Inflammatory Cells in Diffuse Large B Cell Lymphoma
Abstract
:1. Introduction
1.1. Diffuse Large B Cell Lymphoma
1.2. Tumor Microenvironment Immune Cells
1.3. Epithelial Mesenchymal Transition (EMT) and Inflammatory Cells
2. Immune Infiltrating Cells
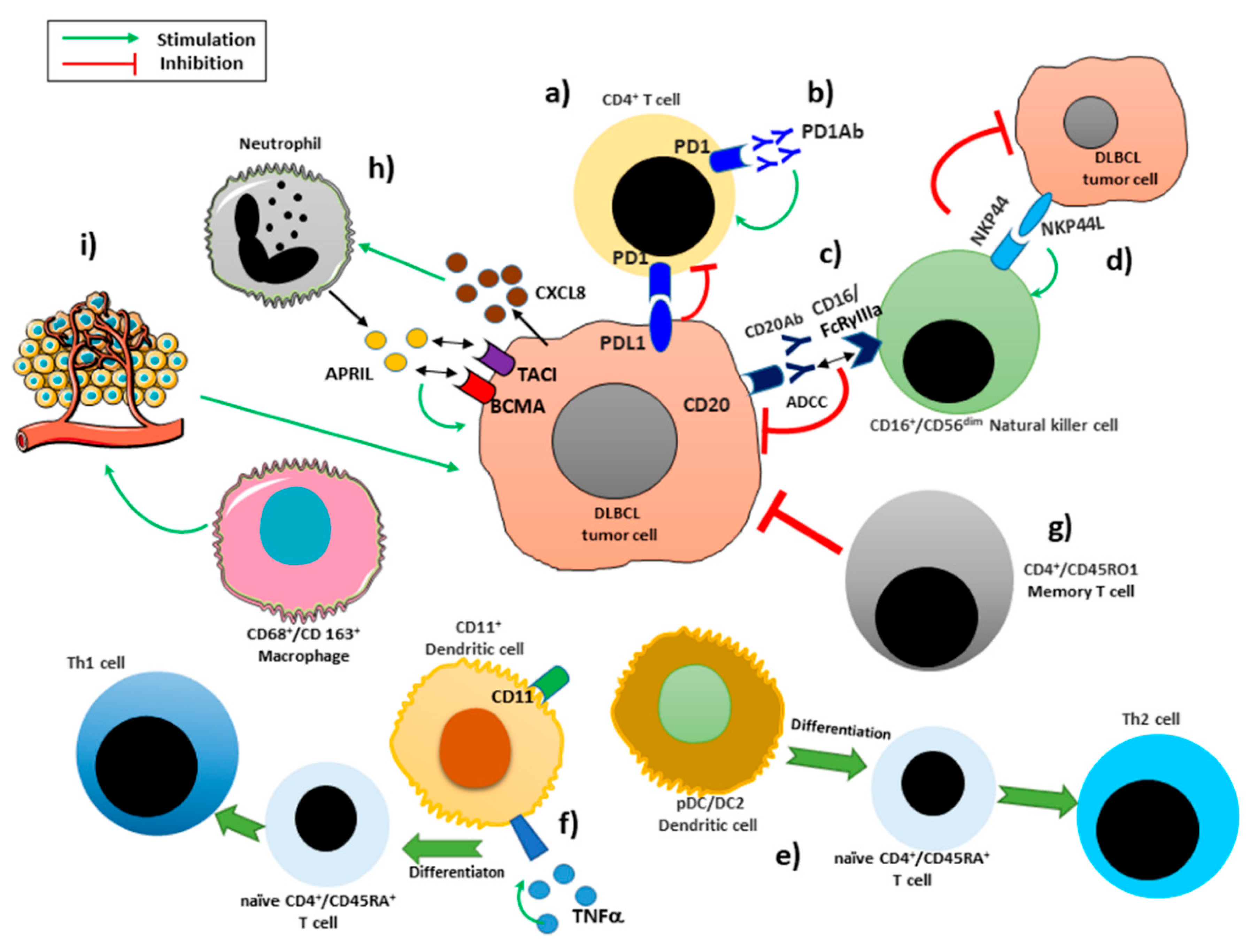
2.1. Macrophages
2.2. Neutrophils
2.3. Dendritic Cells
2.4. T Lymphocytes
PD-1/PD-L1 Blockade Therapy
2.5. Natural Killers Cells
3. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011, 117, 5019–5032. [Google Scholar] [CrossRef] [Green Version]
- Teras, L.R.; DeSantis, C.E.; Cerhan, J.R.; Morton, L.M.; Jemal, A.; Flowers, C.R. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J. Clin. 2016, 66, 443–459. [Google Scholar] [CrossRef]
- Ollila, T.A.; Olszewski, A.J. Extranodal Diffuse Large B Cell Lymphoma: Molecular Features, Prognosis, and Risk of Central Nervous System Recurrence. Curr. Treat. Options Oncol. 2018, 19, 38. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swerdlow, S.H. Lymphoma classification and the tools of our trade: An introduction to the 2012 USCAP Long Course. Mod. Pathol. 2013, 26, S1–S14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, C.R.; Hartsock, R.J. Classifications of lymphoma; reflections of time and technology. Virchows Arch. 2011, 458, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, M.; Gazzola, A.; Mannu, C.; Pileri, S.A.; Sabattini, E.; Pileri, S.A. The role of molecular biology in the diagnosis of lymphoid neoplasms. Front. Biosci. 2014, 19, 1088–1104. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.; Pasqualucci, L.; Dalla-Favera, R. Molecular pathogenesis of diffuse large B-cell lymphoma. Semin. Diagn. Pathol. 2011, 28, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Gasteiger, G.; Fan, X.; Dikiy, S.; Lee, S.Y.; Rudensky, A.Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 2015, 350, 981–985. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.W.; Qiu, S.J.; Fan, J.; Zhou, J.; Gao, Q.; Xiao, Y.S.; Xu, Y.F. Intratumoral neutrophils: A poor prognostic factor for hepatocellular carcinoma following resection. J. Hepatol. 2011, 54, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Patruno, R.; Lionetti, A.; Di Summa, A.; Mattioli, E.; Bufo, P.; Pellecchia, A.; Ribatti, D.; Zizzo, N. Endothelial area and microvascular density in a canine non–Hodgkin’s lymphoma: An interspecies model of tumor angiogenesis. Leuk. Lymphoma 2005, 46, 1639–1643. [Google Scholar] [CrossRef]
- Shain, K.H.; Dalton, W.S.; Tao, J. The tumor microenvironment shapes hallmarks of mature B–cell malignancies. Oncogene 2015, 34, 4673–4682. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Han, J.J.; Stenson, M.; Maurer, M.; Wellik, L.; Hu, G.; Ziesmer, S.; Dogan, A.; Witzig, T.E. Elevated serum IL-10 levels in diffuse large B–cell lymphoma: A mechanism of aberrant JAK2 activation. Blood 2012, 119, 2844–2853. [Google Scholar] [CrossRef]
- Alas, S.; Bonavida, B. Inhibition of constitutive STAT3 activity sensitizes resistant non–Hodgkin’s lymphoma and multiple myeloma to chemotherapeutic drug–mediated apoptosis. Clin. Cancer Res. 2003, 9, 316–326. [Google Scholar]
- Hashwah, H.; Bertram, K.; Stirm, K.; Stelling, A.; Wu, C.T.; Kasser, S.; Manz, M.G.; Theocharides, A.P.; Tzankov, A.; Muller, A. The IL–6 signaling complex is a critical driver, negative prognostic factor, and therapeutic target in diffuse large B–cell lymphoma. EMBO Mol. Med. 2019, 11, e10576. [Google Scholar] [CrossRef]
- Kuper, H.; Adami, H.O.; Trichopoulos, D. Infections as a major preventable cause of human cancer. J. Intern. Med. 2000, 248, 171–183. [Google Scholar] [CrossRef]
