Efficacy of Corticosteroids in Patients with SARS, MERS and COVID-19: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
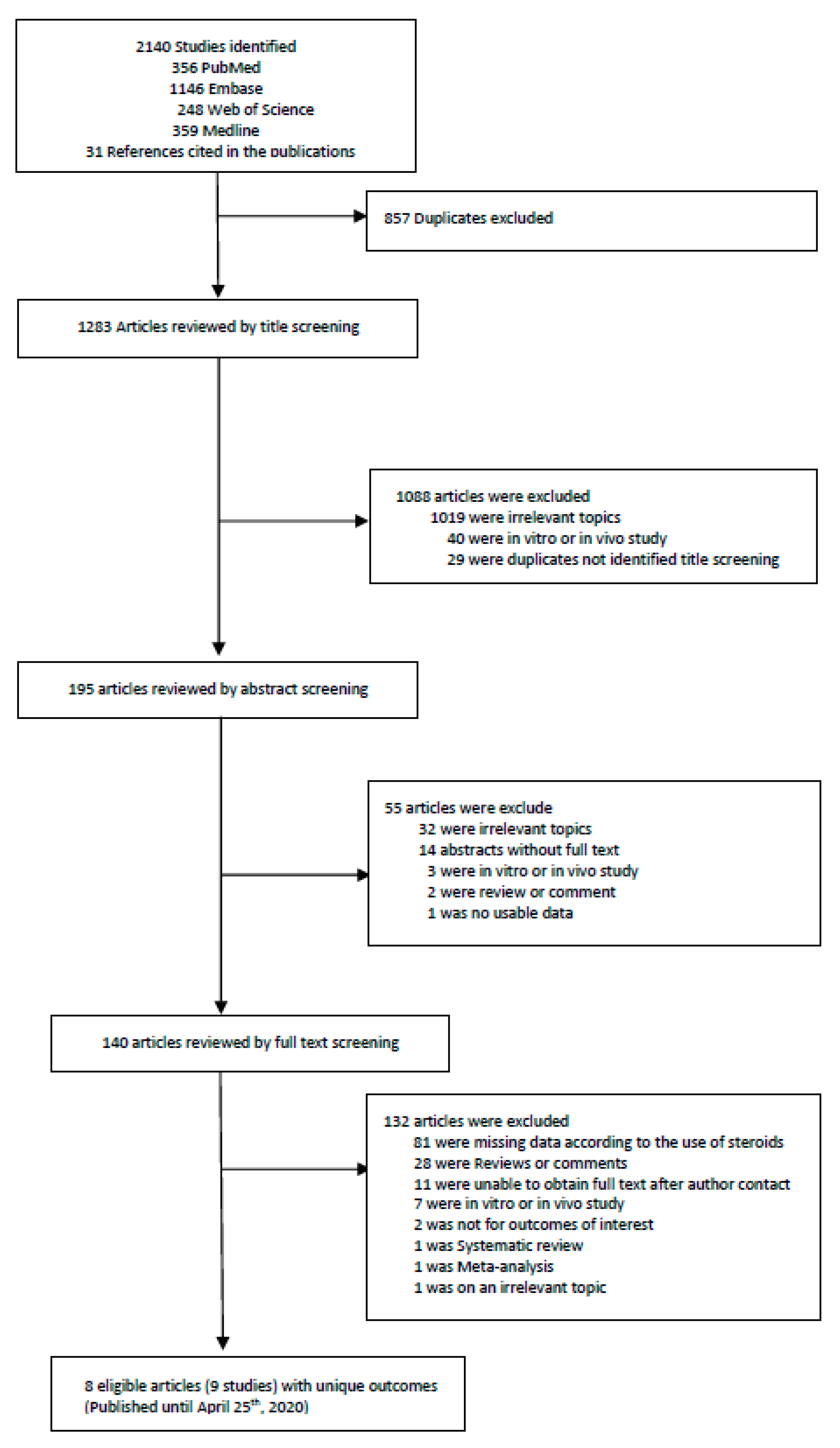
2.1. Literature Search Strategy and Selection Criteria
2.2. Quality Assessment
2.3. Analysis of Studies
2.4. Statistical Analysis
3. Results
3.1. Included Studies and Baseline Characteristics
3.2. Quality Assessment
3.3. Meta-Analyses
3.4. Sensitivity Subset Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease (COVID-19) Outbreak. 2020. Available online: https://covid19.who.int/ (accessed on 16 May 2020).
