Surgery and Perioperative Management in Small Intestinal Neuroendocrine Tumors
Abstract
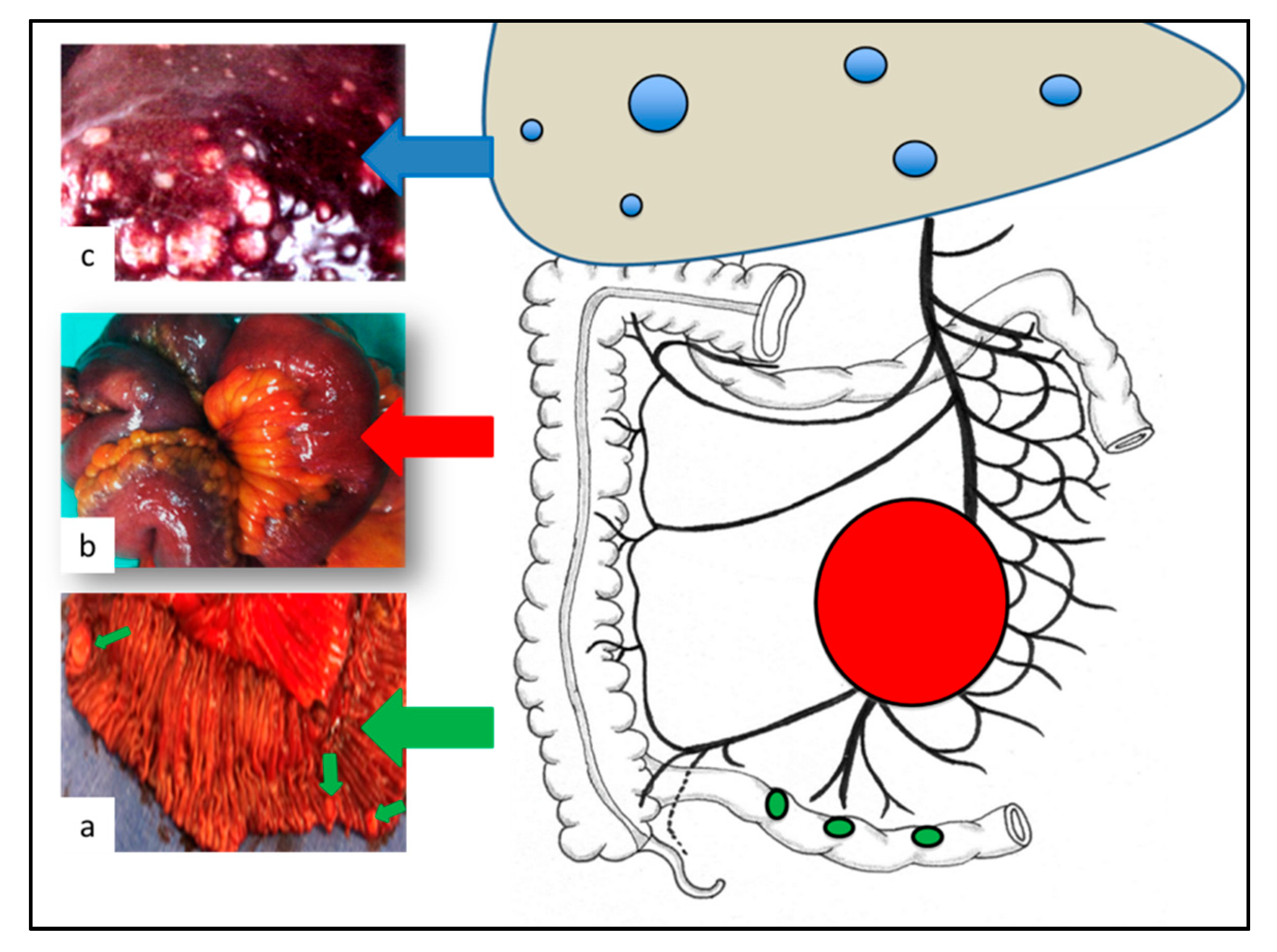
:1. Introduction
2. Preoperative Imaging
2.1. Primary Tumor(s) Imaging
2.2. MLNM Imaging
2.3. Imaging of Liver Metastases
2.4. Peritoneal Carcinomatosis Imaging
3. Pre and Perioperative Management of the Hormonal Syndrome
3.1. Diagnosis and Management of Carcinoid Syndrome
3.2. Cardiac Evaluation
3.3. Prevention and Management of the Carcinoid Crisis
4. Surgery
4.1. Surgery of Primary Tumor and Mesenteric Lymph Node Metastases in Curative Intent
4.1.1. Exploration of the Abdomen
4.1.2. Surgery of MLNM
4.1.3. Emergency Surgery
4.2. Surgery of Metastases in Curative Intent
4.2.1. Curative Surgery for Liver Metastases
4.2.2. Peritoneal Carcinomatosis
4.2.3. Palliative Surgery
Resection of the Local Disease (Primary Tumor + MLNM) when Liver Metastases are Unresectable
Palliative Surgery for Inextirpable Bulky MLNM
Palliative Debulking Surgery for Liver Metastases
4.3. Open vs. Laparoscopic Resections
4.4. Prophylactic Cholecystectomy
4.5. Place of Neoadjuvant Treatment
5. Postoperative Management
5.1. Postoperative Evaluation
5.2. Adjuvant Treatment
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Pape, U.-F.; Berndt, U.; Muller-Nordhorn, J.; Bohmig, M.; Roll, S.; Koch, M.; Willich, S.N.; Wiedenmann, B. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr. Relat. Cancer 2008, 15, 1083–1097. [Google Scholar] [CrossRef]
- Norlén, O.; Stålberg, P.; Öberg, K.; Eriksson, J.; Hedberg, J.; Hessman, O.; Janson, E.T.; Hellman, P.; Åkerström, G. Long-Term Results of Surgery for Small Intestinal Neuroendocrine Tumors at a Tertiary Referral Center. World J. Surg. 2012, 36, 1419–1431. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Weber, J.M.; Feldman, M.; Coppola, D.; Meredith, K.; Kvols, L.K. Prognostic validity of the American Joint Committee on Cancer staging classification for midgut neuroendocrine tumors. J. Clin. Oncol. 2013, 31, 420–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Roux, C.; Lombard-Bohas, C.; Delmas, C.; Dominguez-Tinajero, S.; Ruszniewski, P.; Samalin, E.; Raoul, J.-L.; Renard, P.; Baudin, E.; Robaskiewicz, M.; et al. Relapse factors for ileal neuroendocrine tumours after curative surgery: A retrospective French multicentre study. Dig. Liver Dis. 2011, 43, 828–833. [Google Scholar] [CrossRef]
- Keck, K.J.; Maxwell, J.E.; Utria, A.F.; Bellizzi, A.M.; Dillon, J.S.; O’Dorisio, T.M.; Howe, J.R. The Distal Predilection of Small Bowel Neuroendocrine Tumors. Ann. Surg. Oncol. 2018, 25, 3207–3213. [Google Scholar] [CrossRef]
- Landry, C.S.; Lin, H.Y.; Phan, A.; Charnsangavej, C.; Abdalla, E.K.; Aloia, T.; Nicolas Vauthey, J.; Katz, M.H.G.; Yao, J.C.; Fleming, J.B. Resection of At-Risk Mesenteric Lymph Nodes Is Associated with Improved Survival in Patients with Small Bowel Neuroendocrine Tumors. World J. Surg. 2013, 37, 1695–1700. [Google Scholar] [CrossRef]
- Motz, B.M.; Lorimer, P.D.; Boselli, D.; Hill, J.S.; Salo, J.C. Optimal Lymphadenectomy in Small Bowel Neuroendocrine Tumors: Analysis of the NCDB. J. Gastrointest. Surg. 2018, 22, 117–123. [Google Scholar] [CrossRef]
- Chen, L.; Song, Y.; Zhang, Y.; Chen, M.; Chen, J. Exploration of the Exact Prognostic Significance of Lymphatic Metastasis in Jejunoileal Neuroendocrine Tumors. Ann. Surg. Oncol. 2018, 25, 2067–2074. [Google Scholar] [CrossRef]
- Modlin, I.M.; Oberg, K.; Chung, D.C.; Jensen, R.T.; de Herder, W.W.; Thakker, R.V.; Caplin, M.; Delle Fave, G.; Kaltsas, G.A.; Krenning, E.P.; et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008, 9, 61–72. [Google Scholar] [CrossRef]
