Impact of Incomplete Coronary Revascularization on Late Ischemic and Bleeding Events after Transcatheter Aortic Valve Replacement
Abstract
1. Introduction
2. Materials and Methods
2.1. Calculation of Baseline SYNTAX Score (bSS), Residual SYNTAX Score (rSS) and Syntax Revascularization Index (SRI)
2.2. Collection of Data
2.3. Study Endpoints
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Clinical Outcomes
3.2.1. Ischemic Events
3.2.2. Bleeding Events
3.2.3. Myocardial Infarction Predictors
3.2.4. Predictors of Late Major/Life-Threatening Events
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goel, S.S.; Ige, M.; Tuzcu, E.M.; Ellis, S.G.; Stewart, W.J.; Svensson, L.G.; Lytle, B.W.; Kapadia, S.R. Severe aortic stenosis and coronary artery disease-implications for management in the transcatheter aortic valve replacement era: A comprehensive review. J. Am. Coll. Cardiol. 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Gilard, M.; Eltchaninoff, H.; Iung, B.; Donzeau-Gouge, P.; Chevreul, K.; Fajadet, J.; Leprince, P.; Leguerrier, A.; Lievre, M.; Prat, A.; et al. FRANCE 2 Investigators. Registry of transcatheter aortic-valve implantation in high-risk patients. N. Engl. J. Med. 2012, 366, 1705–1715. [Google Scholar]
- Stewart, B.F.; Siscovick, D.; Lind, B.K.; Gardin, J.M.; Gottdiener, J.S.; Smith, V.E.; Kitzman, D.W.; Otto, C.M. Clinical factors associated with calcific aortic valve disease. Cardiovasc. Health Study J. Am. Coll. Cardiol. 1997, 29, 630–634. [Google Scholar] [CrossRef]
- Lindroos, M.; Kupari, M.; Valvanne, J.; Strandberg, T.; Heikkilä, J.; Tilvis, R. Factors associated with calcific aortic valve degeneration in the elderly. Eur. Heart J. 1994, 15, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Aranki, S.F.; Rizzo, R.J.; Couper, G.S.; Adams, D.H.; Collins, J.J., Jr.; Gildea, J.S.; Kinchla, N.M.; Cohn, L.H. Aortic valve replacement in the elderly effect of gender and coronary artery disease on operativemortality. Circulation 1993, 88, II17–II23. [Google Scholar]
- Falk, V.; Baumgartner, H.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2017, 52, 616–664. [Google Scholar] [PubMed]
- Sianos, G.; Morel, M.A.; Kappetein, A.P.; Morice, M.C.; Colombo, A.; Dawkins, K.; van den Brand, M.; Van Dyck, N.; Russell, M.E.; Mohr, F.W.; et al. The SYNTAX Score: An angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005, 1, 219–227. [Google Scholar] [PubMed]
- Farooq, V.; Serruys, P.W.; Bourantas, C.V.; Zhang, Y.; Muramatsu, T.; Feldman, T.; Holmes, D.R.; Mack, M.; Morice, M.C.; Ståhle, E.; et al. Quantification of incomplete revascularization and its association with five-year mortality in the synergy between percutaneous coronary intervention with taxus and cardiac surgery (SYNTAX) trial validation of the residual SYNTAX score. Circulation 2013, 128, 141–151. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The ValveAcademic Research Consortium-2 consensus document. J. Am. Coll. Cardiol. 2012, 60, 1438–1454. [Google Scholar] [CrossRef]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef]