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Phelan, A.L.; Katz, R.; Gostin, L.O. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA 2020, 323, 709–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Ghinai, I.; McPherson, T.D.; Hunter, J.C.; Kirking, H.L.; Christiansen, D.; Joshi, K.; Rubin, R.; Morales-Estrada, S.; Black, S.R.; Pacilli, M.; et al. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet 2020, 395, 1137–1144. [Google Scholar] [CrossRef]
- Kalil, A.C. Treating COVID-19-Off-Label Drug Use, Compassionate Use, and Randomized Clinical Trials during Pandemics. JAMA 2020, 323, 1897–1898. [Google Scholar] [CrossRef]
- Omer, S.B.; Malani, P.; Del Rio, C. The COVID-19 Pandemic in the US: A Clinical Update. JAMA 2020, 323, 1767–1768. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. A Research and Development Blueprint for Action to Prevent Epidemics. Available online: https://www.who.int/blueprint/en/ (accessed on 11 April 2020).
- World Health Organization. SARS (Severe Acute Respiratory Syndrome). Available online: https://www.who.int/ith/diseases/sars/en/ (accessed on 25 March 2020).
- Peiris, J.S.; Yuen, K.Y.; Osterhaus, A.D.; Stöhr, K. The severe acute respiratory syndrome. N. Engl. J. Med. 2003, 349, 2431–2441. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Available online: https://www.who.int/emergencies/mers-cov/en/ (accessed on 25 March 2020).
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Cheng, V.C.; Lau, S.K.; Woo, P.C.; Yuen, K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007, 20, 660–694. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.F.; Lau, S.K.; To, K.K.; Cheng, V.C.; Woo, P.C.; Yuen, K.Y. Middle East respiratory syndrome coronavirus: Another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 2015, 28, 465–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the Eye of the Cytokine Storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef] [Green Version]
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. HLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Nicholls, J.M.; Poon, L.L.; Lee, K.C.; Ng, W.F.; Lai, S.T.; Leung, C.Y.; Chu, C.M.; Hui, P.K.; Mak, K.L.; Lim, W.; et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet 2003, 361, 1773–1778. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Wang, Y.; Shao, C.; Huang, J.; Gan, J.; Huang, X.; Bucci, E.; Piacentini, M.; Ippolito, G.; Melino, G. COVID-19 infection: The perspectives on immune responses. Cell Death Differ. 2020, 27, 1451–1454. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef]
- Russell, C.D.; Millar, J.E.; Baillie, J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020, 395, 473–475. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection is Suspected. 2020. Available online: https://www.who.int/internal-publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novelcoronavirus-(ncov)-infection-is-suspected (accessed on 25 March 2020).
- Center for Disease Control. Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html (accessed on 25 March 2020).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 28 March 2020).