- Norlén, O.; Edfeldt, K.; Akerstrom, G.; Westin, G.; Hellman, P.; Bjorklund, P.; Stalberg, P. Peritoneal carcinomatosis from small intestinal neuroendocrine tumors: Clinical course and genetic profiling. Surgery 2014, 156, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Manguso, N.; Gangi, A.; Nissen, N.; Harit, A.; Siegel, E.; Hendifar, A.; Amersi, F. Long-Term Outcomes after Elective versus Emergency Surgery for Small Bowel Neuroendocrine Tumors. Am. Surg. 2018, 84, 1570–1574. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Lee, L.; Jensen, R.T. Carcinoid-syndrome: Recent advances, current status and controversies. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Niederle, B.; Pape, U.-F.; Costa, F.; Gross, D.; Kelestimur, F.; Knigge, U.; Öberg, K.; Pavel, M.; Perren, A.; Toumpanakis, C.; et al. ENETS Consensus Guidelines Update for Neuroendocrine Neoplasms of the Jejunum and Ileum. Neuroendocrinology 2016, 103, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Howe, J.R.; Cardona, K.; Fraker, D.L.; Kebebew, E.; Untch, B.R.; Wang, Y.-Z.; Law, C.H.; Liu, E.H.; Kim, M.K.; Menda, Y.; et al. The Surgical Management of Small Bowel Neuroendocrine Tumors: Consensus Guidelines of the North American Neuroendocrine Tumor Society. Pancreas 2017, 46, 715–731. [Google Scholar] [CrossRef]
- Addeo, P.; Poncet, G.; Goichot, B.; Leclerc, L.; Brigand, C.; Mutter, D.; Romain, B.; Namer, I.-J.; Bachellier, P.; Imperiale, A. The Added Diagnostic Value of 18F-Fluorodihydroxyphenylalanine PET/CT in the Preoperative Work-Up of Small Bowel Neuroendocrine Tumors. J. Gastrointest. Surg. 2018, 22, 722–730. [Google Scholar] [CrossRef]
- Pasquer, A.; Walter, T.; Hervieu, V.; Forestier, J.; Scoazec, J.-Y.; Lombard-Bohas, C.; Poncet, G. Surgical Management of Small Bowel Neuroendocrine Tumors: Specific Requirements and Their Impact on Staging and Prognosis. Ann. Surg. Oncol. 2015, 22, 742–749. [Google Scholar] [CrossRef]
- Asnacios, A.; Courbon, F.; Rochaix, P.; Bauvin, E.; Cances-Lauwers, V.; Susini, C.; Schulz, S.; Boneu, A.; Guimbaud, R.; Buscail, L. Indium-111–Pentetreotide Scintigraphy and Somatostatin Receptor Subtype 2 Expression: New Prognostic Factors for Malignant Well-Differentiated Endocrine Tumors. J. Clin. Oncol. 2008, 26, 963–970. [Google Scholar] [CrossRef]
- Campana, D.; Ambrosini, V.; Pezzilli, R.; Fanti, S.; Labate, A.M.M.; Santini, D.; Ceccarelli, C.; Nori, F.; Franchi, R.; Corinaldesi, R.; et al. Standardized uptake values of (68)Ga-DOTANOC PET: A promising prognostic tool in neuroendocrine tumors. J. Nucl. Med. 2010, 51, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Sundin, A.; Vullierme, M.-P.; Kaltsas, G.; Plöckinger, U. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological Examinations. Neuroendocrinology 2009, 90, 167–183. [Google Scholar] [CrossRef]
- Fiebrich, H.-B.; de Jong, J.R.; Kema, I.P.; Koopmans, K.P.; Sluiter, W.; Dierckx, R.A.J.O.; Walenkamp, A.M.; Links, T.P.; Brouwers, A.H.; de Vries, E.G.E. Total 18F-dopa PET tumour uptake reflects metabolic endocrine tumour activity in patients with a carcinoid tumour. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1854–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montravers, F.; Kerrou, K.; Nataf, V.; Huchet, V.; Lotz, J.-P.; Ruszniewski, P.; Rougier, P.; Duron, F.; Bouchard, P.; Grangé, J.-D.; et al. Impact of fluorodihydroxyphenylalanine-18F positron emission tomography on management of adult patients with documented or occult digestive endocrine tumors. J. Clin. Endocrinol. Metab. 2009, 94, 1295–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koopmans, K.P.; de Vries, E.G.; Kema, I.P.; Elsinga, P.H.; Neels, O.C.; Sluiter, W.J.; van der Horst-Schrivers, A.N.; Jager, P.L. Staging of carcinoid tumours with 18F-DOPA PET: A prospective, diagnostic accuracy study. Lancet Oncol. 2006, 7, 728–734. [Google Scholar] [CrossRef]
- Binderup, T.; Knigge, U.; Loft, A.; Federspiel, B.; Kjaer, A. 18F-Fluorodeoxyglucose Positron Emission Tomography Predicts Survival of Patients with Neuroendocrine Tumors. Clin. Cancer Res. 2010, 16, 978–985. [Google Scholar] [CrossRef] [Green Version]