- Horrocks, J.; Thompson, M.E. Modeling event times with multiple outcomes using the Wiener process with drift. Lifetime Data Anal. 2004, 10, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Gray, R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann. Stat. 1988, 16, 1141–1154. [Google Scholar] [CrossRef]
- Masson, J.B.; Lee, M.; Boone, R.H.; Al Ali, A.; Al Bugami, S.; Hamburger, J.; John Mancini, G.B.; Ye, J.; Cheung, A.; Humphries, K.H.; et al. Impact of coronary artery disease on outcomes after transcatheter aortic valve implantation. Catheter Cardiovasc. Interv. 2010, 76, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Van Mieghem, N.M.; van der Boon, R.M.; Faqiri, E.; Diletti, R.; Schultz, C.; van Geuns, R.J.; Serruys, P.W.; Kappetein, A.P.; van Domburg, R.T.; de Jaegere, P.P. Complete revascularization is not a prerequisite for success in current transcatheter aortic valve implantation practice. JACC Cardiovasc. Interv. 2013, 6, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, G.G.; Stortecky, S.; Cao, D.; Rat-Wirtzler, J.; O’Sullivan, C.J.; Gloekler, S.; Buellesfeld, L.; Khattab, A.A.; Nietlispach, F.; Pilgrim, T.; et al. Coronary artery disease severity and aortic stenosis: Clinical outcomes according to SYNTAXscore in patients undergoing transcatheter aortic valve implantation. Eur. Heart J. 2014, 35, 2530–2540. [Google Scholar] [CrossRef] [PubMed]
- Shamekhi, J.; Stundl, A.; Weber, M.; Mellert, F.; Welz, A.; Grube, E.1.; Nickenig, G.; Werner, N.; Sinning, J.M. Impact of coronary artery disease in patients undergoing transfemoral transcatheter aortic valve implantation. Int J. Cardiol. 2017, 245, 215–221. [Google Scholar] [CrossRef]
- Ussia, G.P.; Barbanti, M.; Colombo, A.; Tarantini, G.; Petronio, A.S.; Ettori, F.; Ramondo, A.; Santoro, G.; Klugmann, S.; Bedogni, F.; et al. CoreValve Italian Registry Investigators. Impact of coronary artery disease in elderly patients undergoing transcatheter aortic valve implantation: Insight from the Italian CoreValve Registry. Int. J. Cardiol. 2013, 167, 943–950. [Google Scholar] [CrossRef]
- Hamm, C.W.; Möllmann, H.; Holzhey, D.; Beckmann, A.; Veit, C.; Figulla, H.-R.; Cremer, J.; Kuck, K.-H.; Lange, R.; Zahn, R.; et al. The German Aortic Valve Registry (GARY): In-hospital outcome. Eur. Heart J. 2014, 35, 1588–1598. [Google Scholar] [CrossRef]
- Iung, B.; Drissi, M.F.; Michel, P.L.; de Pamphilis, O.; Tsezana, R.; Cormier, B.; Vahanian, A.; Acar, J. Prognosis of valve replacement foraortic stenosiswith or without coexisting coronary heart disease: A comparative study. J. Heart Valve Dis. 1993, 2, 430–439. [Google Scholar]
- Lund, O.; Nielsen, T.T.; Pilegaard, H.K.; Magnussen, K.; Knudsen, M.A. The influence of coronary artery disease and bypass grafting on early and late survival after valve replacement for aortic stenosis. J. Thorac. Cardiovasc. Surg. 1990, 100, 327–337. [Google Scholar] [CrossRef]
- Mullany, C.J.; Elveback, L.R.; Frye, R.L.; Pluth, J.R.; Edwards, W.D.; Orszulak, T.A.; Nassef, L.A., Jr.; Riner, R.E.; Danielson, G.K. Coronary artery disease and its management: Influence on survival in patients undergoing aortic valve replacement. J. Am. Coll. Cardiol. 1987, 10, 66–72. [Google Scholar] [CrossRef]