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.; Smith, G.D. Sifting the evidence-what’s wrong with significance tests? BMJ 2001, 322, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.; Tarone, R.; McLaughlin, J.K. The false-positive to false-negative ratio in epidemiologic studies. Epidemiology 2011, 22, 450–456. [Google Scholar] [CrossRef]
- Cochran, W.G. The combination of estimates from different experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Li, N.; Ma, J.; Nie, L.; Li, H.; Que, C.; Gao, Z.; Wang, G.; Xu, X.; Lu, H.; Wang, G. Retrospective analysis of the corticosteroids treatment on severe acute respiratory syndrome (SARS). Beijing Da Xue Xue Bao Yi Xue Ban 2003, 35, 16–18. (In Chinese) [Google Scholar]
- Yam, L.Y.; Lau, A.C.; Lai, F.Y.; Shung, E.; Chan, J.; Wong, V. Hong Kong Hospital Authority SARS Collaborative Group (HASCOG). Corticosteroid treatment of severe acute respiratory syndrome in Hong Kong. J. Infect. 2007, 54, 28–39. [Google Scholar]
- Lau, E.H.; Cowling, B.J.; Muller, M.P.; Ho, L.M.; Tsang, T.; Lo, S.V.; Louie, M.; Leung, G.M. Effectiveness of Ribavirin and Corticosteroids for Severe Acute Respiratory Syndrome. Am. J. Med. 2009, 122, 1150.e11–1150.e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arabi, Y.M.; Mandourah, Y.; Al-Hameed, F.; Sindi, A.A.; Almekhlafi, G.A.; Hussein, M.A.; Jose, J.; Pinto, R.; Al-Omari, A.; Kharaba, A.; et al. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am. J. Respir. Crit. Care Med. 2018, 197, 757–767. [Google Scholar] [CrossRef]
- Chen, R.C.; Tang, X.P.; Tan, S.Y.; Liang, B.L.; Wan, Z.Y.; Fang, J.Q.; Zhong, N. Treatment of Severe Acute Respiratory Syndrome with glucosteroids: The Guangzhou experience. Chest 2006, 129, 1441–1452. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020. [Google Scholar] [CrossRef] [Green Version]
- Al Ghamdi, M.; Alghamdi, K.M.; Ghandoora, Y.; Alzahrani, A.; Salah, F.; Alsulami, A.; Bawayan, M.F.; Vaidya, D.; Perl, T.M.; Sood, G.; et al. Treatment outcomes for patients with Middle Eastern Respiratory Syndrome Coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infect. Dis. 2016, 16, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, J.; Zhou, Y.; Zhao, X.; Zhao, Q.; Liu, J. The effect of corticosteroid treatment on patients with coronavirus infection: A systematic review and meta-analysis. J. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Wang, Y.; Zhang, Z.L.; Liu, Y.X.; Le, K.J.; Cui, M.; Yu, Y.T.; Gu, Z.C.; Gao, Y.; Lin, H.W. Efficacy and safety of current therapeutic options for COVID-19—Lessons to be learnt from SARS and MERS epidemic: A systematic review and meta-analysis. Pharmacol. Res. 2020, 104872. [Google Scholar] [CrossRef]
- Lee, D.T.; Wing, Y.K.; Leung, H.C.; Sung, J.J.; Ng, Y.K.; Yiu, G.C.; Chen, R.Y.; Chiu, H.F. Factors associated with psychosis among patients with severe acute respiratory syndrome: A case-control study. Clin. Infect. Dis. 2004, 39, 1247–1249. (In Chinese) [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.Z.; Ma, L.; Gao, J.; Yang, Z.J.; Xing, X.Y.; Zhao, H.C.; Jiao, J.S.; Li, G.W. Glucocorticoid-induced diabetes in severe acute respiratory syndrome: The impact of high dosage and duration of methylprednisolone therapy. Zhonghua Nei Ke Za Zhi 2004, 43, 179–182. (In Chinese) [Google Scholar]