- Wahl, L.M.; Kleinman, H.K. Tumor-associated macrophages as targets for cancer therapy. J. Natl. Cancer Inst. 1998, 90, 1583–1584. [Google Scholar] [CrossRef] [Green Version]
- Karin, M. Nuclear factor–kappaB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.; Hardy, S.D.; Kim, D.; Akhand, S.S.; Jolly, M.K.; Wang, W.H.; Anderson, J.C.; Khodadadi, R.B.; Brown, W.S.; George, J.T.; et al. Spleen Tyrosine Kinase-Mediated Autophagy Is Required for Epithelial-Mesenchymal Plasticity and Metastasis in Breast Cancer. Cancer Res. 2019, 79, 1831–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, S.D.; Shinde, A.; Wang, W.H.; Wendt, M.K.; Geahlen, R.L. Regulation of epithelial–mesenchymal transition and metastasis by TGF-beta, P–bodies, and autophagy. Oncotarget 2017, 8, 103302–103314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libring, S.; Shinde, A.; Chanda, M.K.; Nuru, M.; George, H.; Saleh, A.M.; Abdullah, A.; Kinzer-Ursem, T.L.; Calve, S.; Wendt, M.K.; et al. The Dynamic Relationship of Breast Cancer Cells and Fibroblasts in Fibronectin Accumulation at Primary and Metastatic Tumor Sites. Cancers 2020, 12, 1270. [Google Scholar] [CrossRef]
- Shinde, A.; Libring, S.; Alpsoy, A.; Abdullah, A.; Schaber, J.A.; Solorio, L.; Wendt, M.K. Autocrine Fibronectin Inhibits Breast Cancer Metastasis. Mol. Cancer Res. 2018, 16, 1579–1589. [Google Scholar] [CrossRef] [Green Version]
- Shinde, A.; Paez, J.S.; Libring, S.; Hopkins, K.; Solorio, L.; Wendt, M.K. Transglutaminase-2 facilitates extracellular vesicle-mediated establishment of the metastatic niche. Oncogenesis 2020, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Shinde, A.; Wilmanski, T.; Chen, H.; Teegarden, D.; Wendt, M.K. Pyruvate carboxylase supports the pulmonary tropism of metastatic breast cancer. Breast Cancer Res. 2018, 20, 76. [Google Scholar] [CrossRef] [Green Version]
- Wilmanski, T.; Zhou, X.; Zheng, W.; Shinde, A.; Donkin, S.S.; Wendt, M.; Burgess, J.R.; Teegarden, D. Inhibition of pyruvate carboxylase by 1alpha,25–dihydroxyvitamin D promotes oxidative stress in early breast cancer progression. Cancer Lett. 2017, 411, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Uzunalli, G.; Dieterly, A.M.; Kemet, C.M.; Weng, H.Y.; Soepriatna, A.H.; Goergen, C.J.; Shinde, A.B.; Wendt, M.K.; Lyle, L.T. Dynamic transition of the blood–brain barrier in the development of non-small cell lung cancer brain metastases. Oncotarget 2019, 10, 6334–6348. [Google Scholar] [CrossRef] [Green Version]
- Schoppmann, S.F.; Birner, P.; Stockl, J.; Kalt, R.; Ullrich, R.; Caucig, C.; Kriehuber, E.; Nagy, K.; Alitalo, K.; Kerjaschki, D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 2002, 161, 947–956. [Google Scholar] [CrossRef] [Green Version]
- Tamma, R.; Ruggieri, S.; Annese, T.; Simone, G.; Mangia, A.; Rega, S.; Zito, F.A.; Nico, B.; Ribatti, D. Bcl6/p53 expression, macrophages/mast cells infiltration and microvascular density in invasive breast carcinoma. Oncotarget 2018, 9, 22727–22740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Q.C.; Liao, H.; Lin, S.X.; Xia, Y.; Wang, X.X.; Gao, Y.; Lin, Z.X.; Lu, J.B.; Huang, H.Q. High expression of tumor–infiltrating macrophages correlates with poor prognosis in patients with diffuse large B–cell lymphoma. Med. Oncol. 2012, 29, 2317–2322. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, C.; Huang, H.; Janda, K.; Edgington, T. Overexpression of legumain in tumors is significant for invasion/metastasis and a candidate enzymatic target for prodrug therapy. Cancer Res. 2003, 63, 2957–2964. [Google Scholar] [PubMed]