- Beach, J.M.; Mihaljevic, T.; Svensson, L.G.; Rajeswaran, J.; Marwick, T.; Griffin, B.; Johnston, D.R.; Sabik, J.F., 3rd; Blackstone, E.H. Coronary artery disease outcomes of aortic valve replacement for severe aortic stenosis. J. Am. Coll. Cardiol. 2013, 61, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165, published correction in Eur. Heart J. 2019, 40, 3096. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.S.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, C.D.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Søndergaard, L.; Popma, J.J.; Reardon, M.J.; Van Mieghem, N.M.; Deeb, G.M.; Kodali, S.; George, I.; Williams, M.R.; Yakubov, S.J.; Kappetein, A.P.; et al. Comparison of a Complete Percutaneous versus Surgical Approach to Aortic Valve Replacement and Revascularization in Patients at Intermediate Surgical Risk: Results from the Randomized SURTAVI Trial. Circulation 2019. [Google Scholar] [CrossRef]
- Kibler, M.; Marchandot, B.; Messas, N.; Labreuche, J.; Vincent, F.; Grunebaum, L.; Hoang, V.A.; Reydel, A.; Crimizade, U.; Kindo, M.; et al. Primary Hemostatic Disorders and Late Major Bleeding After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 72, 2139–2148. [Google Scholar] [CrossRef]
- Généreux, P.; Head, S.J.; Van Mieghem, N.M.; Kodali, S.; Kirtane, A.J.; Xu, K.; Smith, C.; Serruys, P.W.; Kappetein, A.P.; Leon, M.B. Clinical outcomes after transcatheter aortic valve replacement using valve academic researchconsortium definitions: A weighted meta-analysis of 3519 patients from 16 studies. J. Am. Coll. Cardiol. 2012, 59, 2317–2326. [Google Scholar] [CrossRef]
- Piccolo, R.; Pilgrim, T.; Franzone, A.; Valgimigli, M.; Haynes, A.; Asami, M.; Lanz, J.; Räber, L.; Praz, F.; Langhammer, B.; et al. Frequency, Timing, and Impact of Access-Site and Non-Access-Site Bleeding on Mortality Among Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 1436–1446. [Google Scholar] [CrossRef]
- Khan, R.; Al-Hawwas, M.; Hatem, R.; Azzalini, L.; Fortier, A.; Joliecoeur, E.M.; Tanguay, J.F.; Lavoie-L’Allier, P.; Ly, H.Q. Prognostic impact of the residual SYNTAX score on in-hospital outcomes in patients undergoing primary percutaneous coronary intervention. Catheter Cardiovasc. Interv. 2016, 88, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, G.; Patialiakas, A.; Piccolo, R.; Thury, A.; Colangelo, S.; Campo, G.; Tebaldi, M.; Ungi, I.; Tondi, S.; Roffi, M.; et al. Impact of angiographic coronary artery disease complexity on ischemic and bleeding risks and on the comparative effectiveness of zotarolimus-eluting vs. bare-metal stents in uncertain drug-eluting stent candidates. Int. J. Cardiol. 2019, 277, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, M.V.; Généreux, P.; Palmerini, T.; Caixeta, A.; Xu, K.; McAndrew, T.C.; Francese, D.P.; Kirtane, A.J.; Mehran, R.; Stone, G.W. The association between the extent of coronary artery disease and major bleeding events after percutaneous coronary intervention: From the ACUITY trial. J. Invasive Cardiol. 2015, 27, 203–211. [Google Scholar] [PubMed]
