Association between Bone Metabolism and Vestibular Problems in the Modified Romberg Test: Data from the 2009–2010 Korean National Health and Nutrition Examination Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Evaluation of Falling, Modified Romberg Test, and Pure Tone Audiometry
2.3. Factors Associated with Dizziness and Bone Metabolism
2.4. Evaluation of Bone Metabolism and Osteoporosis
2.5. Statistical Analysis
3. Results
3.1. Factors Associated with Falling and Vestibular Problems in the Modified Romberg Test
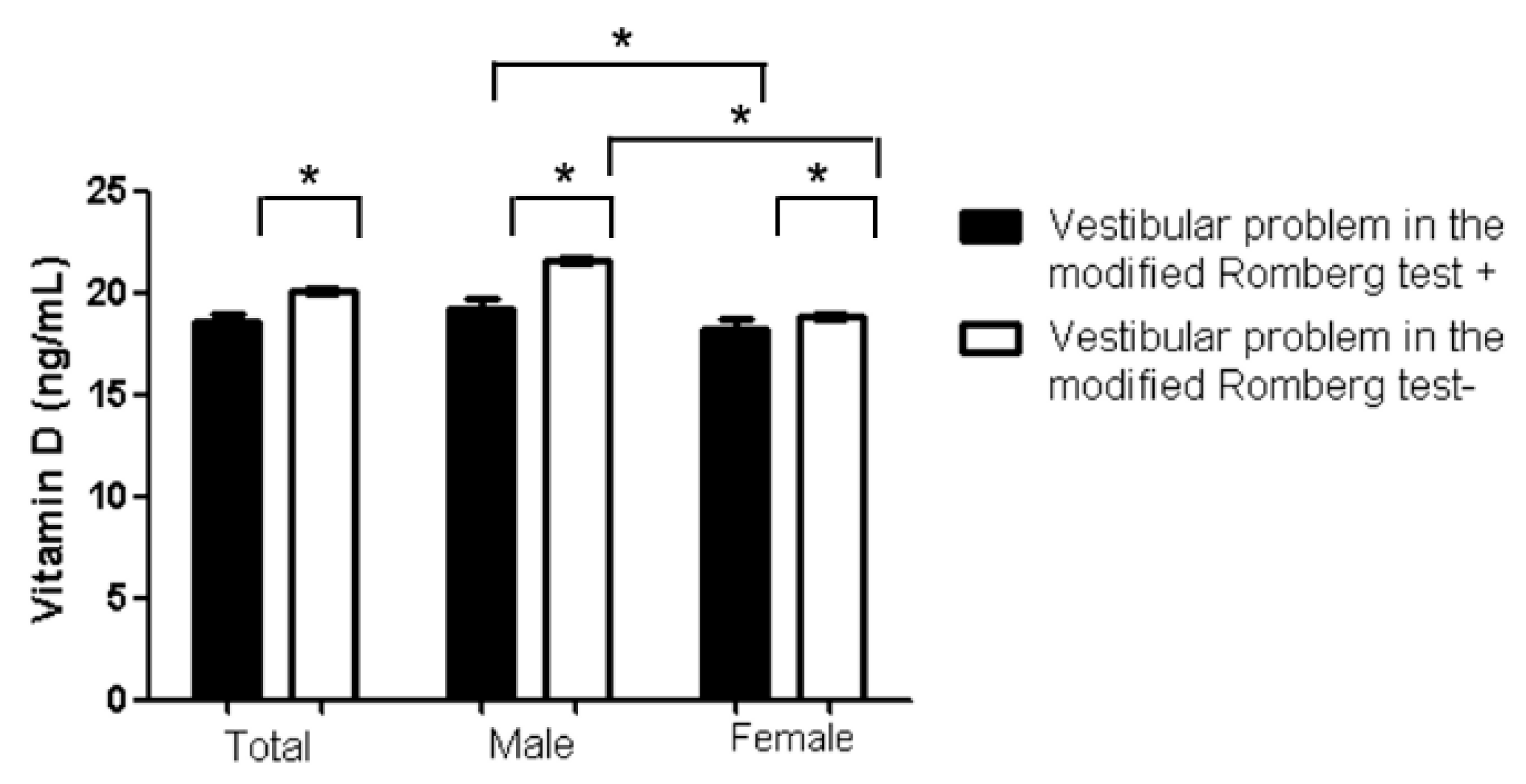
3.2. Sex Differences in Factors Associated with Vestibular Problems in the Modified Romberg Test
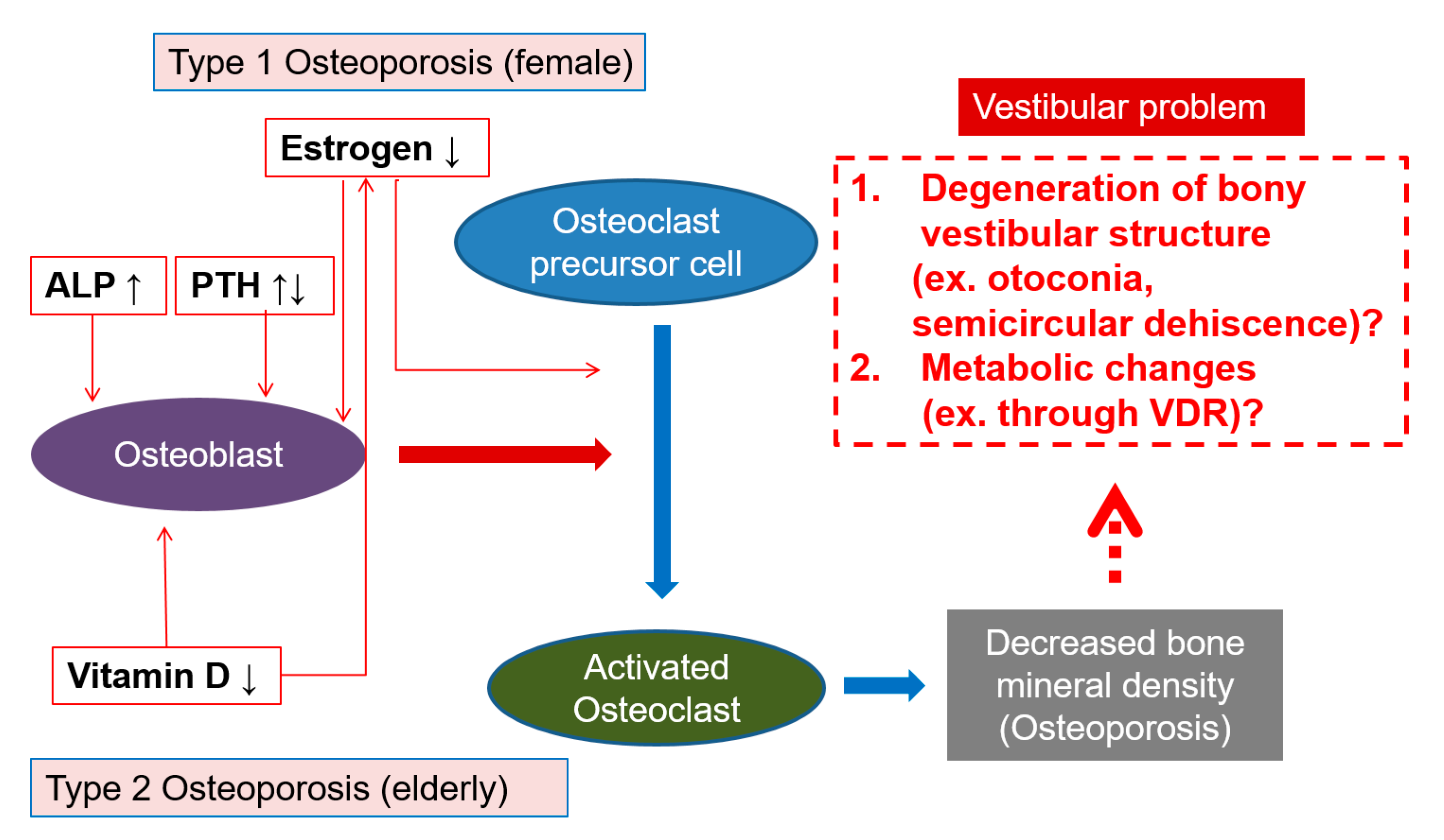
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Delbaere, K.; Close, J.C.; Menz, H.B.; Cumming, R.G.; Cameron, I.D.; Sambrook, P.N.; March, L.M.; Lord, S.R. Development and validation of fall risk screening tools for use in residential aged care facilities. Med. J. Aust. 2008, 189, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Horlings, C.G.; van Engelen, B.G.; Allum, J.H.; Bloem, B.R. A weak balance: The contribution of muscle weakness to postural instability and falls. Nat. Clin. Pract. Neurol. 2008, 4, 504–515. [Google Scholar] [CrossRef]
- Djaldetti, R.; Lorberboym, M.; Melamed, E. Primary postural instability: A cause of recurrent sudden falls in the elderly. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2006, 27, 412–416. [Google Scholar] [CrossRef]
- Miko, I.; Szerb, I.; Szerb, A.; Bender, T.; Poor, G. Effect of a balance-training programme on postural balance, aerobic capacity and frequency of falls in women with osteoporosis: A randomized controlled trial. J. Rehabil. Med. 2018, 50, 542–547. [Google Scholar] [CrossRef] [Green Version]
- Coughlan, T.; Dockery, F. Osteoporosis and fracture risk in older people. Clin. Med. 2014, 14, 187–191. [Google Scholar] [CrossRef]
- Armas, L.A.; Recker, R.R. Pathophysiology of osteoporosis: New mechanistic insights. Endocrinol. Metab. Clin. N. Am. 2012, 41, 475–486. [Google Scholar] [CrossRef]
- Siris, E.S.; Brenneman, S.K.; Barrett-Connor, E.; Miller, P.D.; Sajjan, S.; Berger, M.L.; Chen, Y.T. The effect of age and bone mineral density on the absolute, excess, and relative risk of fracture in postmenopausal women aged 50–99: Results from the National Osteoporosis Risk Assessment (NORA). Osteoporos. Int. 2006, 17, 565–574. [Google Scholar] [CrossRef]
