.5 ng/mL) | WBC (v.n. 3.7-9.7 x103 uL) | Blood Culture | TTE | TEE |
| 1 | 75 | M | ICD | Yes | Yes | 48 | 18.4 | 16 | 5.6 | 0 | Negative | / |
| 2 | 74 | M | PM | Yes | No | 8 | 3.1 | 3.7 | 5.5 | 0 | Negative | / |
| 3 | 73 | M | PM | Yes | No | 18 | 35 | 0.02 | 10.05 | 0 | Negative | / |
| 4 | 83 | M | PM | Yes | Yes | 25 | 1 | 0.03 | 7.65 | Staphyl. Aureus | Negative | Negative |
| 5 | 83 | M | ICD | Yes | Yes | 48 | 3 | 0.04 | 6.57 | Staphyl. Epiderm. | Negative | / |
| 6 | 59 | F | PM | No | Yes | 38 | 0 | 0.02 | 6.7 | 0 | Negative | / |
| 7 | 46 | M | PM | Yes | Yes | 52 | 42.7 | 0.07 | 7.77 | 0 | Negative | Positive |
| 8 | 84 | M | ICD | Yes | No | 50 | 70.6 | 11 | 6.2 | 0 | Negative | / |
| 9 | 56 | M | PM | No | No | 31 | 99 | 0.13 | 8.91 | 0 | Positive | Positive |
| 10 | 63 | M | PM | Yes | No | 50 | 3.1 | 3.4 | 4.35 | 0 | Negative | / |
| 11 | 71 | M | PM | Yes | Yes | 0 | 1 | 0.02 | 5.48 | 0 | Negative | Positive |
| 12 | 53 | M | ICD | No | No | 16 | 1.7 | 0.03 | 6.31 | 0 | Negative | / |
| 13 | 73 | M | ICD | Yes | No | 37 | 38 | 0.07 | 7.04 | 0 | Negative | Positive |
| 14 | 62 | M | ICD | No | Yes | 0 | 0 | 0 | 4.5 | 0 | Negative | / |
| 15 | 78 | M | ICD | No | No | 1 | 1 | 0.03 | 5.93 | Staphyl. Epiderm. | Negative | / |
| 18F-FDG PET/CT Analysis Results | Final Results | ||||||
|---|---|---|---|---|---|---|---|
| ID | Result | Site | SUVmax | SQR | Microbiological Analysis | Clinical Follow-Up | |
| 1 | Positive | 3.65 | 3.80 | Staphyl. haemolyticus | / | ||
| 2 | Positive | 4.96 | 9.54 | / | Negative | ||
| 3 | Negative | 2.19 | 3.37 | Negative | / | ||
| 4 | Positive | 3.32 | 4.61 | Staphyl. aureus | / | ||
| 5 | Positive | 4.31 | 8.71 | Staphyl. epidermidis | / | ||
| 6 | Negative | 2.56 | 3.24 | / | Negative | ||
| 7 | Positive | Lead | 2.80 | 4.41 | Staphyl. epidermidis | / | |
| 8 | Negative | 1.96 | 3.24 | Staphyl. epidermidis | / | ||
| 9 | Positive | Lead | 2.69 | 2.88 | Staphyl. epidermidis | / | |
| 10 | Positive | 6.55 | 9.63 | Staphyl. epidermidis | / | ||
| 11 | Positive | 6.75 | 10.38 | Staphyl. epidermidis | / | ||
| 12 | Negative | 1.82 | 1.43 | / | Negative | ||
| 13 | Positive | 2.20 | 4.58 | Staphyl. epidermidis | / | ||
| 14 | Positive | 4.61 | 8.46 | Staphyl. epidermidis | / | ||
| 15 | Positive | 7.33 | 11.63 | Staphyl. epidermidis | / | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubini, G.; Ferrari, C.; Carretta, D.; Santacroce, L.; Ruta, R.; Iuele, F.; Lavelli, V.; Merenda, N.; D’Agostino, C.; Sardaro, A.; et al. Usefulness of 18F-FDG PET/CT in Patients with Cardiac Implantable Electronic Device Suspected of Late Infection. J. Clin. Med. 2020, 9, 2246. https://doi.org/10.3390/jcm9072246
Rubini G, Ferrari C, Carretta D, Santacroce L, Ruta R, Iuele F, Lavelli V, Merenda N, D’Agostino C, Sardaro A, et al. Usefulness of 18F-FDG PET/CT in Patients with Cardiac Implantable Electronic Device Suspected of Late Infection. Journal of Clinical Medicine. 2020; 9(7):2246. https://doi.org/10.3390/jcm9072246
Chicago/Turabian StyleRubini, Giuseppe, Cristina Ferrari, Domenico Carretta, Luigi Santacroce, Rossella Ruta, Francesca Iuele, Valentina Lavelli, Nunzio Merenda, Carlo D’Agostino, Angela Sardaro, and et al. 2020. "Usefulness of 18F-FDG PET/CT in Patients with Cardiac Implantable Electronic Device Suspected of Late Infection" Journal of Clinical Medicine 9, no. 7: 2246. https://doi.org/10.3390/jcm9072246
APA StyleRubini, G., Ferrari, C., Carretta, D., Santacroce, L., Ruta, R., Iuele, F., Lavelli, V., Merenda, N., D’Agostino, C., Sardaro, A., & Niccoli Asabella, A. (2020). Usefulness of 18F-FDG PET/CT in Patients with Cardiac Implantable Electronic Device Suspected of Late Infection. Journal of Clinical Medicine, 9(7), 2246. https://doi.org/10.3390/jcm9072246