- Li, Y.M.; Wang, S.X.; Gao, H.S.; Wang, J.G.; Wei, C.S.; Chen, L.M.; Hui, W.L.; Yuan, S.L.; Jiao, Z.S.; Yang, Z.; et al. Factors of avascular necrosis of femoral head and osteoporosis in SARS patients’ convalescence. Zhonghua Yi Xue Za Zhi 2004, 84, 1348–1353. (In Chinese) [Google Scholar]
- Ni, Y.N.; Chen, G.; Sun, J.; Liang, B.M.; Liang, Z.A. The effect of corticosteroids on mortality of patients with influenza pneumonia: A systematic review and meta-analysis. Crit. Care 2019, 23, 99. [Google Scholar] [CrossRef] [Green Version]
- McGee, S.; Hirschmann, J. Use of corticosteroids in treating infectious diseases. Arch. Intern. Med. 2008, 168, 1034–1046. [Google Scholar] [CrossRef]
- Corneli, H.M.; Zorc, J.J.; Mahajan, P.; Shaw, K.N.; Holubkov, R.; Reeves, S.D.; Ruddy, R.M.; Malik, B.; Nelson, K.A.; Bregstein, J.S.; et al. A multicenter, randomized, controlled trial of dexamethasone for bronchiolitis. N. Engl. J. Med. 2007, 357, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, J.P. The Mass Production of Redundant, Misleading, and Conflicted Systematic Reviews and Meta-analyses. Milbank. Q. 2016, 94, 485–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.; Chan, K.A.; Hui, D.S.; Ng, E.K.; Wu, A.; Chiu, R.W.; Wong, V.W.; Chan, P.K.; Wong, K.T.; Wong, E.; et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J. Clin. Virol. 2004, 31, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Zhao, J.; Hu, Y.; Du, R.; Cao, B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 2020, 395, 683–684. [Google Scholar] [CrossRef] [Green Version]
- Russell, B.; Moss, C.; George, G.; Santaolalla, A.; Cope, A.; Papa, S.; Van Hemelrijck, M. Associations between immune-suppressive and stimulating drugs and novel Covid-19—A systematic review of current evidence. Ecancermedicalscience 2020, 14, 1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, B.; Moss, C.; Rigg, A.; Van Hemelrijck, M. COVID-19 and treatment with NSAIDs and corticosteroids: Should we be limiting their use in the clinical setting? Ecancermedicalscience 2020, 14, 1023. [Google Scholar] [CrossRef] [Green Version]
- National Health Commission of the People’s Republic of China. The 5th Trial Version of Diagnosis and Treatment Scheme for Pneumonitis with 2019-nCoV Infection. Available online: http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a.shtml (accessed on 25 March 2020). (In Chinese)
- Horby, P.; Lim, W.S.; Emberson, J.; Mafham, M.; Bell, J.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19: Preliminary Report. medRxiv 2020. [Google Scholar] [CrossRef]
- Villar, J.; Ferrando, C.; Martínez, D.; Ambrós, A.; Muñoz, T.; Soler, J.A.; Aguilar, G.; Alba, F.; González-Higueras, E.; Conesa, L.A.; et al. Dexamethasone Treatment for the Acute Respiratory Distress Syndrome: A Multicentre, Randomised Controlled Trial. Lancet Respir. Med. 2020, 8, 267–276. [Google Scholar] [CrossRef]



| Authors | Disease | Location/Country | Hospitals, n | Sample Size, n | Patients Used Steroids, n | Controls, n | Deaths in Steroid Group, n (%) | Deaths in Controls, n (%) | Description | Comment |