- Yamanaka, T.; Shirota, S.; Sawai, Y.; Murai, T.; Fujita, N.; Hosoi, H. Osteoporosis as a risk factor for the recurrence of benign paroxysmal positional vertigo. Laryngoscope 2013, 123, 2813–2816. [Google Scholar] [CrossRef]
- Park, R.J.; Kim, Y.H. Association Between Osteoporosis/Osteopenia and Vestibular Dysfunction in South Korean Adults. Ear Hear. 2016, 37, 615–619. [Google Scholar] [CrossRef]
- Vibert, D.; Sans, A.; Kompis, M.; Travo, C.; Muhlbauer, R.C.; Tschudi, I.; Boukhaddaoui, H.; Hausler, R. Ultrastructural changes in otoconia of osteoporotic rats. Audiol. Neurootol. 2008, 13, 293–301. [Google Scholar] [CrossRef]
- Mendy, A.; Vieira, E.R.; Albatineh, A.N.; Nnadi, A.K.; Lowry, D.; Gasana, J. Low bone mineral density is associated with balance and hearing impairments. Ann. Epidemiol. 2014, 24, 58–62. [Google Scholar] [CrossRef]
- Cangussu, L.M.; Nahas-Neto, J.; Petri Nahas, E.A.; Rodrigues Barral, A.B.; Buttros Dde, A.; Uemura, G. Evaluation of postural balance in postmenopausal women and its relationship with bone mineral density--a cross sectional study. BMC Musculoskelet. Disord. 2012, 13, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynn, S.G.; Sinaki, M.; Westerlind, K.C. Balance characteristics of persons with osteoporosis. Arch. Phys. Med. Rehabil. 1997, 78, 273–277. [Google Scholar] [CrossRef]
- Migliaccio, S.; Greco, E.A.; Aversa, A.; Lenzi, A. Age-associated (cardio)metabolic diseases and cross-talk between adipose tissue and skeleton: Endocrine aspects. Horm. Mol. Biol. Clin. Investig. 2014, 20, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; O’Brien, C.A.; Almeida, M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 2013, 9, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.E.; Troisi, R.; Wise, L.A.; Palmer, J.R.; Titus-Ernstoff, L.; Strohsnitter, W.C.; Hatch, E.E. Menarche, menopause, years of menstruation, and the incidence of osteoporosis: The influence of prenatal exposure to diethylstilbestrol. J. Clin. Endocrinol. Metab. 2014, 99, 594–601. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.S.; Choi, S.H.; Park, K.H.; Park, H.J.; Kim, J.W.; Moon, I.J.; Rhee, C.S.; Kim, K.S.; Sun, D.I.; Lee, S.H.; et al. Prevalence of otolaryngologic diseases in South Korea: Data from the Korea national health and nutrition examination survey 2008. Clin. Exp. Otorhinolaryngol. 2010, 3, 183–193. [Google Scholar] [CrossRef]