- Kotsia, A.; Brilakis, E.S.; Held, C.; Cannon, C.; Steg, G.P.; Meier, B.; Cools, F.; Claeys, M.J.; Cornel, J.H.; Aylward, P.; et al. Extent of coronary artery disease and outcomes after ticagrelor administration in patients with an acute coronary syndrome: Insights from the PLATelet inhibition and patient Outcomes (PLATO) trial. Am. Heart J. 2014, 168, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Cohen, D.J.; Williams, M.R.; Mack, M.; Kodali, S.K.; Svensson, L.G.; Kirtane, A.J.; Xu, K.; McAndrew, T.C.; Makkar, R.; et al. Bleeding complications after surgical aortic valve replacement compared with transcatheter aortic valve replacement: Insights from the PARTNER I Trial (Placement of Aortic Transcatheter Valve). J. Am. Coll. Cardiol. 2014, 63, 1100–1109. [Google Scholar] [CrossRef]
- Vincentelli, A.; Susen, S.; Le Tourneau, T.; Six, I.; Fabre, O.; Juthier, F.; Bauters, A.; Decoene, C.; Goudemand, J.; Prat, A.; et al. Acquired von Willebrand syndrome in aortic stenosis. N. Engl. J. Med. 2003, 349, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Blackshear, J.L.; McRee, C.W.; Safford, R.E.; Pollak, P.M.; Stark, M.E.; Thomas, C.S.; Rivera, C.E.; Wysokinska, E.M.; Chen, D. von Willebrand Factor Abnormalities and Heyde Syndrome in Dysfunctional Heart Valve Prostheses. JAMA Cardiol. 2016, 1, 198–204. [Google Scholar] [CrossRef]
- Caspar, T.; Jesel, L.; Desprez, D.; Grunebaum, L.; Samet, H.; Trinh, A.; Petit-Eisenmann, H.; Kindo, M.; Ohlmann, P.; Morel, O. Effects of transcutaneous aortic valve implantation on aortic valve disease-related hemostatic disorders involving von Willebrand factor. Can. J. Cardiol. 2015, 31, 738–743. [Google Scholar] [CrossRef]
- Spangenberg, T.; Budde, U.; Schewel, D.; Frerker, C.; Thielsen, T.; Kuck, K.H.; Schäfer, U. Treatment of acquired von Willebrand syndrome in aortic stenosis with transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 2015, 8, 692–700. [Google Scholar] [CrossRef]
- Van Belle, E.; Rauch, A.; Vincent, F.; Robin, E.; Kibler, M.; Labreuche, J.; Jeanpierre, E.; Levade, M.; Hurt, C.; Rousse, N.; et al. Von Willebrand Factor Multimers during Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2016, 375, 335–344. [Google Scholar] [CrossRef]
- Kibler, M.; Marchandot, B.; Messas, N.; Caspar, T.; Vincent, F.; Von Hunolstein, J.J.; Grunebaum, L.; Reydel, A.; Rauch, A.; Crimizade, U.; et al. CT-ADP Point-of-Care Assay Predicts 30-Day Paravalvular Aortic Regurgitation and Bleeding Events following Transcatheter Aortic Valve Replacement. Thromb. Haemost. 2018, 118, 893–905. [Google Scholar] [CrossRef] [PubMed]


| Variables | rSS ≤ 8 | rSS > 8 | p Value |
|---|---|---|---|
| Clinical Parameters | |||
| Age (Median IQR)–year | 85 (82–89) | 85 (80–86) | 0.127 |
| Male sex–no./total no. (%) | 124 (45.4%) | 12 (31.6%) | 0.107 |
| EuroScore (Median IQR)–% | 17 (11–25) | 19(13–28) | 0.263 |
| BMI (Median IQR) | 26.6 (23.4–30.0) | 25.1 (22.0–26.9) | 0.022 |