- de Mestier, L.; Lepage, C.; Baudin, E.; Coriat, R.; Courbon, F.; Couvelard, A.; Do Cao, C.; Frampas, E.; Gaujoux, S.; Gincul, R.; et al. Digestive Neuroendocrine Neoplasms (NEN): French Intergroup clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, GTE, RENATEN, TENPATH, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig. Liver Dis. 2020, 52, 473–492. [Google Scholar] [CrossRef]
- Chan, D.L.; Pavlakis, N.; Schembri, G.P.; Bernard, E.J.; Hsiao, E.; Hayes, A.; Barnes, T.; Diakos, C.; Khasraw, M.; Samra, J.; et al. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics 2017, 7, 1149–1158. [Google Scholar] [CrossRef]
- Zagorowicz, E.S. Small bowel tumors detected and missed during capsule endoscopy: Single center experience. World J. Gastroenterol. 2013, 19, 9043. [Google Scholar] [CrossRef]
- Kamaoui, I.; De-Luca, V.; Ficarelli, S.; Mennesson, N.; Lombard-Bohas, C.; Pilleul, F. Value of CT Enteroclysis in Suspected Small-Bowel Carcinoid Tumors. Am. J. Roentgenol. 2010, 194, 629–633. [Google Scholar] [CrossRef]
- Lardière-Deguelte, S.; de Mestier, L.; Appéré, F.; Vullierme, M.-P.; Zappa, M.; Hoeffel, C.; Noaves, M.; Brixi, H.; Hentic, O.; Ruszniewski, P.; et al. Toward a Preoperative Classification of Lymph Node Metastases in Patients with Small Intestinal Neuroendocrine Tumors in the Era of Intestinal-Sparing Surgery. Neuroendocrinology 2016, 103, 552–559. [Google Scholar] [CrossRef]
- d’Assignies, G.; Fina, P.; Bruno, O.; Vullierme, M.-P.; Tubach, F.; Paradis, V.; Sauvanet, A.; Ruszniewski, P.; Vilgrain, V. High sensitivity of diffusion-weighted MR imaging for the detection of liver metastases from neuroendocrine tumors: Comparison with T2-weighted and dynamic gadolinium-enhanced MR imaging. Radiology 2013, 268, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Moryoussef, F.; de Mestier, L.; Belkebir, M.; Deguelte-Lardière, S.; Brixi, H.; Kianmanesh, R.; Hoeffel, C.; Cadiot, G. Impact of Liver and Whole-Body Diffusion-Weighted MRI for Neuroendocrine Tumors on Patient Management: A Pilot Study. Neuroendocrinology 2017, 104, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W.E.; Gonzalez, R.S.; Cates, J.M.M.; Liu, E.; Shi, C. Hepatic micrometastases are associated with poor prognosis in patients with liver metastases from neuroendocrine tumors of the digestive tract. Hum. Pathol. 2018, 79, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Fossmark, R.; Balto, T.M.; Martinsen, T.C.; Grønbech, J.E.; Munkvold, B.; Mjønes, P.G.; Waldum, H.L. Hepatic micrometastases outside macrometastases are present in all patients with ileal neuroendocrine primary tumour at the time of liver resection. Scand. J. Gastroenterol. 2019, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Lefevre, J.H.; Duvillard, P.; Goéré, D.; Dromain, C.; Dumont, F.; Baudin, E. Hepatic metastases from neuroendocrine tumors with a “thin slice” pathological examination: They are many more than you think. Ann. Surg. 2010, 251, 307–310. [Google Scholar] [CrossRef]
- Frilling, A.; Modlin, I.M.; Kidd, M.; Russell, C.; Breitenstein, S.; Salem, R.; Kwekkeboom, D.; Lau, W.; Klersy, C.; Vilgrain, V.; et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014, 15, e8–e21. [Google Scholar] [CrossRef]
- Norlén, O.; Montan, H.; Hellman, P.; Stålberg, P.; Sundin, A. Preoperative 68Ga-DOTA-Somatostatin Analog-PET/CT Hybrid Imaging Increases Detection Rate of Intra-abdominal Small Intestinal Neuroendocrine Tumor Lesions. World J. Surg. 2018, 42, 498–505. [Google Scholar] [CrossRef] [Green Version]
- Halperin, D.M.; Shen, C.; Dasari, A.; Xu, Y.; Chu, Y.; Zhou, S.; Shih, Y.-C.T.; Yao, J.C. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: A population-based study. Lancet Oncol. 2017, 18, 525–534. [Google Scholar] [CrossRef]
- Mota, J.M.; Sousa, L.G.; Riechelmann, R.P. Complications from carcinoid syndrome: Review of the current evidence. Ecancer 2016, 10. [Google Scholar] [CrossRef] [Green Version]
- Feldman, J.M.; O’dorisio, T.M. Role of neuropeptides and serotonin in the diagnosis of carcinoid tumors. Am. J. Med. 1986, 81, 41–48. [Google Scholar] [CrossRef]