- Hong, S.K.; Park, J.H.; Kwon, S.Y.; Kim, J.S.; Koo, J.W. Clinical efficacy of the Romberg test using a foam pad to identify balance problems: A comparative study with the sensory organization test. Eur. Arch. Otorhinolaryngol. 2014. [Google Scholar] [CrossRef]
- Suh, M.J.Y.; Yi, H.J.; Kim, H.J.; Kim, S.H. Is Asymmetric Hearing Loss a Risk Factor for Vestibular Dysfunction? Lesson From Big Data Analysis Based on the Korean National Health and Nutrition Survey. Otol. Neurotol. 2019, 40, 1339–1345. [Google Scholar] [CrossRef]
- Wei, E.X.; Agrawal, Y. Vestibular Dysfunction and Difficulty with Driving: Data from the 2001–2004 National Health and Nutrition Examination Surveys. Front. Neurol. 2017, 8, 557. [Google Scholar] [CrossRef] [Green Version]
- AmericanAcademy of Otolaryngology-Head and Neck Surgery Ffoundation, Inc. Committee on Hearing and Equilibrium guidelines for the evaluation of results of treatment of conductive hearing loss. Otolaryngol. Head Neck Surg. 1995, 113, 186–187. [Google Scholar] [CrossRef]
- Korea Centers for Disease Control and Prevention. The Fourth Korea National Health and Nutrition Examination Survey IV. Available online: http://knhanes.cdc.go.kr (accessed on 20 January 2012).
- Hurst, N.P.; Kind, P.; Ruta, D.; Hunter, M.; Stubbings, A. Measuring health-related quality of life in rheumatoid arthritis: Validity, responsiveness and reliability of EuroQol (EQ-5D). Br. J. Rheumatol. 1997, 36, 551–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewiecki, E.M.; Gordon, C.M.; Baim, S.; Leonard, M.B.; Bishop, N.J.; Bianchi, M.L.; Kalkwarf, H.J.; Langman, C.B.; Plotkin, H.; Rauch, F.; et al. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone 2008, 43, 1115–1121. [Google Scholar] [CrossRef]
- Orimo, H.; Hayashi, Y.; Fukunaga, M.; Sone, T.; Fujiwara, S.; Shiraki, M.; Kushida, K.; Miyamoto, S.; Soen, S.; Nishimura, J.; et al. Diagnostic criteria for primary osteoporosis: Year 2000 revision. J. Bone Miner. Metab. 2001, 19, 331–337. [Google Scholar] [CrossRef]
- Gargeshwari, A.; Jha, R.H.; Singh, N.K.; Kumar, P. Behavioural and objective vestibular assessment in persons with osteoporosis and osteopenia: A preliminary investigation. Braz. J. Otorhinolaryngol. 2018, 84, 744–753. [Google Scholar] [CrossRef]
- Singh, N.K.; Jha, R.H.; Gargeshwari, A.; Kumar, P. Altered auditory and vestibular functioning in individuals with low bone mineral density: A systematic review. Eur. Arch. Otorhinolaryngol. 2018, 275, 1–10. [Google Scholar] [CrossRef]