| NYHA Class before TAVR–no./total no. (%) | |||
| NYHA 2 | 81 (29.7%) | 8 (21.1%) | 0.271 |
| NYHA 3 | 161 (59%) | 24 (63.2%) | 0.623 |
| NYHA 4 | 31 (11.4%) | 6 (15.8%) | 0.429 |
| Cardiovascular Risk Factor and Medical History | |||
| Hypertension–no./total no. (%) | 230 (84.2%) | 31 (81.6%) | 0.675 |
| Diabetes mellitus–no./total no. (%) | 97 (35.7%) | 13 (34.2%) | 0.861 |
| Dyslipidemia–no./total no. (%) | 151 (55.5%) | 23 (60.5%) | 0.56 |
| Current smoking–no./total no. (%) | 10 (3.7%) | 2 (5.3%) | 0.631 |
| Current dialysis–no./total no. (%) | 5 (1.8%) | 2 (5.6%) | 0.16 |
| Family history of cardiovascular disease–no./total no. (%) | 12 (4.4%) | 3 (7.9%) | 0.349 |
| Previous angioplasty–no./total no. (%) | 35 (12.8%) | 11 (28.9%) | 0.009 |
| History of myocardial infarction–no./total no. (%) | 37 (13.6%) | 9 (23.7%) | 0.102 |
| History of atrial fibrillation–no./total no. (%) | 116 (42.5%) | 17 (44.72%) | 0.793 |
| Chronic kidney disease (serum creatinine > 150 µmol L) | 59 (21.6%) | 10 (26.3%) | 0.513 |
| Prior bleeding events–no./total no. (%) | 34 (12.5%) | 7 (18.4%) | 0.308 |
| Pre-hospital Antithrombotic Management | |||
| Single APT–no./total no. (%) | 147 (53.8%) | 29 (76.3%) | 0.009 |
| Dual APT–no./total no. (%) | 51 (18.7%) | 19 (50%) | <0.001 |
| Loading dose Clopidogrel–no./total no. (%) | 123 (45.1%) | 11 (28.9%) | 0.06 |
| Anticoagulant therapy–no./total no. (%) | 113 (41.4%) | 14 (36.8%) | 0.593 |
| Imaging Parameters | |||
| Mean aortic gradient–mmHg ± DS | 49.3 ± 12.9 | 45.5 ± 11.1 | 0.085 |
| Aortic valve calcium score (Median IQR)–AU | 2781.5 (2051–3806) | 2713.0 (1790.5–3270.0) | 0.399 |
| CT aortic surface (Median IQR)–mm2 ± DS | 476 (414–537) | 436 (391–539) | 0.386 |
| Echocardiography Parameters | |||
| LVEF pre-TAVR (Median IQR)–(%) | 58 (50–64) | 53 (40–63) | 0.099 |
| LVEF 1 month post-TAVR (Median IQR)–(%) | 60 (54–66) | 55 (48–65) | 0.102 |
| LVEDD (Median IQR)–mm ± DS | 49 (45–54) | 50 (44–56 | 0.646 |
| LVESD (Median IQR)–mm ± DS | 33 (28–39) | 38 (30–43) | 0.039 |
| Outflow chamber of the left ventricle (Median IQR)–mm ± DS | 21 (20–23) | 21(20–23) | 0.703 |
| AVA baseline (Median IQR)–cm² ± DS | 0.720 (0.590–0.860) | 0.720 (0.552–0.867) | 0.706 |
| Mean Aortic Gradient (Median IQR)–mmHg ± DS | 48 (41–58) | 45 (39–51) | 0.102 |
| Systolic PAP (Median IQR)–mmHg ± DS | 38 (30–47) | 37 (31–44) | 0.769 |
| Baseline Biological Characteristics | |||
| CT ADP Baseline (Median IQR) | 186 (130–300) | 175 (126–300) | 0.533 |
| CT ADP Day 1 Post TAVR (Median IQR) | 121 (97–177) | 141 (100–182) | 0.388 |
| PRI VASP Day 1 Post TAVR (Median IQR) | 71 (60–78) | 63 (46–76) | 0.023 |
| Coronary AngiographyCharacteristics | |||
| Baseline SYNTAX score (bSS) (Median IQR) | 0 (0–5) | 19 (13–25) | <0.001 |
| Residual SYNTAX score (rSS) (Median IQR) | 0 (0–2) | 13 (10–16) | <0.001 |
| Angioplasty–no./total no. (%) | 67 (24.5%) | 22 (57.9%) | <0.001 |
| Left main angioplasty–no./total no. (%) | 6 (2.2%) | 3 (7.9%) | 0.05 |
| Left anterior descending angioplasty–no./total no. (%) | 39 (14.3%) | 11 (28.9%) | 0.021 |