- Tellez, M.R.; Mamikunian, G.; O’Dorisio, T.M.; Vinik, A.I.; Woltering, E.A. A Single Fasting Plasma 5-HIAA Value Correlates with 24-Hour Urinary 5-HIAA Values and Other Biomarkers in Midgut Neuroendocrine Tumors (NETs). Pancreas 2013, 42, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, K.; Kaye, A.D.; Boudreaux, J.P.; Fox, C.J.; Lang, P.; Kalarickal, P.L.; Gomez, S.; Primeaux, P.J. Carcinoid syndrome and perioperative anesthetic considerations. J. Clin. Anesth. 2011, 23, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Muller, H.-H.; Arnold, R. For the PROMID Study Group Placebo Controlled, Double Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results on Long Term Survival. Neuroendocrinology 2016, 104, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Davar, J.; Connolly, H.M.; Caplin, M.E.; Pavel, M.; Zacks, J.; Bhattacharyya, S.; Cuthbertson, D.J.; Dobson, R.; Grozinsky-Glasberg, S.; Steeds, R.P.; et al. Diagnosing and Managing Carcinoid Heart Disease in Patients with Neuroendocrine Tumors. J. Am. Coll. Cardiol. 2017, 69, 1288–1304. [Google Scholar] [CrossRef]
- Ram, P.; Penalver, J.L.; Lo, K.B.U.; Rangaswami, J.; Pressman, G.S. Carcinoid Heart Disease: Review of Current Knowledge. Tex. Heart Inst. J. 2019, 46, 21–27. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Toumpanakis, C.; Chilkunda, D.; Caplin, M.E.; Davar, J. Risk Factors for the Development and Progression of Carcinoid Heart Disease. Am. J. Cardiol. 2011, 107, 1221–1226. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Toumpanakis, C.; Caplin, M.E.; Davar, J. Analysis of 150 Patients with Carcinoid Syndrome Seen in a Single Year at One Institution in the First Decade of the Twenty-First Century. Am. J. Cardiol. 2008, 101, 378–381. [Google Scholar] [CrossRef]
- Zuetenhorst, J.M.; Korse, C.M.; Bonfrer, J.M.G.; Bakker, R.H.; Taal, B.G. Role of natriuretic peptides in the diagnosis and treatment of patients with carcinoid heart disease. Br. J. Cancer 2004, 90, 2073–2079. [Google Scholar] [CrossRef]
- Condron, M.E.; Jameson, N.E.; Limbach, K.E.; Bingham, A.E.; Sera, V.A.; Anderson, R.B.; Schenning, K.J.; Yockelson, S.; Harukuni, I.; Kahl, E.A.; et al. A prospective study of the pathophysiology of carcinoid crisis. Surgery 2019, 165, 158–165. [Google Scholar] [CrossRef]
- Massimino, K.; Harrskog, O.; Pommier, S.; Pommier, R. Octreotide LAR and bolus octreotide are insufficient for preventing intraoperative complications in carcinoid patients: Preventing Intraoperative Complications in Carcinoid Patients. J. Surg. Oncol. 2013, 107, 842–846. [Google Scholar] [CrossRef]
- Kwon, D.H.; Paciorek, A.; Mulvey, C.K.; Chan, H.; Fidelman, N.; Meng, L.; Nakakura, E.K.; Zhang, L.; Bergsland, E.K.; Van Loon, K. Periprocedural Management of Patients Undergoing Liver Resection or Embolotherapy for Neuroendocrine Tumor Metastases. Pancreas 2019, 48, 496–503. [Google Scholar] [CrossRef]
- Condron, M.E.; Pommier, S.J.; Pommier, R.F. Continuous infusion of octreotide combined with perioperative octreotide bolus does not prevent intraoperative carcinoid crisis. Surgery 2016, 159, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Kinney, M.A.O.; Warner, M.E.; Nagorney, D.M.; Rubin, J.; Schroeder, D.R.; Maxson, P.M.; Warner, M.A. Perianaesthetic risks and outcomes of abdominal surgery for metastatic carcinoid tumours. Br. J. Anaesth. 2001, 87, 447–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinney, M.A.O.; Nagorney, D.M.; Clark, D.F.; O’Brien, T.D.; Turner, J.D.; Marienau, M.E.; Schroeder, D.R.; Martin, D.P. Partial hepatic resections for metastatic neuroendocrine tumors: Perioperative outcomes. J. Clin. Anesth. 2018, 51, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Woltering, E.A.; Wright, A.E.; Stevens, M.A.; Wang, Y.-Z.; Boudreaux, J.P.; Mamikunian, G.; Riopelle, J.M.; Kaye, A.D. Development of effective prophylaxis against intraoperative carcinoid crisis. J. Clin. Anesth. 2016, 32, 189–193. [Google Scholar] [CrossRef]