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention (OR, not-adjusted time): steroids were used in more severe cases in 3/5 included studies | ||||||||||
| Li et al. (2003) [32] | SARS | Beijing/China | 1 | 43 | 39 | 4 | 1 (2.6) | 0 (0.0) | Steroid (n = 39 (91%)); Non-steroid (n = 4 (9%)) | MP was effective for SARS patients. |
| Yam et al. (2007) [33] | SARS | Hong Kong/China | 14 | 1287 | 1188 | 99 | 202 (17.0) | 28 (28.3) | Ribavirin + Steroid (n = 1188 (92%)); Ribavirin (n = 99 (8%)) | Ribavirin + Steroid group received more ICU care than control |
| Lau et al. (2009)_H † [34] | SARS | Hong Kong/China | - | 1743 | 51 | 751 | 15 (1.9) | 175 (18.4) | Steroid (n = 51 (3%)); No therapy (n = 751 (43%)] | Steroid group was older and had more comorbidities than control |
| 739 | 202 | 93 (11.8) | 18 (1.9) | Ribavirin + Steroid (n = 739 (42%)); Ribavirin (n = 202 (12%)) | ||||||
| Lau et al. (2009)_T † [34] | SARS | Toronto/Canada | - | 191 | 42 ‡ | 149 ‡ | 6 (14.3) | 19 (12.8) | Ribavirin + Steroid (n = 39 (42%)); Ribavirin (n = 107 (56%)) | Longer delay between symptom onset and admission, hazy chest radiograph were more likely to be treated with combination therapy |
| Arabi et al. (2018) * [35] | MERS | All/Saudi Arabia | 14 | 309 | 151 | 158 | 117 (77.5) | 91 (57.6) | Steroid (n = 151 (49%)); Non-steroid (n = 158 (51%)) | Steroid was not associated 90-day mortality; Steroid group is more severe than control group |
| Intervention (HR, adjusted time): adjusted for comorbidities or time-dependent conducted studies | ||||||||||
| Chen et al. (2006) [36] | SARS | Guangzhou/China | - | 152 | 121 | 31 | 18 (14.9) | 7 (22.6) | Steroid (n = 121 (80%)); Non-steroid (n = 31 (20%)) HR 0.372 (95% CI 0.139–0.998) among critical patients in ICU (n = 152) | Steroid significantly reduced the case fatality among critical SARS after death-related variables were adjusted |
| Wu et al. (2020) [37] | COVID-19 | Wuhan/China | 1 | 84 § | 50 § | 34 § | 23 (46.0) § | 21 (61.8) § | Steroid (n = 62 (31%)]: Non-steroid (n = 139 (69%)) HR 0.38 (95% CI 0.20–0.72) among patients with ARDS (n = 201) | MP decreased the risk of death among patients with ARDS |
| Risk factor (not-adjusted time): steroid users had more severe underlying diseases | ||||||||||
| Al Ghamdi et al. (2016) [38] | MERS | Jeddah/Saudi Arabia | 1 | 51 | 5 | 46 | 3 (60.0) | 16 (34.8) | Survival (n = 2/32 (6%)); Death (n = 3/19 (16%)): steroid-used patients/each groups (p = 0.348) | All deaths received ICU care and all survivors were non ICU patients; non-survivors were more severe |
| Zhou et al. (2020) [39] | COVID-19 | Wuhan/China | 2 | 191 | 57 | 134 | 26 (45.6) | 28 (20.9) | Survival (n = 31/137 (23%)); Death (n = 26/54 (48%)): steroid-used patients/each groups (p = 0.0005) | Steroid was used in both groups after ARDS; Non-survivors were older and had more comorbidities than survivors |
| Authors | Patient Group | Mean Age (SD), Years | Male: Female | Type of Steroids | Duration of Steroids, Day | Mean Duration between Onset of Illness and Steroid Initiation, Day (SD) | ICU Care in Steroids | ICU Care in Controls | Ventilator in Steroids | Ventilator in Controls | ALI/ARDS in Steroids | ALI/ARDS in Controls | Quality Assessment ‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention (OR, not adjusted time) | |||||||||||||