| Diagonal angioplasty–no./total no. (%) | 2 (0.7%) | 3 (7.9%) | 0.001 |
| Intermediate angioplasty–no./total no. (%) | 2 (0.7%) | 0 (0%) | 0.597 |
| Circumflex angioplasty–no./total no. (%) | 14 (5.1%) | 6 (15.8%) | 0.012 |
| Marginal angioplasty–no./total no. (%) | 7 (2.6%) | 2 (5.3%) | 0.352 |
| Right coronary artery angioplasty–no./total no. (%) | 23 (8.4%) | 8 (21.1%) | 0.015 |
| Variables | rSS ≤ 8 | rSS > 8 | p Value |
|---|---|---|---|
| Valve | |||
| Sapien–no./total no. (%) | 170 (62.3%) | 26 (68.4%) | 0.462 |
| CoreValve–no./total no. (%) | 103 (37.7%) | 12 (31.6%) | 0.462 |
| Transfemoral approach–no./total no. (%) | 247 (90.5%) | 35 (92.1%) | 0.746 |
| Size of the Introducer | |||
| 14F | 166 (60.8%) | 27 (71.1%) | 0.223 |
| 16F | 41 (15%) | 5 (13.2%) | 0.762 |
| 18F | 66 (24.2%) | 6 (15.8%) | 0.251 |
| Reimpaction for significant paravalvular aortic regurgitation–no./total no. (%) | 30 (11%) | 4 (10.5%) | 0.932 |
| Size Valve | |||
| 23 mm–no./total no. (%) | 83 (30.4%) | 14 (36.8%) | 0.422 |
| 26 mm–no./total no. (%) | 103 (37.7%) | 14 (36.8%) | 0.916 |
| 29 mm–no./total no. (%) | 76 (27.8%) | 9 (23.7%) | 0.59 |
| 31 mm–no./total no. (%) | 11 (4%) | 1 (2.6%) | 0.675 |
| Discharge Antithrombotic Medication | |||
| ASA–no./total no. (%) | 268 (98.2%) | 35 (92.1%) | 0.027 |
| DAPT–no./total no. (%) | 152 (55.7%) | 27 (71.1%) | 0.072 |
| Clopidogrel–no./total no. (%) | 151 (55.3%) | 27 (71.1%) | 0.066 |
| Anticoagulant therapy–no./total no. (%) | 121 (44.3%) | 17 (44.7%) | 0.962 |
| No DAPT nor anticoagulant therapy–no./total no. (%) | 7 (87.5%) | 1 (12.5%) | 0.948 |
| Duration of DAPT (Median IQR)–Days | 60 (60–90) | 60 (37–90) | 0.765 |
| Variables | rSS ≤ 8 | rSS > 8 | p Value | bSS ≤ 22 | bSS > 22 | p Value |
|---|---|---|---|---|---|---|
| Primary Endpoint–no./total no. (%) | ||||||
| MACE = cardiovascular death and/or myocardial infarction and/or rehospitalization for heart failure and/or stroke | 103 (37.9%) | 18 (47.4%) | 0.261 | 109 (37.3%) | 12 (66.7%) | 0.013 |
| Secondary Endpoint–no./total no. (%) | ||||||
| Death from any cause | 87 (31.9%) | 10 (26.3%) | 0.489 | 92 (31.4%) | 5 (27.8%) | 0.748 |
| Cardiovascular death | 40 (14.7%) | 5 (13.2%) | 0.806 | 42 (14.3% | 3 (16.7%) | 0.785 |
| Rehospitalization for heart failure | 72 (26.4%) | 11 (28.9%) | 0.737 | 75 (25.6%) | 8 (44.4%) | 0.079 |
| Myocardial infarction | 7 (2.6%) | 5 (13.2%) | 0.001 | 9 (3.1%) | 3 (16.7%) | 0.004 |
| Stroke | 25 (9.2%) | 4 (10.5%) | 0.786 | 25 (8.5%) | 4 (22.2%) | 0.053 |
| Bleeding–no./total no. (%) | ||||||
| Immediate post-procedural major and life threating bleeding | 54 (19.8%) | 13 (34.2%) | 0.043 | 60 (20.5%) | 7 (38.9%) | 0.065 |
| Immediate post-procedural major bleeding | 39 (14.3%) | 13 (34.2%) | 0.002 | 45 (15.4%) | 7 (38.9%) | 0.009 |
| Immediate post-procedural life threatening bleeding | 15 (5.5%) | 0 (0%) | 0.139 | 15 (5.1%) | 0 (0%) | 0.325 |
| Bleeding requiring red blood cell transfusion >2 U | 55 (20.1%) | 12 (31.6%) | 0.108 | 60 (20.5%) | 7 (38.9%) | 0.065 |