- Hudspeth, A.J.; Gillespie, P.G. Pulling springs to tune transduction: Adaptation by hair cells. Neuron 1994, 12, 1–9. [Google Scholar] [CrossRef]
- Yamauchi, D.; Nakaya, K.; Raveendran, N.N.; Harbidge, D.G.; Singh, R.; Wangemann, P.; Marcus, D.C. Expression of epithelial calcium transport system in rat cochlea and vestibular labyrinth. BMC Physiol. 2010, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Carlberg, C.; Seuter, S. The vitamin D receptor. Dermatologic Clin. 2007, 25, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C. The effects of calcitriol on falls and fractures and physical performance tests. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 497–501. [Google Scholar] [CrossRef]
- Zou, J.; Minasyan, A.; Keisala, T.; Zhang, Y.; Wang, J.H.; Lou, Y.R.; Kalueff, A.; Pyykko, I.; Tuohimaa, P. Progressive hearing loss in mice with a mutated vitamin D receptor gene. Audiol. Neurootol. 2008, 13, 219–230. [Google Scholar] [CrossRef]
- Yamauchi, D.; Raveendran, N.N.; Pondugula, S.R.; Kampalli, S.B.; Sanneman, J.D.; Harbidge, D.G.; Marcus, D.C. Vitamin D upregulates expression of ECaC1 mRNA in semicircular canal. Biochem. Biophys. Res. Commun. 2005, 331, 1353–1357. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Lou, Y.R.; Laaksi, I.; Tuohimaa, P. Impaired motor performance in mice lacking neurosteroid vitamin D receptors. Brain Res. Bull. 2004, 64, 25–29. [Google Scholar] [CrossRef]
- Minasyan, A.; Keisala, T.; Zou, J.; Zhang, Y.; Toppila, E.; Syvala, H.; Lou, Y.R.; Kalueff, A.V.; Pyykko, I.; Tuohimaa, P. Vestibular dysfunction in vitamin D receptor mutant mice. J. Steroid Biochem. Mol. Biol. 2009, 114, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ogun, O.A.; Buki, B.; Cohn, E.S.; Janky, K.L.; Lundberg, Y.W. Menopause and benign paroxysmal positional vertigo. Menopause 2014, 21, 886–889. [Google Scholar] [CrossRef] [Green Version]
- Kamel, H.K. Postmenopausal osteoporosis: Etiology, current diagnostic strategies, and nonprescription interventions. J. Manag. Care Pharm. JMCP 2006, 12, S4–S9, quiz S26–28. [Google Scholar]
- Ekblad, S.; Lonnberg, B.; Berg, G.; Odkvist, L.; Ledin, T.; Hammar, M. Estrogen effects on postural balance in postmenopausal women without vasomotor symptoms: A randomized masked trial. Obstet. Gynecol. 2000, 95, 278–283. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Bermudez Rey, M.C.; Clark, T.K.; Merfeld, D.M. Balance Screening of Vestibular Function in Subjects Aged 4 Years and Older: A Living Laboratory Experience. Front. Neurol. 2017, 8, 631. [Google Scholar] [CrossRef] [Green Version]