| Li et al. (2003) [32] | Non-ICU | - | - | MP, pulse | - | - | 0 | 0 | 0 | 0 | - | - | Low |
| Yam et al. (2007) [33] | ICU/Non-ICU | - | 553:734 | HC, MP, PL, pulse | 15–21 | 5 (1) | 243 | 4 | 161 | 4 | 7 | 49 | Low |
| Lau et al. (2009)_H † [34] | ICU/Non-ICU | - | 773:970 | CS | - | - | - | - | - | - | - | - | Low |
| Lau et al. (2009)_T † [34] | ICU/Non-ICU | - | 74:117 | CS | - | - | - | - | - | - | - | - | Low |
| Arabi et al. (2018) * [35] | ICU | - | 213:96 | HC, DX, MP, PL | 3–21 | 10 (3) | 151 | 158 | 141 | 121 | - | - | Low |
| Intervention (HR, adjusted time) | |||||||||||||
| Chen et al. (2006) [36] | ICU | 40.2(14.6) | 68:84 | HC, MP, PL | - | 4.9 (3.6) | 152 | 0 | - | - | - | - | High |
| Wu et al. (2020) [37] | ICU | 58.5 | 60:24 | MP | - | - | - | - | - | - | - | - | High |
| Risk factor (not adjusted time) | |||||||||||||
| Al Ghamdi et al. (2016) [38] | ICU/Non-ICU | 54 | 40:11 | HC | - | - | 3 | 16 | - | - | - | - | Low |
| Zhou et al. (2020) [39] | ICU/Non-ICU | 56 | 119:72 | CS | - | 12 (4) | - | - | - | - | - | - | Low |
| Variables | SARS n (%) | MERS, n (%) | COVID-19, n (%) |
|---|---|---|---|
| No. of patients | 3416 | 360 | 275 |
| Steroid used | 2180 (63.8) | 156 (43.3) | 107 (38.9) |
| control | 1236 (36.2) | 204 (56.7) | 168 (61.1) |
| Sex * | |||
| Male | 1468 | 253 | 179 |
| Female | 1905 | 107 | 96 |
| Country | |||
| China | 3225 (94.4) | - | 275 (100) |
| Canada | 191 (5.6) | - | - |
| Saudi-Arabia | - | 360 (100) | - |
| Onset of steroid * | 2 | 1 | 1 |
| Early (<7 days from onset of illness) | 2 | 0 | 0 |
| Late (>7 days from onset of illness) | 0 | 1 | 1 |
| ICU care * | 399 | 19 | - |
| Steroid group | 395 | 3 | - |
| Controls | 4 | 16 | - |
| Ventilator * | 165 | 262 | - |
| Steroid group | 161 | 141 | - |
| Controls | 4 | 121 | - |
| ALI/ARDS * | 56 | - | - |
| Steroid group | 7 | - | - |
| Controls | 49 | - | - |
| Deaths | 582 | 227 | 98 |
| Steroid group | 335 (57.6) | 120 (52.9) | 49 (50.0) |
| Controls | 247 (42.4) | 107 (47.1) | 49 (50.0) |
| Deaths/No. | 582/3416 (17.0) | 227/360 (63.1) | 98/275 (35.6) |
| Steroid group | 335/2180 (15.4) | 120/156 (76.9) | 49/107 (45.8) |
| Controls | 247/1236 (20.0) | 107/204 (52.5) | 49/168 (29.2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.H.; Yoon, S.; Jeong, G.H.; Kim, J.Y.; Han, Y.J.; Hong, S.H.; Ryu, S.; Kim, J.S.; Lee, J.Y.; Yang, J.W.; et al. Efficacy of Corticosteroids in Patients with SARS, MERS and COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2392. https://doi.org/10.3390/jcm9082392
Lee KH, Yoon S, Jeong GH, Kim JY, Han YJ, Hong SH, Ryu S, Kim JS, Lee JY, Yang JW, et al. Efficacy of Corticosteroids in Patients with SARS, MERS and COVID-19: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2020; 9(8):2392. https://doi.org/10.3390/jcm9082392
Chicago/Turabian StyleLee, Keum Hwa, Sojung Yoon, Gwang Hun Jeong, Jong Yeob Kim, Young Joo Han, Sung Hwi Hong, Seohyun Ryu, Jae Seok Kim, Jun Young Lee, Jae Won Yang, and et al. 2020. "Efficacy of Corticosteroids in Patients with SARS, MERS and COVID-19: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 9, no. 8: 2392. https://doi.org/10.3390/jcm9082392