- Fouché, M.; Bouffard, Y.; Le Goff, M.-C.; Prothet, J.; Malavieille, F.; Sagnard, P.; Christin, F.; Hayi-Slayman, D.; Pasquer, A.; Poncet, G.; et al. Intraoperative carcinoid syndrome during small-bowel neuroendocrine tumour surgery. Endocr. Connect. 2018, 7, 1245–1250. [Google Scholar] [CrossRef] [Green Version]
- Moris, D.; Ntanasis-Stathopoulos, I.; Tsilimigras, D.I.; Vagios, S.; Karamitros, A.; Karaolanis, G.; Griniatsos, J.; Papalampros, A.; Papaconstantinou, I.; Glantzounis, G.K.; et al. Update on Surgical Management of Small Bowel Neuroendocrine Tumors. Anticancer Res. 2018, 38, 1267–1278. [Google Scholar]
- Clift, A.K.; Faiz, O.; Al-Nahhas, A.; Bockisch, A.; Liedke, M.O.; Schloericke, E.; Wasan, H.; Martin, J.; Ziprin, P.; Moorthy, K.; et al. Role of Staging in Patients with Small Intestinal Neuroendocrine Tumours. J. Gastrointest. Surg. 2016, 20, 180–188. [Google Scholar] [CrossRef]
- Pasquer, A.; Walter, T.; Rousset, P.; Hervieu, V.; Forestier, J.; Lombard-Bohas, C.; Poncet, G. Lymphadenectomy during Small Bowel Neuroendocrine Tumor Surgery: The Concept of Skip Metastases. Ann. Surg. Oncol. 2016, 23, 804–808. [Google Scholar] [CrossRef]
- Figueiredo, M.N.; Maggiori, L.; Gaujoux, S.; Couvelard, A.; Guedj, N.; Ruszniewski, P.; Panis, Y. Surgery for small-bowel neuroendocrine tumors: Is there any benefit of the laparoscopic approach? Surg. Endosc. 2014, 28, 1720–1726. [Google Scholar] [CrossRef]
- Walsh, J.C.; Schaeffer, D.F.; Kirsch, R.; Pollett, A.; Manzoni, M.; Riddell, R.H.; Albarello, L. Ileal “carcinoid” tumors—Small size belies deadly intent: High rate of nodal metastasis in tumors ≤1 cm in size. Hum. Pathol. 2016, 56, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Z.; Carrasquillo, J.P.; McCord, E.; Vidrine, R.; Lobo, M.L.; Zamin, S.A.; Boudreaux, P.; Woltering, E. Reappraisal of lymphatic mapping for midgut neuroendocrine patients undergoing cytoreductive surgery. Surgery 2014, 156, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Hubalewska-Dydejczyk, A.; Kulig, J.; Szybinski, P.; Mikolajczak, R.; Pach, D.; Sowa-Staszczak, A.; Fröss-Baron, K.; Huszno, B. Radio-guided surgery with the use of [99mTc-EDDA/HYNIC]octreotate in intra-operative detection of neuroendocrine tumours of the gastrointestinal tract. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Arigami, T.; Uenosono, Y.; Yanagita, S.; Okubo, K.; Kijima, T.; Matsushita, D.; Amatatsu, M.; Hagihara, T.; Haraguchi, N.; Mataki, Y.; et al. Sentinel node navigation surgery for gastroduodenal neuroendocrine tumors: Two case reports. Medicine 2016, 95, e4063. [Google Scholar] [CrossRef]
- Behrend, C.; Jeppesen, P.B.; Mortensen, P.B. Vitamin B12 absorption after ileorectal anastomosis for Crohn’s disease: Effect of ileal resection and time span after surgery. Eur. J. Gastroenterol. Hepatol. 1995, 7, 397–400. [Google Scholar]
- Malik, P.; Pinto, C.; Naparst, M.S.; Ward, S.C.; Aronson, A.; Aalberg, J.J.; Divino, C.M.; Kim, M.K. Impact of Mesenteric Mass in Patients with Midgut Neuroendocrine Tumors. Pancreas 2019, 48, 682–685. [Google Scholar] [CrossRef]
- Blažević, A.; Zandee, W.T.; Franssen, G.J.H.; Hofland, J.; van Velthuysen, M.-L.F.; Hofland, L.J.; Feelders, R.A.; de Herder, W.W. Mesenteric fibrosis and palliative surgery in small intestinal neuroendocrine tumours. Endocr. Relat. Cancer 2018, 25, 245–254. [Google Scholar] [CrossRef]
- Partelli, S.; Bartsch, D.K.; Capdevila, J.; Chen, J.; Knigge, U.; Niederle, B.; Nieveen van Dijkum, E.J.M.; Pape, U.-F.; Pascher, A.; Ramage, J.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2017, 105, 255–265. [Google Scholar] [CrossRef]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.-F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef]
- Addeo, P.; Bertin, J.-B.; Imperiale, A.; Averous, G.; Terrone, A.; Goichot, B.; Bachellier, P. Outcomes of Simultaneous Resection of Small Bowel Neuroendocrine Tumors with Synchronous Liver Metastases. World J. Surg. 2020, 44, 2377–2384. [Google Scholar] [CrossRef]