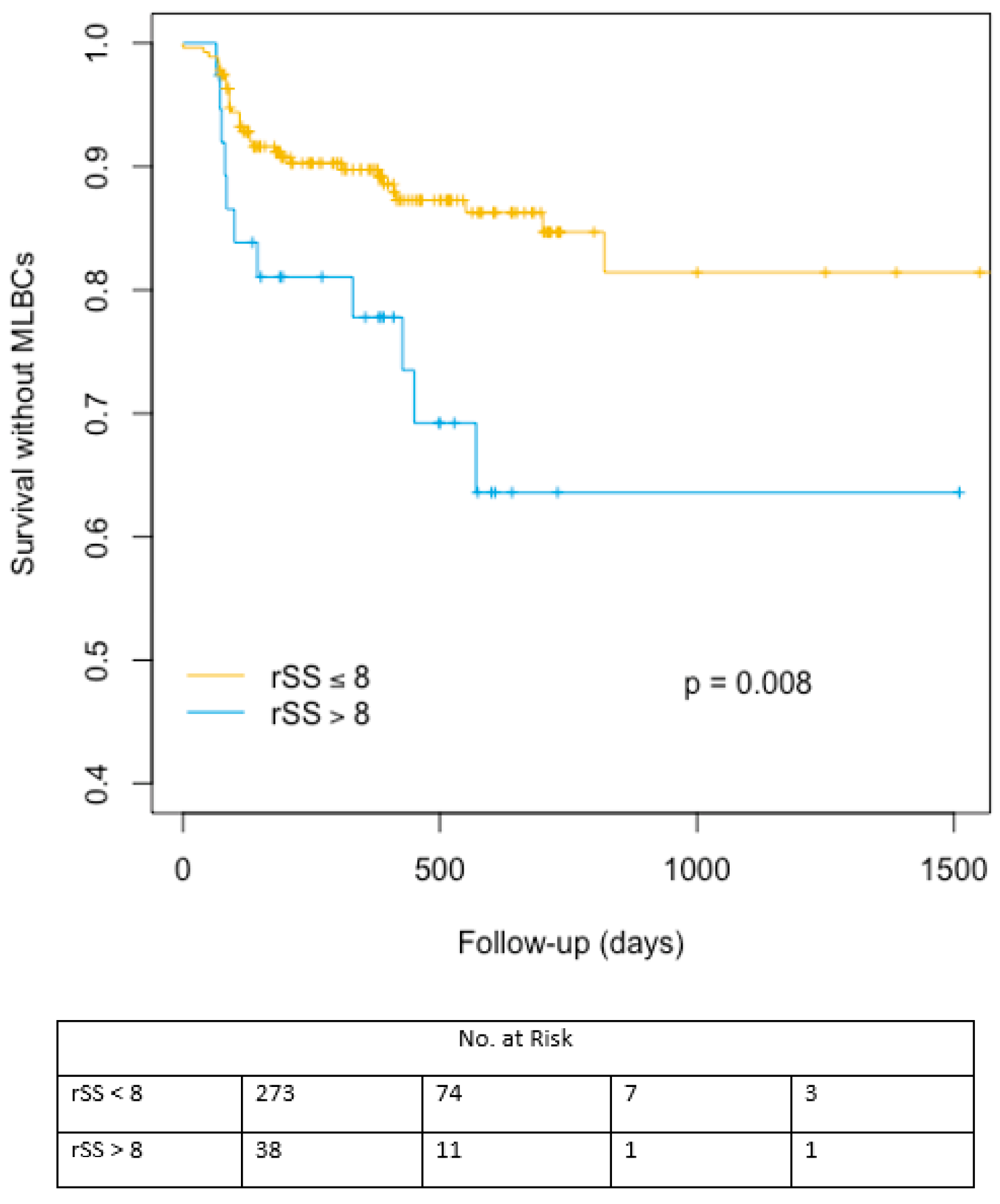
| Late major and life threating bleeding | 33 (12.1%) | 11 (28.9%) | 0.005 | 38 (13%) | 6 (33.3%) | 0.016 |
| Late major bleeding | 27 (9.9%) | 8 (21.1%) | 0.041 | 31 (10.6%) | 4 (22.2%) | 0.129 |
| Late life-threatening bleeding | 6 (2.2%) | 1 (2.6%) | 0.866 | 6 (2%) | 1 (5.6%) | 0.33 |
| Late transfusion ≥ 2 U | 32 (11.7%) | 11 (28.9%) | 0.004 | 38 (13%) | 5 (27.8%) | 0.077 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | sHR | CI 95% | p Value | sHR | CI 95% | p Value |
| Coronary parameters | ||||||
| bSS > 22 | 6.318 | 1.724–23.150 | 0.005 | 1.416 | 0.147–13.677 | 0.764 |
| rSS > 8 | 6.134 | 1.896–19.850 | 0.002 | 2.654 | 0.297–23.744 | 0.383 |
| Angioplasty | 6.283 | 1.681–23.482 | 0.006 | 1.654 | 0.319–8.571 | 0.589 |
| Aortic valve calcium score | 0.999 | 0.999–1.000 | 0.011 | |||
| Clinical Parameters | ||||||
| Age | 0.949 | 0.913–0.986 | 0.007 | 0.917 | 0.861–0.978 | 0.008 |
| EuroScore > 20 | 2.509 | 0.714–8.815 | 0.151 | |||
| Male sex | 0.713 | 0.210–2.420 | 0.588 | |||
| Body mass index (BMI) | 1.056 | 0.980–1.139 | 0.154 | |||
| Hypertension | 1.911 | 0.246–14.870 | 0.536 | |||
| Diabetes mellitus | 3.281 | 0.965–11.160 | 0.057 | 5.502 | 0.936–32.325 | 0.059 |
| Dyslipidemia | 3.578 | 0.778–16.441 | 0.101 | |||
| Current smoking | 2.941 | 0.453–19.062 | 0.258 | |||
| Cardiovascular disease heredity | 4.453 | 0.900–22.029 | 0.067 | 11.940 | 0.842–169.272 | 0.067 |
| Peripheral artery disease | 1.124 | 0.298–4.246 | 0.863 | |||
| History of atrial fibrillation | 1.700 | 0.522–5.538 | 0.379 | |||
| Chronic kidney disease (creatinine level >150 µmol.L) | 2.856 | 0.881–9.265 | 0.080 | 2.968 | 0.508–17.347 | 0.227 |
| COPD | 0.945 | 0.207–4.320 | 0.942 | |||
| Stroke history | 1.952 | 0.518–7.345 | 0.323 | |||
| Bleeding history | 1.348 | 0.309–5.881 | 0.691 | |||
| Echocardiography Parameters | ||||||
| LVEF pre TAVR | 0.987 | 0.940; 1.037 | 0.611 | |||
| LVEF 1 month post TAVR | 0.977 | 0.930; 1.025 | 0.342 | |||
| Biological Parameters | ||||||
| CT ADP > 180 | 1.213 | 0.360–4.093 | 0.755 | |||
| PRI VASP Post TAVR | 0.968 | 0.941–0.997 | 0.028 | 0.970 | 0.944–0.998 | 0.033 |
| Discharge Antithrombotic Medication | ||||||
| DAPT | 3.251 | 0.709–14.910 | 0.129 | |||
| Clopidogrel | 1.945 | 0.526–7.195 | 0.319 | |||
| Anticoagulant therapy | 1.072 | 0.330–3.482 | 0.908 | |||
| Univariate | |||
|---|---|---|---|
| Variables | sHR | CI 95% | p Value |
| Coronary Parameters | |||
| bSS > 22 | 2.704 | 1.179–6.203 | 0.019 |
| rSS > 8 | 2.502 | 1.278–4.900 | 0.007 |
| Angioplasty | 1.502 | 0.819–2.754 | 0.188 |
| Clinical Parameters | |||
| Age | 1.001 | 0.961–1.042 | 0.978 |
| Aortic valve calcium score | 1.006 | 0.976–1.036 | 0.716 |
| EuroScore > 20 | 2.077 | 1.143–3.776 | 0.016 |
| Male sex | 1.123 | 0.622–2.028 | 0.700 |
| Body mass index (BMI) | 0.965 | 0.916–1.016 | 0.178 |
| Hypertension | 0.869 | 0.415–1.820 | 0.709 |
| Diabetes mellitus | 1.400 | 0.773–2.537 | 0.267 |
| Peripheral artery disease | 0.733 | 0.354–1.517 | 0.402 |
| Bleeding history | 2.100 | 1.079–4.086 | 0.029 |