- Karmali, F.; Bermudez Rey, M.C.; Clark, T.K.; Wang, W.; Merfeld, D.M. Multivariate Analyses of Balance Test Performance, Vestibular Thresholds, and Age. Front. Neurol. 2017, 8, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Vestibular Problems in the Modified Romberg test (+) (n = 158) | Vestibular Problems in the Modified Romberg test (−) (n = 3896) | p-Value c | OR | 95% CI | Falling (+) (n = 626) | Falling (−) (n = 3428) | p-Value c | OR | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 70.98 ± 8.19 | 62.55 ± 8.37 | <0.001 * | 1.097 | 1.067–1.127 | 65.32 ± 8.67 | 62.43 ± 8.42 | 0.012 * | 1.018 | 1.004–1.032 |
| Male | 3.4 a | 96.6 a | 0.1182 b | 1.248 | 0.901–1.729 | 11.0 | 89.0 | <0.001 *,b | 1.645 | 1.307–2.071 |
| Female | 4.3 a | 95.7 a | 19.0 | 81.0 | ||||||
| BMI (kg/m2) | 22.75 ± 3.13 | 24.07 ± 3.04 | <0.001 * | 0.863 | 0.809–0.920 | 23.76 ± 3.31 | 24.07 ± 3.00 | 0.004 | 0.952 | 0.921–0.985 |
| % a | Vestibular Problems in the Modified Romberg Test (+) | Vestibular Problems in the Modified Romberg Test (−) | The Univariable Analysis | The Multivariable Analysis † | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | OR | 95% CI | p-Value | OR | 95% CI | |||||||
| Otolaryngologic conditions (physical examination and questionnaire) | ||||||||||||
| Tinnitus | ||||||||||||
| No (%) | 75.5 | 3.7 | 96.3 | Referent | ||||||||
| Yes (%) | 24.5 | 4.5 | 95.5 | 0.238 | 1.237 | 0.869–1.760 | ||||||
| Hearing loss | ||||||||||||
| No (%) | 76.3 | 2.6 | 97.4 | Referent | Referent | |||||||
| Yes (%) | 23.7 | 8.1 | 91.9 | <0.001 * | 3.337 | 2.421–4.599 | 0.010 * | 1.63 | 1.123–2.365 | |||
| Falling | ||||||||||||
| No (%) | 84.6 | 2.9 | 97.1 | Referent | ||||||||
| Yes (%) | 15.4 | 9.1 | 90.9 | <0.001 * | 3.3 | 2.357–4.621 | ||||||
| General conditions for activity | ||||||||||||
| Limitation of activity | ||||||||||||
| No (%) | 78.6 | 2.7 | 97.3 | Referent | Referent | |||||||
| Yes (%) | 21.4 | 8.4 | 91.6 | <0.001 * | 3.355 | 2.431–4.631 | 0.003 * | 1.891 | 1.244–2.874 | |||
| Stress | ||||||||||||
| No (%) | 75.6 | 3.5 | 96.5 | Referent | ||||||||
| Yes (%) | 24.4 | 5.1 | 94.9 | 0.031 * | 1.46 | 1.036–2.057 | 0.451 | 1.169 | 0.779–1.753 | |||
| EQ-5D index (Mean) b,† | 0.91 ± 0.14 | 0.81 ± 0.21 | 0.91 ± 0.14 | <0.001 * | 0.046 | 0.022–0.096 | 0.11 | 0.404 | 0.133–1.226 | |||