- Steinmüller, T.; Kianmanesh, R.; Falconi, M.; Scarpa, A.; Taal, B.; Kwekkeboom, D.J.; Lopes, J.M.; Perren, A.; Nikou, G.; Yao, J.; et al. Consensus Guidelines for the Management of Patients with Liver Metastases from Digestive (Neuro)endocrine Tumors: Foregut, Midgut, Hindgut, and Unknown Primary. Neuroendocrinology 2008, 87, 47–62. [Google Scholar] [CrossRef]
- Kianmanesh, R.; Sauvanet, A.; Hentic, O.; Couvelard, A.; Lévy, P.; Vilgrain, V.; Ruszniewski, P.; Belghiti, J. Two-step surgery for synchronous bilobar liver metastases from digestive endocrine tumors: A safe approach for radical resection. Ann. Surg. 2008, 247, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, J.M.; Heywood, G.; Rubin, J.; Ilstrup, D.M.; Nagorney, D.M.; Que, F.G. Surgical treatment of neuroendocrine metastases to the liver. J. Am. Coll. Surg. 2003, 197, 29–37. [Google Scholar] [CrossRef]
- Norlén, O.; Stålberg, P.; Zedenius, J.; Hellman, P. Outcome after resection and radiofrequency ablation of liver metastases from small intestinal neuroendocrine tumours: Treatment of liver metastases from small intestinal neuroendocrine tumours. Br. J. Surg. 2013, 100, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hardacre, J.M.; Uzar, A.; Cameron, J.L.; Choti, M.A. Isolated liver metastases from neuroendocrine tumors: Does resection prolong survival? J. Am. Coll. Surg. 1998, 187, 88–92. [Google Scholar] [CrossRef]
- Chamberlain, R.S.; Canes, D.; Brown, K.T.; Saltz, L.; Jarnagin, W.; Fong, Y.; Blumgart, L.H. Hepatic neuroendocrine metastases: Does intervention alter outcomes? J. Am. Coll. Surg. 2000, 190, 432–445. [Google Scholar] [CrossRef]
- Jaeck, D.; Oussoultzoglou, E.; Bachellier, P.; Lemarque, P.; Weber, J.-C.; Nakano, H.; Wolf, P. Hepatic Metastases of Gastroenteropancreatic Neuroendocrine Tumors: Safe Hepatic Surgery. World J. Surg. 2001, 25, 689–692. [Google Scholar] [CrossRef]
- Elias, D.; Lasser, P.; Ducreux, M.; Duvillard, P.; Ouellet, J.-F.; Dromain, C.; Schlumberger, M.; Pocard, M.; Boige, V.; Miquel, C.; et al. Liver resection (and associated extrahepatic resections) for metastatic well-differentiated endocrine tumors: A 15-year single center prospective study. Surgery 2003, 133, 375–382. [Google Scholar] [CrossRef]
- Scigliano, S.; Lebtahi, R.; Maire, F.; Stievenart, J.L.; Kianmanesh, R.; Sauvanet, A.; Vullierme, M.P.; Couvelard, A.; Belghiti, J.; Ruszniewski, P.; et al. Clinical and imaging follow-up after exhaustive liver resection of endocrine metastases: A 15-year monocentric experience. Endocr. Relat. Cancer 2009, 16, 977–990. [Google Scholar] [CrossRef] [Green Version]
- Bertani, E.; Falconi, M.; Grana, C.; Botteri, E.; Chiappa, A.; Misitano, P.; Spada, F.; Ravizza, D.; Bazolli, B.; Fazio, N. Small intestinal neuroendocrine tumors with liver metastases and resection of the primary: Prognostic factors for decision making. Int. J. Surg. 2015, 20, 58–64. [Google Scholar] [CrossRef]
- Kim, J.; Zimmerman, M.A.; Hong, J.C. Liver transplantation in the treatment of unresectable hepatic metastasis from neuroendocrine tumors. J. Gastrointest. Oncol. 2020, 11, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Merola, E.; Prasad, V.; Pascher, A.; Pape, U.-F.; Arsenic, R.; Denecke, T.; Fehrenbach, U.; Wiedenmann, B.; Pavel, M.E. Peritoneal Carcinomatosis in Gastro-Entero-Pancreatic Neuroendocrine Neoplasms: Clinical Impact and Effectiveness of the Available Therapeutic Options. Neuroendocrinology 2020, 110, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Kianmanesh, R.; Ruszniewski, P.; Rindi, G.; Kwekkeboom, D.; Pape, U.-F.; Kulke, M.; Sevilla Garcia, I.; Scoazec, J.-Y.; Nilsson, O.; Fazio, N.; et al. ENETS Consensus Guidelines for the Management of Peritoneal Carcinomatosis from Neuroendocrine Tumors. Neuroendocrinology 2010, 91, 333–340. [Google Scholar] [CrossRef] [PubMed]