- Lenz, G.; Wright, G.; Dave, S.S.; Xiao, W.; Powell, J.; Zhao, H.; Xu, W.; Tan, B.; Goldschmidt, N.; Iqbal, J.; et al. Stromal gene signatures in large-B–cell lymphomas. N. Engl. J. Med. 2008, 359, 2313–2323. [Google Scholar] [CrossRef] [Green Version]
- Hasselblom, S.; Hansson, U.; Sigurdardottir, M.; Nilsson-Ehle, H.; Ridell, B.; Andersson, P.O. Expression of CD68+ tumor-associated macrophages in patients with diffuse large B–cell lymphoma and its relation to prognosis. Pathol. Int. 2008, 58, 529–532. [Google Scholar] [CrossRef]
- Marinaccio, C.; Ingravallo, G.; Gaudio, F.; Perrone, T.; Ruggieri, S.; Opinto, G.; Nico, B.; Maiorano, E.; Specchia, G.; Ribatti, D. T cells, mast cells and microvascular density in diffuse large B–cell lymphoma. Clin. Exp. Med. 2016, 16, 301–306. [Google Scholar] [CrossRef]
- Zizzo, N.; Patruno, R.; Zito, F.A.; Di Summa, A.; Tinelli, A.; Troilo, S.; Misino, A.; Ruggieri, E.; Goffredo, V.; Gadaleta, C.D.; et al. Vascular endothelial growth factor concentrations from platelets correlate with tumor angiogenesis and grading in a spontaneous canine non-Hodgkin lymphoma model. Leuk. Lymphoma 2010, 51, 291–296. [Google Scholar] [CrossRef]
- Shen, L.; Li, H.; Shi, Y.; Wang, D.; Gong, J.; Xun, J.; Zhou, S.; Xiang, R.; Tan, X. M2 tumour–associated macrophages contribute to tumour progression via legumain remodelling the extracellular matrix in diffuse large B–cell lymphoma. Sci. Rep. 2016, 6, 30347. [Google Scholar] [CrossRef]
- Marchesi, F.; Cirillo, M.; Bianchi, A.; Gately, M.; Olimpieri, O.M.; Cerchiara, E.; Renzi, D.; Micera, A.; Balzamino, B.O.; Bonini, S.; et al. High density of CD68+/CD163+ tumour-associated macrophages (M2–TAM) at diagnosis is significantly correlated to unfavorable prognostic factors and to poor clinical outcomes in patients with diffuse large B–cell lymphoma. Hematol. Oncol. 2015, 33, 110–112. [Google Scholar] [CrossRef]
- Nam, S.J.; Go, H.; Paik, J.H.; Kim, T.M.; Heo, D.S.; Kim, C.W.; Jeon, Y.K. An increase of M2 macrophages predicts poor prognosis in patients with diffuse large B–cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk. Lymphoma 2014, 55, 2466–2476. [Google Scholar] [CrossRef]
- Tamma, R.; Ingravallo, G.; Gaudio, F.; Annese, T.; Albano, F.; Ruggieri, S.; Dicataldo, M.; Maiorano, E.; Specchia, G.; Ribatti, D. STAT3, tumor microenvironment, and microvessel density in diffuse large B–cell lymphomas. Leuk. Lymphoma 2020, 61, 567–574. [Google Scholar] [CrossRef]
- Mu, S.; Ai, L.; Fan, F.; Qin, Y.; Sun, C.; Hu, Y. Prognostic role of neutrophil–to–lymphocyte ratio in diffuse large B cell lymphoma patients: An updated dose–response meta–analysis. Cancer Cell Int. 2018, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Stefaniuk, P.; Szymczyk, A.; Podhorecka, M. The Neutrophil to Lymphocyte and Lymphocyte to Monocyte Ratios as New Prognostic Factors in Hematological Malignancies—A Narrative Review. Cancer Manag. Res. 2020, 12, 2961–2977. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Fridlender, Z.G.; Granot, Z. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer Microenviron. 2015, 8, 125–158. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor–associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Fridlender, Z.G.; Albelda, S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef] [Green Version]
- Scapini, P.; Lapinet-Vera, J.A.; Gasperini, S.; Calzetti, F.; Bazzoni, F.; Cassatella, M.A. The neutrophil as a cellular source of chemokines. Immunol. Rev. 2000, 177, 195–203. [Google Scholar] [CrossRef]