| Visual disturbance | ||||||||||||
| No (%) | 98.6 | 3.8 | 96.2 | Referent | Referent | |||||||
| Yes (%) | 1.4 | 10.9 | 89.1 | 0.007 * | 3.099 | 1.307–7.346 | 0.503 | 0.699 | 0.245–1.994 | |||
| Underlying diseases | ||||||||||||
| Diagnosis of stroke | ||||||||||||
| No (%) | 97.1 | 3.9 | 96.1 | Referent | ||||||||
| Yes (%) | 2.9 | 5.1 | 94.9 | 0.5 | 1.334 | 0.577–3.080 | ||||||
| Diagnosis of osteoarthritis | ||||||||||||
| No (%) | 78.9 | 3.4 | 79.3 | Referent | Referent | |||||||
| Yes (%) | 21.1 | 5.7 | 94.3 | 0.002 * | 1.718 | 1.216–2.428 | 0.343 | 1.223 | 0.807–1.851 | |||
| Depressive mood | ||||||||||||
| No (%) | 83.3 | 3.7 | 96.3 | Referent | ||||||||
| Yes (%) | 16.7 | 4.7 | 95.3 | 0.23 | 1.275 | 0.857–1.897 | ||||||
| Diagnosis of depression | ||||||||||||
| No (%) | 95 | 3.9 | 96.1 | Referent | ||||||||
| Yes (%) | 5 | 4 | 96 | 0.962 | 1.018 | 0.493–2.103 | ||||||
| Diagnosis of Hypertension | ||||||||||||
| No (%) | 61.2 | 3 | 97 | Referent | Referent | |||||||
| Yes (%) | 38.8 | 5.3 | 94.7 | <0.001 * | 1.839 | 1.337–2.530 | 0.115 | 1.349 | 0.929–1.958 | |||
| Diagnosis of Diabetes | ||||||||||||
| No (%) | 86.1 | 3.6 | 96.4 | Referent | Referent | |||||||
| Yes (%) | 13.9 | 5.7 | 94.3 | 0.019 * | 1.613 | 1.083–2.403 | 0.18 | 1.35 | 0.870–2.095 | |||
| Diagnosis of Anemia | ||||||||||||
| No (%) | 90.7 | 3.6 | 96.4 | Referent | Referent | |||||||
| Yes (%) | 9.3 | 6.4 | 93.6 | 0.010 * | 1.803 | 1.152–2.822 | 0.361 | 0.785 | 0.467–1.319 | |||
| Osteoporosis | ||||||||||||
| Normal | 27.5 | 1.4 | 98.6 | Referent | ||||||||
| Osteopenia | 50.5 | 4 | 96 | <0.001 * | 2.953 | 1.659–5.256 | 0.15 | 1.555 | 0.853–2.835 | |||
| Osteoporosis | 22 | 6.8 | 93.2 | <0.001 * | 5.205 | 2.873–9.430 | 0.43 | 1.303 | 0.675–2.513 | |||
| DEXA T score (total femur) | −0.35 ± 1.03 | −0.97 ± 1.14 | −0.32 ± 1.01 | <0.001 * | 0.531 | 0.452–0.624 | ||||||
| DEXA T score (femur neck) | −1.24 ± 1.06 | −1.89 ± 1.15 | −1.21±1.05 | <0.001 * | 0.523 | 0.444–0.615 | ||||||
| DEXA T score (lumbar) | −1.22 ± 1.36 | −1.64 ± 1.49 | −1.20 ± 1.35 | <0.001 * | 0.776 | 0.683–0.882 | ||||||
| vitamin D (ng/mL) | 20.05 ± 7.22 | 18.57 ± 6.78 | 20.11 ± 7.23 | <0.001 * | 0.969 | 0.946–0.992 | <0.001 * | 0.951 | 0.926–0.976 | |||
| alkaline phosphatase (IU/L) | 247.18 ± 75.81 | 270.26 ± 94.72 | 246.24 ± 74.81 | <0.001 * | 1.003 | 1.002–1.005 | 0.108 | 1.002 | 1.000–1.004 | |||