- de Mestier, L.; Lardière-Deguelte, S.; Brixi, H.; O’Toole, D.; Ruszniewski, P.; Cadiot, G.; Kianmanesh, R. Updating the Surgical Management of Peritoneal Carcinomatosis in Patients with Neuroendocrine Tumors. Neuroendocrinology 2015, 101, 105–111. [Google Scholar] [CrossRef]
- Elias, D.; David, A.; Sourrouille, I.; Honoré, C.; Goéré, D.; Dumont, F.; Stoclin, A.; Baudin, E. Neuroendocrine carcinomas: Optimal surgery of peritoneal metastases (and associated intra-abdominal metastases). Surgery 2014, 155, 5–12. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar]
- Au, J.T.; Levine, J.; Aytaman, A.; Weber, T.; Serafini, F. Management of peritoneal metastasis from neuroendocrine tumors. J. Surg. Oncol. 2013, 108, 385–386. [Google Scholar] [CrossRef]
- Hellman, P.; Lundström, T.; Öhrvall, U.; Eriksson, B.; Skogseid, B.; Öberg, K.; Tiensuu Janson, E.; Åkerström, G. Effect of Surgery on the Outcome of Midgut Carcinoid Disease with Lymph Node and Liver Metastases. World J. Surg. 2002, 26, 991–997. [Google Scholar] [CrossRef]
- Almond, L.M.; Hodson, J.; Ford, S.J.; Gourevitch, D.; Roberts, K.J.; Shah, T.; Isaac, J.; Desai, A. Role of palliative resection of the primary tumour in advanced pancreatic and small intestinal neuroendocrine tumours: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2017, 43, 1808–1815. [Google Scholar] [CrossRef] [Green Version]
- Daskalakis, K.; Karakatsanis, A.; Hessman, O.; Stuart, H.C.; Welin, S.; Tiensuu Janson, E.; Öberg, K.; Hellman, P.; Norlén, O.; Stålberg, P. Association of a Prophylactic Surgical Approach to Stage IV Small Intestinal Neuroendocrine Tumors with Survival. JAMA Oncol. 2017, 4, 183–189. [Google Scholar] [CrossRef]
- Boudreaux, J.P.; Klimstra, D.S.; Hassan, M.M.; Woltering, E.A.; Jensen, R.T.; Goldsmith, S.J.; Nutting, C.; Bushnell, D.L.; Caplin, M.E.; Yao, J.C. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: Well-differentiated neuroendocrine tumors of the jejunum, ileum, appendix, and cecum. Pancreas 2010, 39, 753–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulke, M.H.; Shah, M.H.; Benson, A.B.; Bergsland, E.; Berlin, J.D.; Blaszkowsky, L.S.; Emerson, L.; Engstrom, P.F.; Fanta, P.; Giordano, T.; et al. Neuroendocrine tumors, version 1.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 78–108. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.J.; Pasieka, J.L.; Dixon, E.; Rorstad, O. The palliative benefit of aggressive surgical intervention for both hepatic and mesenteric metastases from neuroendocrine tumors. Surgery 2008, 144, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Boudreaux, J.P.; Putty, B.; Frey, D.J.; Woltering, E.; Anthony, L.; Daly, I.; Ramcharan, T.; Lopera, J.; Castaneda, W. Surgical Treatment of Advanced-Stage Carcinoid Tumors: Lessons Learned. Ann. Surg. 2005, 241, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Hellman, P.; Hessman, O.; Åkerström, G.; Stålberg, P.; Hennings, J.; Björck, M.; Eriksson, L.-G. Stenting of the Superior Mesenteric Vein in Midgut Carcinoid Disease with Large Mesenteric Masses. World J. Surg. 2010, 34, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Woltering, E.A.; Voros, B.A.; Beyer, D.T.; Wang, Y.-Z.; Thiagarajan, R.; Ryan, P.; Wright, A.; Ramirez, R.A.; Ricks, M.J.; Boudreaux, J.P. Aggressive Surgical Approach to the Management of Neuroendocrine Tumors: A Report of 1,000 Surgical Cytoreductions by a Single Institution. J. Am. Coll. Surg. 2017, 224, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Systematic review of D2 lymphadenectomy versus D2 with para-aortic nodal dissection for advanced gastric cancer. World J. Gastroenterol. 2010, 16, 1138. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL). Electronic address: Easloffice@easloffice.eu EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J. Hepatol. 2016, 65, 146–181. [Google Scholar] [CrossRef] [Green Version]
- Trendle, M.C.; Moertel, C.G.; Kvols, L.K. Incidence and morbidity of cholelithiasis in patients receiving chronic octreotide for metastatic carcinoid and malignant islet cell tumors. Cancer 1997, 79, 830–834. [Google Scholar] [CrossRef]