- Schrub, J.C.; Courtois, H.; Vuillermet, P.; Viardot, N.; Mezaize, D. Weight loss of obese patients and fatigue. Study of muscular performance and weight changes. Sem. Hop. 1978, 54, 942–946. [Google Scholar]
- Liang, S.C.; Long, A.J.; Bennett, F.; Whitters, M.J.; Karim, R.; Collins, M.; Goldman, S.J.; Dunussi-Joannopoulos, K.; Williams, C.M.; Wright, J.F.; et al. An IL–17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J. Immunol. 2007, 179, 7791–7799. [Google Scholar] [CrossRef] [Green Version]
- Tecchio, C.; Scapini, P.; Pizzolo, G.; Cassatella, M.A. On the cytokines produced by human neutrophils in tumors. Semin. Cancer Biol. 2013, 23, 159–170. [Google Scholar] [CrossRef]
- Kimberley, F.C.; Medema, J.P.; Hahne, M. APRIL in B–cell malignancies and autoimmunity. Results Probl. Cell Differ. 2009, 49, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Schwaller, J.; Schneider, P.; Mhawech-Fauceglia, P.; McKee, T.; Myit, S.; Matthes, T.; Tschopp, J.; Donze, O.; Le Gal, F.A.; Huard, B. Neutrophil–derived APRIL concentrated in tumor lesions by proteoglycans correlates with human B–cell lymphoma aggressiveness. Blood 2007, 109, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Boone, T.; Delaney, J.; Hawkins, N.; Kelley, M.; Ramakrishnan, M.; McCabe, S.; Qiu, W.R.; Kornuc, M.; Xia, X.Z.; et al. APRIL and TALL–I and receptors BCMA and TACI: System for regulating humoral immunity. Nat. Immunol. 2000, 1, 252–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.F.; Anderson, K.C.; Tai, Y.T. Targeting B–Cell Maturation Antigen (BCMA) in Multiple Myeloma: Potential Uses of BCMA–Based Immunotherapy. Front. Immunol. 2018, 9, 1821. [Google Scholar] [CrossRef] [PubMed]
- Hagner, P.R.; Waldman, M.; Gray, F.D.; Yura, R.; Hersey, S.; Chan, H.; Zhang, M.; Boss, I.; Gandhi, A.K. Targeting B-Cell Maturation Antigen (BCMA) with CC-93269, a 2+1 T Cell Engager, Elicits Significant Apoptosis in Diffuse Large B–Cell Lymphoma Preclinical Models. Blood 2019, 134, 1580. [Google Scholar] [CrossRef]
- Friedman, K.M.; Garrett, T.E.; Evans, J.W.; Horton, H.M.; Latimer, H.J.; Seidel, S.L.; Horvath, C.J.; Morgan, R.A. Effective Targeting of Multiple B–Cell Maturation Antigen–Expressing Hematological Malignances by Anti–B–Cell Maturation Antigen Chimeric Antigen Receptor T Cells. Hum. Gene Ther. 2018, 29, 585–601. [Google Scholar] [CrossRef] [Green Version]
- Manfroi, B.; McKee, T.; Mayol, J.F.; Tabruyn, S.; Moret, S.; Villiers, C.; Righini, C.; Dyer, M.; Callanan, M.; Schneider, P.; et al. CXCL–8/IL8 Produced by Diffuse Large B–cell Lymphomas Recruits Neutrophils Expressing a Proliferation–Inducing Ligand APRIL. Cancer Res. 2017, 77, 1097–1107. [Google Scholar] [CrossRef] [Green Version]
- Nie, M.; Yang, L.; Bi, X.; Wang, Y.; Sun, P.; Yang, H.; Liu, P.; Li, Z.; Xia, Y.; Jiang, W. Neutrophil Extracellular Traps Induced by IL8 Promote Diffuse Large B–cell Lymphoma Progression via the TLR9 Signaling. Clin. Cancer Res. 2019, 25, 1867–1879. [Google Scholar] [CrossRef]
- Robinson, S.P.; Patterson, S.; English, N.; Davies, D.; Knight, S.C.; Reid, C.D. Human peripheral blood contains two distinct lineages of dendritic cells. Eur. J. Immunol. 1999, 29, 2769–2778. [Google Scholar] [CrossRef]
- O’Neill, D.W.; Adams, S.; Bhardwaj, N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood 2004, 104, 2235–2246. [Google Scholar] [CrossRef] [Green Version]