| PTH (pg/mL) | 68.89 ± 29.99 | 73.59 ± 32.58 | 68.70 ± 29.86 | 0.047 * | 1.004 | 1.000–1.008 | ||||||
| % a | Falling (+) | Falling (−) | The Univariable Analysis | The Multivariable Analysis † | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | OR | 95% CI | p-Value | OR | 95% CI | |||||||
| Otolaryngologic conditions (Physical examination and questionnaire) | ||||||||||||
| Tinnitus | ||||||||||||
| No (%) | 75.5 | 24.5 | 75.5 | Referent | Referent | |||||||
| Yes (%) | 24.5 | 12.5 | 87.5 | <0.001 * | 2.281 | 1.905–2.730 | <0.001 * | 1.907 | 1.562–2.328 | |||
| Hearing loss | ||||||||||||
| No (%) | 76.3 | 13.7 | 86.3 | Referent | Referent | |||||||
| Yes (%) | 23.7 | 21.0 | 79.0 | <0.001 * | 1.666 | 1.383–2.007 | 0.152 | 1.176 | 0.942–1.468 | |||
| General conditions for activity | ||||||||||||
| Limitation of activity | ||||||||||||
| No (%) | 78.6 | 12.6 | 81.3 | Referent | Referent | |||||||
| Yes (%) | 21.4 | 26.1 | 73.9 | <0.001 * | 2.457 | 2.043–2.954 | 0.002 * | 1.451 | 1.145–1.840 | |||
| Stress | ||||||||||||
| No (%) | 75.6 | 13.0 | 87.0 | Referent | Referent | |||||||
| Yes (%) | 24.4 | 22.5 | 77.5 | <0.001 * | 1.937 | 1.614–2.326 | 0.004 * | 1.391 | 1.110–1.743 | |||
| EQ-5D index (Mean) † | 0.90 ± 0.15 b | 0.84 ± 0.19 b | 0.92±0.13 b | <0.001 * | 0.051 | 0.030–0.086 | <0.001* | 0.286 | 0.141–0.578 | |||
| Visual disturbance | ||||||||||||
| No (%) | 98.6 | 15.2 | 84.8 | Referent | ||||||||
| Yes (%) | 1.4 | 36.4 | 63.6 | <0.001 * | 3.199 | 1.835–5.580 | 0.373 | 1.342 | 0.702–2.566 | |||
| Underlying diseases | ||||||||||||
| Diagnosis of Stroke | ||||||||||||
| No (%) | 97.1 | 15.3 | 84.7 | Referent | ||||||||
| Yes (%) | 2.9 | 21.2 | 78.8 | 0.082 | 1.492 | 0.951–2.339 | ||||||
| Diagnosis of Osteoarthritis | ||||||||||||
| No (%) | 78.9 | 13.9 | 86.1 | Referent | Referent | |||||||
| Yes (%) | 21.1 | 21.4 | 78.6 | <0.001 * | 1.688 | 1.393–2.045 | 0.707 | 1.046 | 0.827–1.324 | |||
| Depressive mood | ||||||||||||
| No (%) | 83.3 | 13.9 | 86.1 | Referent | Referent | |||||||
| Yes (%) | 16.7 | 23.0 | 77.0 | <0.001 * | 1.844 | 1.504–2.260 | 0.337 | 1.132 | 0.879–1.459 | |||
| Diagnosis of Hypertension | ||||||||||||
| No (%) | 61.2 | 13.6 | 86.4 | Referent | ||||||||
| Yes (%) | 38.8 | 18.3 | 81.7 | <0.001 * | 1.425 | 1.200–1.691 | 0.045 * | 1.230 | 1.005–1.504 | |||
| Diagnosis of Diabetes | ||||||||||||
| No (%) | 86.1 | 15.0 | 85.0 | Referent | Referent | |||||||