| Valve sapien | 0.665 | 0.367–1.204 | 0.178 |
| Discharge Antithrombotic Medication | |||
| ASA | 0.556 | 0.149–2.075 | 0.383 |
| DAPT | 1.175 | 0.643–2.147 | 0.600 |
| Clopidogrel | 0.887 | 0.492–1.600 | 0.691 |
| Anticoagulant therapy | 1.348 | 0.745–2.438 | 0.324 |
| Duration of DAPT | 1.002 | 0.998–1.005 | 0.362 |
| Biological Parameters | |||
| PVL >1/4 at 1 month follow up | 29.090 | 10.334–81.911 | <0.001 |
| Post TAVR PRI VASP | 0.989 | 0.969–1.011 | 0.625 |
| CT ADP > 180 | 1.748 | 0.958–3.188 | 0.069 |
| Echocardiography Parameters | |||
| LVEF pre TAVR | 0.960 | 0.941; 0.980 | <0.001 |
| LVEF 1 month post TAVR | 0.974 | 0.951; 0.999 | 0.041 |
| Model 1: All Candidates Predictors Except Significant Post-TAVR PVL at 1 Month and bSS > 22 | |||
| Variable | Hazard Ratio | CI 95% | p Value |
| Residual SYNTAX score: rSS > 8 | 2.345 | 1.171–4.700 | 0.016 |
| EuroScore > 20 | 2.081 | 1.102–3.931 | 0.024 |
| CT-ADP > 180 | 3.302 | 1.257–4.216 | 0.007 |
| Prior bleeding events | 1.835 | 0.959–3.513 | 0.067 |
| LVEF 1-month post-TAVR | 0.975 | 0.953–0.998 | 0.034 |
| Model 2: All Candidates Predictors Except Post-TAVR CT-ADP > 180 s and bSS > 22 | |||
| Variable | Hazard Ratio | IC 95% | p Value |
| Residual SYNTAX score: rSS > 8 | 2.391 | 1.150–4.973 | 0.020 |
| EuroScore > 20 | 1.703 | 0.882–3.288 | 0.113 |
| PVL >1/4 at 1 month follow up | 29.238 | 8.116–105.329 | <0.001 |
| Prior bleeding events | 1.873 | 0.920–3.816 | 0.084 |
| LVEF 1-month post-TAVR | 0.985 | 0.959–1.012 | 0.276 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona, A.; Marchandot, B.; Severac, F.; Kibler, M.; Trimaille, A.; Heger, J.; Peillex, M.; Matsushita, K.; Ristorto, J.; Hoang, V.A.; et al. Impact of Incomplete Coronary Revascularization on Late Ischemic and Bleeding Events after Transcatheter Aortic Valve Replacement. J. Clin. Med. 2020, 9, 2267. https://doi.org/10.3390/jcm9072267
Carmona A, Marchandot B, Severac F, Kibler M, Trimaille A, Heger J, Peillex M, Matsushita K, Ristorto J, Hoang VA, et al. Impact of Incomplete Coronary Revascularization on Late Ischemic and Bleeding Events after Transcatheter Aortic Valve Replacement. Journal of Clinical Medicine. 2020; 9(7):2267. https://doi.org/10.3390/jcm9072267
Chicago/Turabian StyleCarmona, Adrien, Benjamin Marchandot, François Severac, Marion Kibler, Antonin Trimaille, Joe Heger, Marilou Peillex, Kensuke Matsushita, Jessica Ristorto, Viet Anh Hoang, and et al. 2020. "Impact of Incomplete Coronary Revascularization on Late Ischemic and Bleeding Events after Transcatheter Aortic Valve Replacement" Journal of Clinical Medicine 9, no. 7: 2267. https://doi.org/10.3390/jcm9072267
APA StyleCarmona, A., Marchandot, B., Severac, F., Kibler, M., Trimaille, A., Heger, J., Peillex, M., Matsushita, K., Ristorto, J., Hoang, V. A., Hess, S., Jesel, L., Ohlmann, P., & Morel, O. (2020). Impact of Incomplete Coronary Revascularization on Late Ischemic and Bleeding Events after Transcatheter Aortic Valve Replacement. Journal of Clinical Medicine, 9(7), 2267. https://doi.org/10.3390/jcm9072267