- Rissoan, M.C.; Soumelis, V.; Kadowaki, N.; Grouard, G.; Briere, F.; de Waal Malefyt, R.; Liu, Y.J. Reciprocal control of T helper cell and dendritic cell differentiation. Science 1999, 283, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Huang, G.C.; Jones, D.; Lin, Y.H. Distribution patterns of dendritic cells and T cells in diffuse large B–cell lymphomas correlate with prognoses. Clin. Cancer Res. 2007, 13, 6666–6672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Kim, D.H.; Oh, S.Y.; Kim, S.Y.; Koh, M.S.; Lee, J.H.; Lee, S.; Kim, S.H.; Kwak, J.Y.; Pak, M.G.; et al. Clinicopathologic significance of tumor microenvironment CD11c, and FOXP3 expression in diffuse large B–cell lymphoma patients receiving rituximab, cyclophosphamide, anthracycline, vincristine, and prednisone (R–CHOP) combination chemotherapy. Korean J. Intern. Med. 2017, 32, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.; Engleman, E.G. Dendritic cells in cancer immunotherapy. Annu. Rev. Immunol. 2000, 18, 245–273. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, J.M.; Levy, R. Dendritic cell vaccines for cancer immunotherapy. Annu. Rev. Med. 1999, 50, 507–529. [Google Scholar] [CrossRef]
- Hsu, F.J.; Benike, C.; Fagnoni, F.; Liles, T.M.; Czerwinski, D.; Taidi, B.; Engleman, E.G.; Levy, R. Vaccination of patients with B–cell lymphoma using autologous antigen–pulsed dendritic cells. Nat. Med. 1996, 2, 52–58. [Google Scholar] [CrossRef]
- Di Nicola, M.; Zappasodi, R.; Carlo–Stella, C.; Mortarini, R.; Pupa, S.M.; Magni, M.; Devizzi, L.; Matteucci, P.; Baldassari, P.; Ravagnani, F.; et al. Vaccination with autologous tumor–loaded dendritic cells induces clinical and immunologic responses in indolent B–cell lymphoma patients with relapsed and measurable disease: A pilot study. Blood 2009, 113, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Zappasodi, R.; Pupa, S.M.; Ghedini, G.C.; Bongarzone, I.; Magni, M.; Cabras, A.D.; Colombo, M.P.; Carlo-Stella, C.; Gianni, A.M.; Di Nicola, M. Improved clinical outcome in indolent B–cell lymphoma patients vaccinated with autologous tumor cells experiencing immunogenic death. Cancer Res. 2010, 70, 9062–9072. [Google Scholar] [CrossRef] [Green Version]
- Winkler, C.; Steingrube, D.S.; Altermann, W.; Schlaf, G.; Max, D.; Kewitz, S.; Emmer, A.; Kornhuber, M.; Banning-Eichenseer, U.; Staege, M.S. Hodgkin’s lymphoma RNA-transfected dendritic cells induce cancer/testis antigen–specific immune responses. Cancer Immunol. Immunother. 2012, 61, 1769–1779. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pages, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef] [Green Version]
- Pages, F.; Kirilovsky, A.; Mlecnik, B.; Asslaber, M.; Tosolini, M.; Bindea, G.; Lagorce, C.; Wind, P.; Marliot, F.; Bruneval, P.; et al. In situ cytotoxic and memory T cells predict outcome in patients with early–stage colorectal cancer. J. Clin. Oncol. 2009, 27, 5944–5951. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Remark, R.; Goc, J.; Giraldo, N.A.; Becht, E.; Hammond, S.A.; Damotte, D.; Dieu-Nosjean, M.C.; Sautes-Fridman, C. The immune microenvironment: A major player in human cancers. Int. Arch. Allergy Immunol. 2014, 164, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Stenson, M.; Habermann, T.M.; Jelinek, D.F.; Witzig, T.E. Cd4+ T–cell immune response to large B–cell non-Hodgkin’s lymphoma predicts patient outcome. J. Clin. Oncol. 2001, 19, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.M.; Spier, C.M.; Miller, T.P.; Slymen, D.J.; Rybski, J.A.; Grogan, T.M. Tumor–infiltrating T–lymphocytes in B–cell diffuse large cell lymphoma related to disease course. Mod. Pathol. 1990, 3, 361–367. [Google Scholar]
- Lauritzsen, G.F.; Weiss, S.; Dembic, Z.; Bogen, B. Naive idiotype–specific CD4+ T–cells and immunosurveillance of B–cell tumors. Proc. Natl. Acad. Sci. USA 1994, 91, 5700–5704. [Google Scholar] [CrossRef] [Green Version]