| Yes (%) | 13.9 | 18.3 | 81.7 | 0.042 * | 1.274 | 1.009–1.608 | 0.423 | 1.112 | 0.857–1.443 | |||
| Diagnosis of Anemia | ||||||||||||
| No (%) | 90.7 | 14.8 | 85.2 | Referent | Referent | |||||||
| Yes (%) | 9.3 | 21.5 | 78.5 | 0.001 * | 1.578 | 1.214–2.052 | 0.910 | 1.018 | 0.747–1.387 | |||
| Osteoporosis | ||||||||||||
| Normal | 27.5 | 10.4 | 89.6 | Referent | ||||||||
| Osteopenia | 50.5 | 15.5 | 84.5 | <0.001 * | 1.582 | 1.247–2.007 | 0.552 | 1.083 | 0.833–1.407 | |||
| Osteoporosis | 22.0 | 21.9 | 78.1 | <0.001 * | 2.422 | 1.864–3.147 | 0.574 | 1.099 | 0.790–1.529 | |||
| DEXA T score (total femur) | −0.35 ± 1.03 b | −0.59 ± 1.06 b | −0.30 ± 1.01 b | <0.001 * | 0.760 | 0.699–0.828 | ||||||
| DEXA T score (femur neck) | −1.24 ± 1.06 b | −1.56 ± 1.07 b | −1.18 ± 1.05 b | <0.001 * | 0.706 | 0.649–0.769 | ||||||
| DEXA T score (lumbar) | −1.22 ± 1.36 b | −1.52 ± 1.38 b | −1.16 ± 1.35 b | <0.001 * | 0.818 | 0.766–0.875 | ||||||
| vitamin D (ng/mL) | 20.05 ± 7.22 b | 19.79 ± 6.89 b | 20.10 ± 7.28 b | 0.329 | 0.994 | 0.982–1.006 | ||||||
| alkaline phosphatase (IU/L) | 247.18 ± 75.81 b | 251.01 ± 79.11 b | 246.48 ± 75.19 b | 0.169 | 1.001 | 1.000–1.002 | ||||||
| PTH (pg/mL) | 68.89 ± 29.99 b | 67.66 ± 29.27 b | 69.11 ± 30.11 b | 0.266 | 0.998 | 0.995–1.001 | ||||||
| Model | R | R Square | F | p |
|---|---|---|---|---|
| Vitamin D | 0.052 | 0.003 | 9.929 | 0.002 * |
| Vitamin D * Sex | 0.057 | 0.003 | 5.909 | 0.003 * |
| Vitamin D * Sex * Osteoporosis | 0.108 | 0.012 | 14.374 | <0.001 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.Y.; Cho, Y.-S.; Kim, J.-S.; Koo, J.-W. Association between Bone Metabolism and Vestibular Problems in the Modified Romberg Test: Data from the 2009–2010 Korean National Health and Nutrition Examination Survey. J. Clin. Med. 2020, 9, 2415. https://doi.org/10.3390/jcm9082415
Kim SY, Cho Y-S, Kim J-S, Koo J-W. Association between Bone Metabolism and Vestibular Problems in the Modified Romberg Test: Data from the 2009–2010 Korean National Health and Nutrition Examination Survey. Journal of Clinical Medicine. 2020; 9(8):2415. https://doi.org/10.3390/jcm9082415
Chicago/Turabian StyleKim, So Young, Yang-Sun Cho, Ji-Soo Kim, and Ja-Won Koo. 2020. "Association between Bone Metabolism and Vestibular Problems in the Modified Romberg Test: Data from the 2009–2010 Korean National Health and Nutrition Examination Survey" Journal of Clinical Medicine 9, no. 8: 2415. https://doi.org/10.3390/jcm9082415