- Norlén, O.; Hessman, O.; Stålberg, P.; Åkerström, G.; Hellman, P. Prophylactic Cholecystectomy in Midgut Carcinoid Patients. World J. Surg. 2010, 34, 1361–1367. [Google Scholar] [CrossRef]
- Brighi, N.; Lamberti, G.; Maggio, I.; Manuzzi, L.; Ricci, C.; Casadei, R.; Santini, D.; Mosconi, C.; Lisotti, A.; Ambrosini, V.; et al. Biliary stone disease in patients receiving somatostatin analogs for neuroendocrine neoplasms. A retrospective observational study. Dig. Liver Dis. 2019, 51, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Goet, J.C.; Beelen, E.M.J.; Biermann, K.E.; Gijsbers, A.H.; Schouten, W.R.; van der Woude, C.J.; de Vries, A.C. Cholecystectomy Risk in Crohn’s Disease Patients After Ileal Resection: A Long-term Nationwide Cohort Study. J. Gastrointest. Surg. 2019, 23, 1840–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcacuzco Quinto, A.; Nutu, O.-A.; San Román Manso, R.; Justo Alonso, I.; Calvo Pulido, J.; Manrique Municio, A.; García-Sesma, Á.; Loinaz Segurola, C.; Martínez Caballero, J.; Jiménez Romero, L.C. Complications of transarterial chemoembolization (TACE) in the treatment of liver tumors. Cir. Esp. 2018, 96, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Sinnamon, A.J.; Neuwirth, M.G.; Vining, C.C.; Sharoky, C.E.; Yang, Y.-X.; Kelz, R.R.; Fraker, D.L.; Roses, R.E.; Karakousis, G.C. Prophylactic Cholecystectomy at Time of Surgery for Small Bowel Neuroendocrine Tumor Does Not Increase Postoperative Morbidity. Ann. Surg. Oncol. 2018, 25, 239–245. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Frilling, A.; Al-Nahhas, A.; Clift, A.K. Transplantation and Debulking Procedures for Neuroendocrine Tumors. Front. Horm. Res. 2015, 44, 164–176. [Google Scholar]
- Arnold, R.; Chen, Y.-J.; Costa, F.; Falconi, M.; Gross, D.; Grossman, A.B.; Hyrdel, R.; Kos-Kudła, B.; Salazar, R.; Plöckinger, U. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Follow-Up and Documentation. Neuroendocrinology 2009, 90, 227–233. [Google Scholar] [CrossRef]






| Author | Study Period | Patients (n) | Disease Stage | Presence of MLNM * (%) | Presence of MLNM When Primary Tumor <1 cm * (%) | Patients Without Any Lymph Nodes Resection (%) |
|---|---|---|---|---|---|---|
| Chen [9] | 2004–2014 | 1925 | Stage I-III | 80.3 | - | - |
| Landry [7] | 1997–2004 | 1364 | Stage I-IV | 82 | - | 16.2 |
| Motz [8] | 1998–2013 | 11,852 | Stage I-III | 79.3 | 46.7 | 19.2 |
| Norlén [3] | 1985–2010 | 517 | Stage I-IV | 93 | - | - |
| Author | Year | Patients (n) | Length of Follow-Up (Years) | Relapse (%) |
|---|---|---|---|---|
| Chen [75] | 1998 | 3 | 5 | 67 |
| Chamberlain [76] | 2000 | 28 | 5 | 89 |
| Jaeck [77] | 2001 | 4 | 3 | 31 |
| Sarmiento [73] | 2003 | 90 | 10 | 94 |
| Elias [78] | 2003 | 14 | 10 | 89 |
| Kianmanesh [72] | 2008 | 23 | 4 | 48 |
| Scigliano [79] | 2009 | 41 | 5 | 78 |
| Bertani [80] | 2015 | 78 | 8 | 81 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deguelte, S.; Perrier, M.; Hammoutene, C.; Cadiot, G.; Kianmanesh, R. Surgery and Perioperative Management in Small Intestinal Neuroendocrine Tumors. J. Clin. Med. 2020, 9, 2319. https://doi.org/10.3390/jcm9072319
Deguelte S, Perrier M, Hammoutene C, Cadiot G, Kianmanesh R. Surgery and Perioperative Management in Small Intestinal Neuroendocrine Tumors. Journal of Clinical Medicine. 2020; 9(7):2319. https://doi.org/10.3390/jcm9072319
Chicago/Turabian StyleDeguelte, Sophie, Marine Perrier, Cheryne Hammoutene, Guillaume Cadiot, and Reza Kianmanesh. 2020. "Surgery and Perioperative Management in Small Intestinal Neuroendocrine Tumors" Journal of Clinical Medicine 9, no. 7: 2319. https://doi.org/10.3390/jcm9072319
APA StyleDeguelte, S., Perrier, M., Hammoutene, C., Cadiot, G., & Kianmanesh, R. (2020). Surgery and Perioperative Management in Small Intestinal Neuroendocrine Tumors. Journal of Clinical Medicine, 9(7), 2319. https://doi.org/10.3390/jcm9072319