- Jacob, M.C.; Favre, M.; Lemarc’Hadour, F.; Sotto, M.F.; Bonnefoix, T.; Sotto, J.J.; Bensa, J.C. CD45RA expression by CD4 T lymphocytes in tumors invaded by B–cell non–Hodgkin’s lymphoma (NHL) or Hodgkin’s disease (HD). Am. J. Hematol. 1992, 39, 45–51. [Google Scholar] [CrossRef]
- Ramiscal, R.R.; Vinuesa, C.G. T–cell subsets in the germinal center. Immunol. Rev. 2013, 252, 146–155. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD–1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, S.; Hansmann, M.L. Large B–cell lymphoma rich in PD–1+ T–cells: An overlooked subtype of diffuse large B–cell lymphoma? Am. J. Clin. Pathol. 2014, 142, 142–143. [Google Scholar] [CrossRef] [Green Version]
- Ahearne, M.J.; Bhuller, K.; Hew, R.; Ibrahim, H.; Naresh, K.; Wagner, S.D. Expression of PD–1 (CD279) and FoxP3 in diffuse large B–cell lymphoma. Virchows. Arch. 2014, 465, 351–358. [Google Scholar] [CrossRef]
- Kiyasu, J.; Miyoshi, H.; Hirata, A.; Arakawa, F.; Ichikawa, A.; Niino, D.; Sugita, Y.; Yufu, Y.; Choi, I.; Abe, Y.; et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B–cell lymphoma. Blood 2015, 126, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Armand, P.; Engert, A.; Younes, A.; Fanale, M.; Santoro, A.; Zinzani, P.L.; Timmerman, J.M.; Collins, G.P.; Ramchandren, R.; Cohen, J.B.; et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow–Up of the Multicohort Single–Arm Phase II CheckMate 205 Trial. J. Clin. Oncol. 2018, 36, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, J.; Tumuluru, S.; Bao, R.; Leukam, M.; Venkataraman, G.; Phillip, J.; Fitzpatrick, C.; McElherne, J.; MacNabb, B.W.; Orlowski, R.; et al. PD–L1 gene alterations identify a subset of diffuse large B–cell lymphoma harboring a T–cell-inflamed phenotype. Blood 2019, 133, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zinzani, P.L.; Fanale, M.A.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 2125–2132. [Google Scholar] [CrossRef]
- Pericart, S.; Tosolini, M.; Gravelle, P.; Rossi, C.; Traverse-Glehen, A.; Amara, N.; Franchet, C.; Martin, E.; Bezombes, C.; Laurent, G.; et al. Profiling Immune Escape in Hodgkin’s and Diffuse large B–Cell Lymphomas Using the Transcriptome and Immunostaining. Cancers 2018, 10, 415. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Lu, W.; Sun, R.; Jin, X.; Cheng, L.; He, X.; Wang, L.; Yuan, T.; Lyu, C.; Zhao, M. Anti-CD19 Chimeric Antigen Receptor T Cells in Combination With Nivolumab Are Safe and Effective Against Relapsed/Refractory B–Cell Non–hodgkin Lymphoma. Front. Oncol. 2019, 9, 767. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ranganathan, R.; Jiang, S.; Fang, C.; Sun, J.; Kim, S.; Newick, K.; Lo, A.; June, C.H.; Zhao, Y.; et al. A Chimeric Switch-Receptor Targeting PD1 Augments the Efficacy of Second–Generation CAR T Cells in Advanced Solid Tumors. Cancer Res. 2016, 76, 1578–1590. [Google Scholar] [CrossRef] [Green Version]
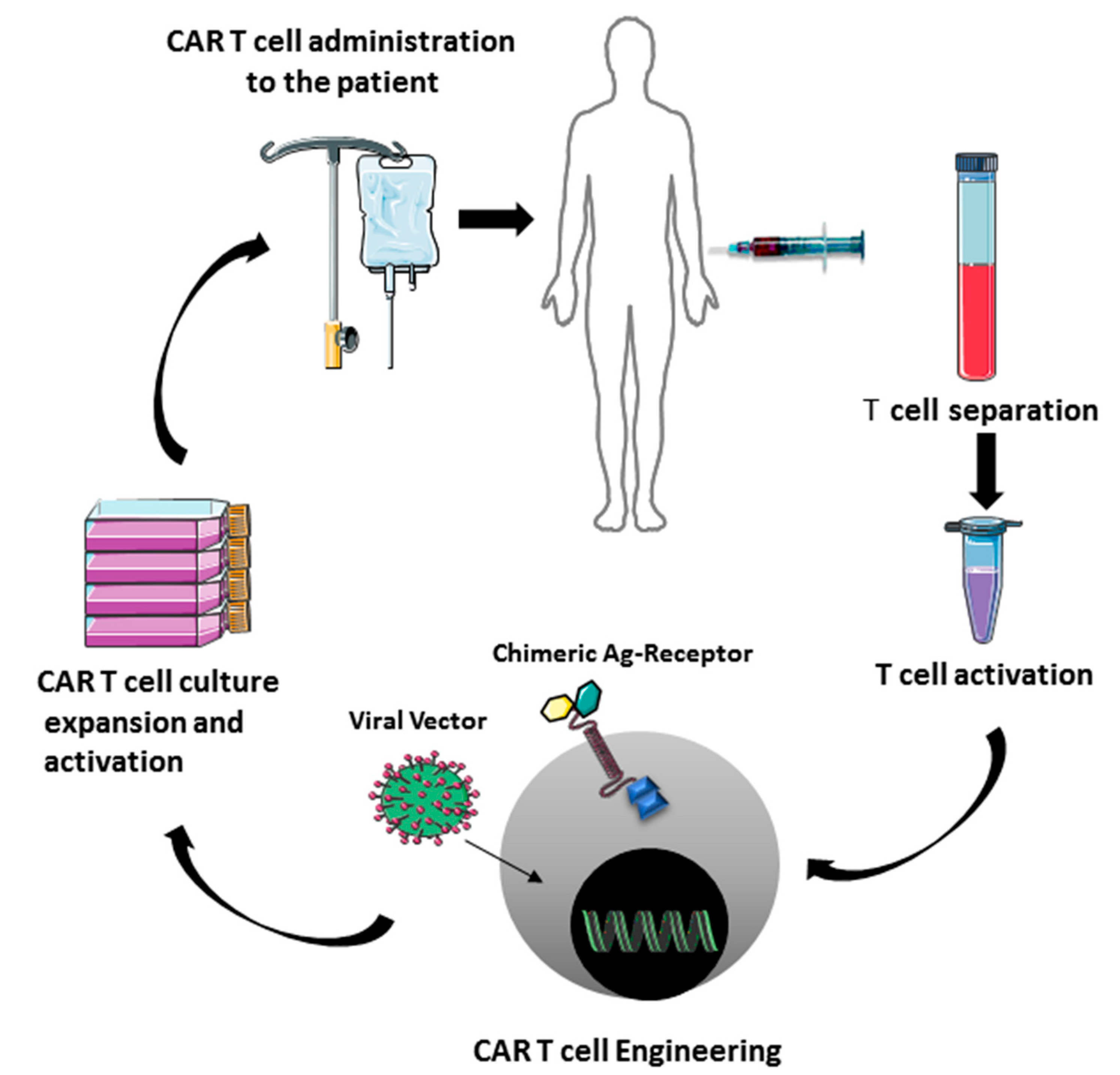
- Fesnak, A.D.; June, C.H.; Levine, B.L. Engineered T–cells: The promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 2016, 16, 566–581. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Chimeric antigen receptor T–cell therapies for lymphoma. Nat. Rev. Clin. Oncol. 2018, 15, 31–46. [Google Scholar] [CrossRef]
- Marin, V.; Pizzitola, I.; Agostoni, V.; Attianese, G.M.; Finney, H.; Lawson, A.; Pule, M.; Rousseau, R.; Biondi, A.; Biagi, E. Cytokine–induced killer cells for cell therapy of acute myeloid leukemia: Improvement of their immune activity by expression of CD33–specific chimeric receptors. Haematologica 2010, 95, 2144–2152. [Google Scholar] [CrossRef]
- Till, B.G.; Jensen, M.C.; Wang, J.; Qian, X.; Gopal, A.K.; Maloney, D.G.; Lindgren, C.G.; Lin, Y.; Pagel, J.M.; Budde, L.E.; et al. CD20–specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4–1BB domains: Pilot clinical trial results. Blood 2012, 119, 3940–3950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef]
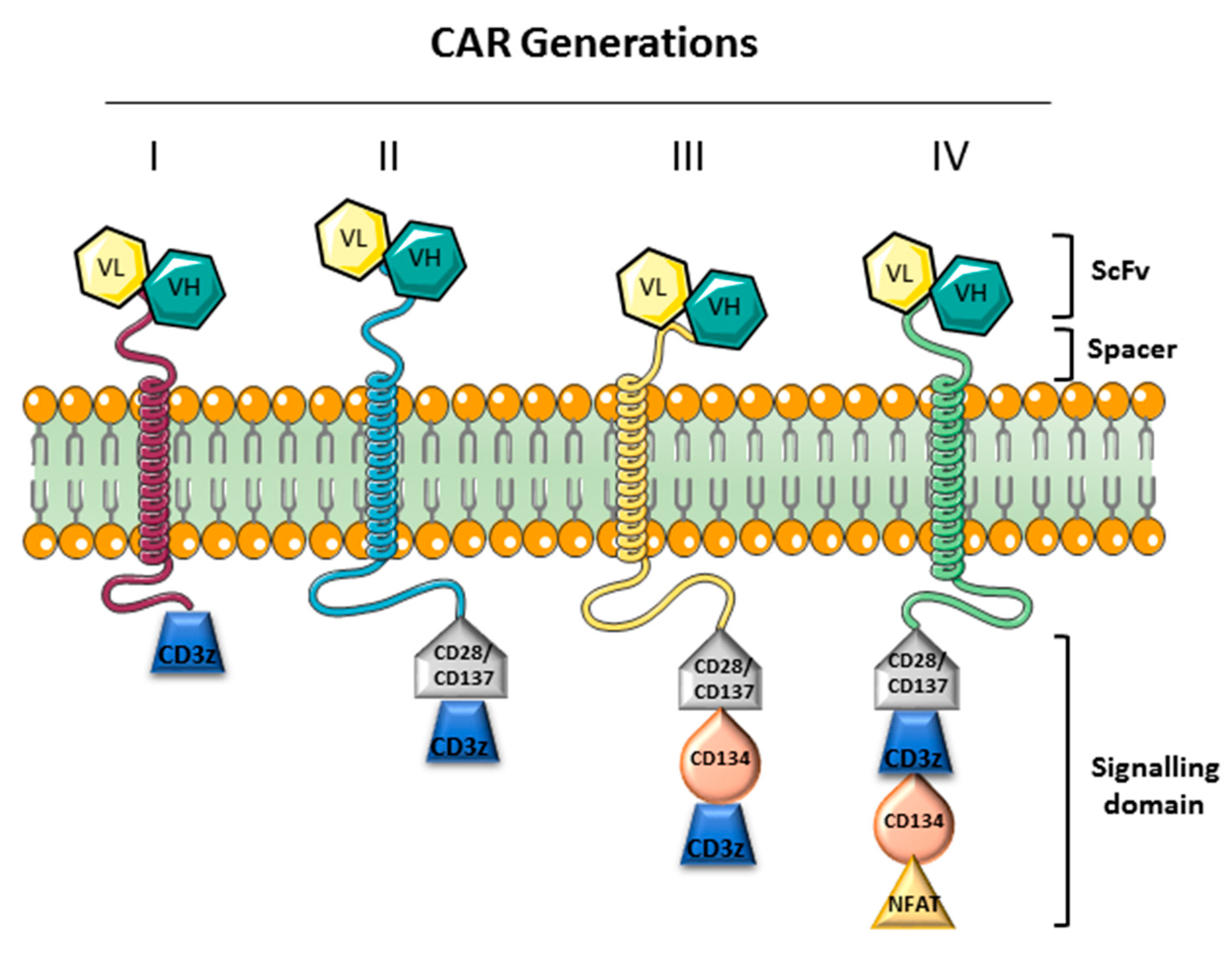
- Chmielewski, M.; Abken, H. TRUCKs: The fourth generation of CARs. Expert Opin. Biol. Ther. 2015, 15, 1145–1154. [Google Scholar] [CrossRef]
- Sha, H.H.; Wang, D.D.; Yan, D.L.; Hu, Y.; Yang, S.J.; Liu, S.W.; Feng, J.F. Chimaeric antigen receptor T–cell therapy for tumour immunotherapy. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [Green Version]
- Sommermeyer, D.; Hudecek, M.; Kosasih, P.L.; Gogishvili, T.; Maloney, D.G.; Turtle, C.J.; Riddell, S.R. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia 2016, 30, 492–500. [Google Scholar] [CrossRef] [Green Version]
- Milone, M.C.; Fish, J.D.; Carpenito, C.; Carroll, R.G.; Binder, G.K.; Teachey, D.; Samanta, M.; Lakhal, M.; Gloss, B.; Danet-Desnoyers, G.; et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 2009, 17, 1453–1464. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric antigen receptor T–cell therapy—assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef]
- Morgan, R.A.; Chinnasamy, N.; Abate-Daga, D.; Gros, A.; Robbins, P.F.; Zheng, Z.; Dudley, M.E.; Feldman, S.A.; Yang, J.C.; Sherry, R.M.; et al. Cancer regression and neurological toxicity following anti–MAGE–A3 TCR gene therapy. J. Immunother. 2013, 36, 133–151. [Google Scholar] [CrossRef] [Green Version]
- Maus, M.V.; Haas, A.R.; Beatty, G.L.; Albelda, S.M.; Levine, B.L.; Liu, X.; Zhao, Y.; Kalos, M.; June, C.H. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol. Res. 2013, 1, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Gardner, R.; Wu, D.; Cherian, S.; Fang, M.; Hanafi, L.A.; Finney, O.; Smithers, H.; Jensen, M.C.; Riddell, S.R.; Maloney, D.G.; et al. Acquisition of a CD19–negative myeloid phenotype allows immune escape of MLL–rearranged B–ALL from CD19 CAR–T–cell therapy. Blood 2016, 127, 2406–2410. [Google Scholar] [CrossRef] [Green Version]
- Orlando, E.J.; Han, X.; Tribouley, C.; Wood, P.A.; Leary, R.J.; Riester, M.; Levine, J.E.; Qayed, M.; Grupp, S.A.; Boyer, M.; et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat. Med. 2018, 24, 1504–1506. [Google Scholar] [CrossRef]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Morvan, M.G.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Chen, Y.; Xiao, W.; Sun, R.; Tian, Z. NK cell–based immunotherapy for malignant diseases. Cell Mol. Immunol. 2013, 10, 230–252. [Google Scholar] [CrossRef] [PubMed]
- Plonquet, A.; Haioun, C.; Jais, J.P.; Debard, A.L.; Salles, G.; Bene, M.C.; Feugier, P.; Rabian, C.; Casasnovas, O.; Labalette, M.; et al. Peripheral blood natural killer cell count is associated with clinical outcome in patients with aaIPI 2–3 diffuse large B–cell lymphoma. Ann. Oncol. 2007, 18, 1209–1215. [Google Scholar] [CrossRef]
- Baier, C.; Fino, A.; Sanchez, C.; Farnault, L.; Rihet, P.; Kahn-Perles, B.; Costello, R.T. Natural killer cells modulation in hematological malignancies. Front. Immunol. 2013, 4, 459. [Google Scholar] [CrossRef] [Green Version]
- Browning, J.L. B cells move to centre stage: Novel opportunities for autoimmune disease treatment. Nat. Rev. Drug Discov. 2006, 5, 564–576. [Google Scholar] [CrossRef]
- Borghaei, H.; Smith, M.R.; Campbell, K.S. Immunotherapy of cancer. Eur. J. Pharmacol. 2009, 625, 41–54. [Google Scholar] [CrossRef]
- Sconocchia, G.; Titus, J.A.; Segal, D.M. Signaling pathways regulating CD44–dependent cytolysis in natural killer cells. Blood 1997, 90, 716–725. [Google Scholar] [CrossRef]
- Essa, E.S.; Tawfeek, G.A.; El Hassanin, S.A.; Emara, K.G.M. Modulation the expression of natural killer cell activating receptor (NKp44) in the peripheral blood of diffuse large B–cell lymphoma patients and the correlation with clinic pathological features. Clin. Immunol. 2018, 188, 38–44. [Google Scholar] [CrossRef]
- Cheng, M.; Ma, J.; Chen, Y.; Zhang, J.; Zhao, W.; Zhang, J.; Wei, H.; Ling, B.; Sun, R.; Tian, Z. Establishment, characterization, and successful adaptive therapy against human tumors of NKG cell, a new human NK cell line. Cell Transpl. 2011, 20, 1731–1746. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhu, B. T–cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef] [Green Version]
- Challa-Malladi, M.; Lieu, Y.K.; Califano, O.; Holmes, A.B.; Bhagat, G.; Murty, V.V.; Dominguez-Sola, D.; Pasqualucci, L.; Dalla-Favera, R. Combined genetic inactivation of beta2–Microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B–cell lymphoma. Cancer Cell 2011, 20, 728–740. [Google Scholar] [CrossRef] [Green Version]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD–1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef]
- Arpon, D.R.; Gandhi, M.K.; Martin, J.H. A new frontier in haematology—combining pharmacokinetic with pharmacodynamic factors to improve choice and dose of drug. Br. J. Clin. Pharmacol. 2014, 78, 274–281. [Google Scholar] [CrossRef]
- Vari, F.; Arpon, D.; Keane, C.; Hertzberg, M.S.; Talaulikar, D.; Jain, S.; Cui, Q.; Han, E.; Tobin, J.; Bird, R.; et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood 2018, 131, 1809–1819. [Google Scholar] [CrossRef] [Green Version]
| Diffuse large B-cell lymphoma, NOS | GCB versus ABC/non-GCB |
| MYC and BCL2 double expressor | |
| CD5+ | |
| DLBCL subtypes | T-cell/histiocyte-rich large B-cell lymphoma |
| Primary DLBCL of the central nervous system | |
| Primary cutaneous DLBCL, leg type | |
| EBV positive DLBCL, NOS | |
| Other lymphomas of large B-cells | Primary mediastinal (thymic) large B-cell lymphoma |
| Intravascular large B-cell lymphoma | |
| DLBCL associated with chronic inflammation | |
| Lymphomatoid granulomatosis | |
| ALK-positive DLBCL | |
| Plasmablastic lymphoma | |
| HHV8+ DLBCL, NOS | |
| Primary effusion lymphoma | |
| Borderline cases | High-grade B-cell lymphoma, with MYC and BCL2 and/or BCL6 translocations |
| High-grade B-cell lymphoma, NOS | |
| B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and classical Hodgkin lymphoma |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamma, R.; Ranieri, G.; Ingravallo, G.; Annese, T.; Oranger, A.; Gaudio, F.; Musto, P.; Specchia, G.; Ribatti, D. Inflammatory Cells in Diffuse Large B Cell Lymphoma. J. Clin. Med. 2020, 9, 2418. https://doi.org/10.3390/jcm9082418
Tamma R, Ranieri G, Ingravallo G, Annese T, Oranger A, Gaudio F, Musto P, Specchia G, Ribatti D. Inflammatory Cells in Diffuse Large B Cell Lymphoma. Journal of Clinical Medicine. 2020; 9(8):2418. https://doi.org/10.3390/jcm9082418
Chicago/Turabian StyleTamma, Roberto, Girolamo Ranieri, Giuseppe Ingravallo, Tiziana Annese, Angela Oranger, Francesco Gaudio, Pellegrino Musto, Giorgina Specchia, and Domenico Ribatti. 2020. "Inflammatory Cells in Diffuse Large B Cell Lymphoma" Journal of Clinical Medicine 9, no. 8: 2418. https://doi.org/10.3390/jcm9082418







