The Use of Bispectral Index Monitoring Does Not Change Intraoperative Exposure to Volatile Anesthetics in Children
Abstract
1. Introduction
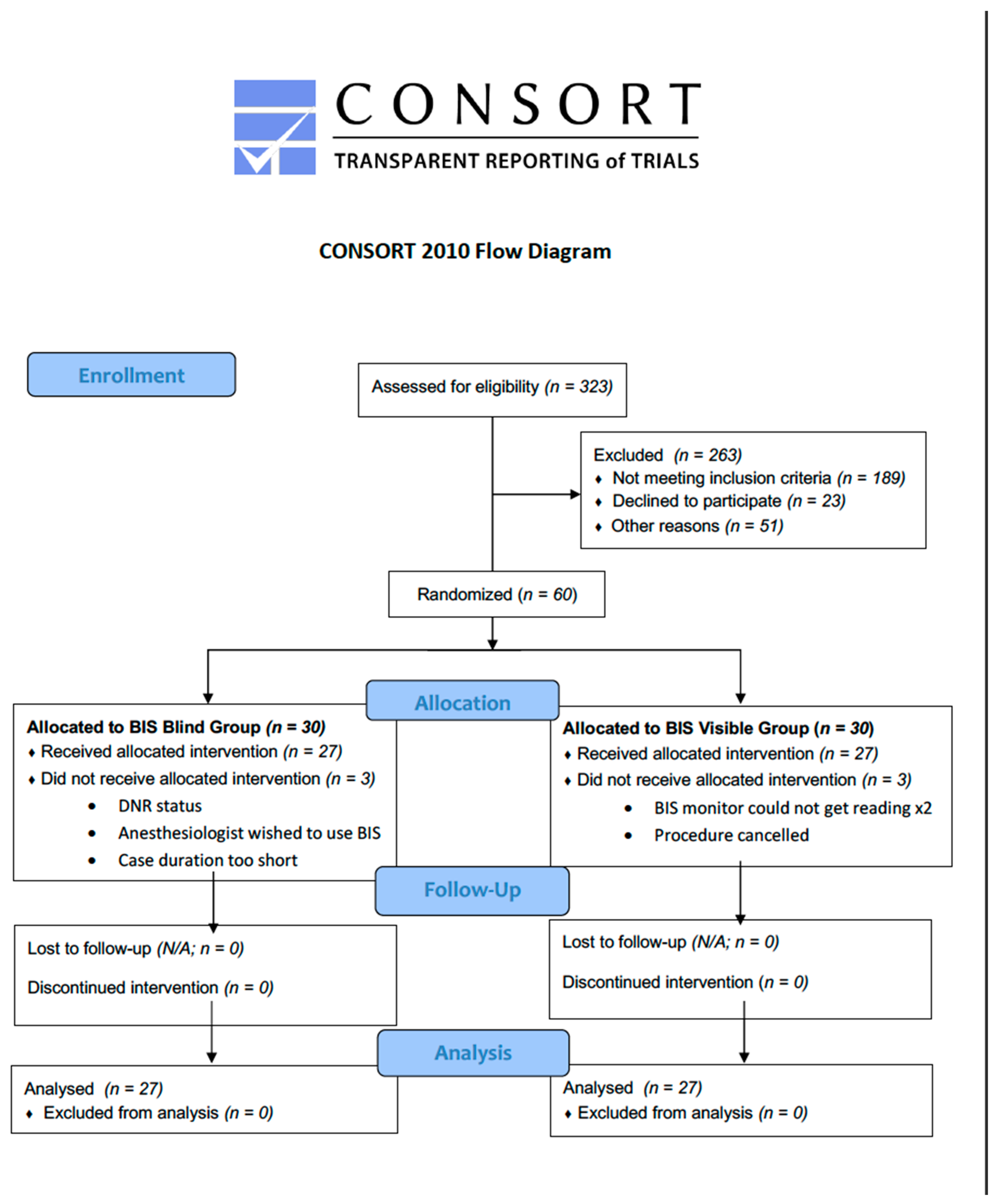
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Intra-Operative Management
3.3. Recovery Profiles
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McCann, M.E.; Soriano, S.G. Does general anesthesia affect neurodevelopment in infants and children? Br. Med. J. 2019, 367, l6459. [Google Scholar] [CrossRef] [PubMed]
- Andropoulos, D.B. Effect of Anesthesia on the Developing Brain: Infant and Fetus. Fetal Diagn. Ther. 2018, 43, 1–11. [Google Scholar] [CrossRef]
- Andropoulos, D.B.; Greene, M.F. Anesthesia and Developing Brains—Implications of the FDA Warning. N. Engl. J. Med. 2017, 376, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, J.W.; Stopp, C.; Wypij, D.; Andropoulos, D.B.; Atallah, J.; Atz, A.M.; Beca, J.; Donofrio, M.T.; Duncan, K.; Ghanayem, N.S.; et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics 2015, 135, 816–825. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA Approves Label Changes for Use of General Anesthetic and Sedation Drugs in Young Children. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-approves-label-changes-use-general-anesthetic-and-sedation-drugs (accessed on 12 June 2020).
- Wodey, E.; Tirel, O.; Bansard, J.Y.; Terrier, A.; Chanavaz, C.; Harris, R.; Ecoffey, C.; Senhadji, L. Impact of age on both BIS values and EEG bispectrum during anaesthesia with sevoflurane in children. Br. J. Anaesth. 2005, 94, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Oh, A.Y.; Kim, C.S.; Kim, S.D.; Seo, K.S.; Kim, J.H. Correlation of bispectral index with end-tidal sevoflurane concentration and age in infants and children. Br. J. Anaesth. 2005, 95, 362–366. [Google Scholar] [CrossRef]
- Rodriguez, R.A.; Hall, L.E.; Duggan, S.; Splinter, W.M. The bispectral index does not correlate with clinical signs of inhalational anesthesia during sevoflurane induction and arousal in children. Can. J. Anaesth. 2004, 51, 472–480. [Google Scholar] [CrossRef]
- Schwartz, D.; Wu, A.; Han, D.; Gibson, C.; Connelly, N. BIS in children during maintenance anesthesia. Rom. Anest. Ter. Intensiv 2011, 18, 95–100. [Google Scholar]
- Short, T.G.; Campbell, D.; Frampton, C.; Chan, M.T.V.; Myles, P.S.; Corcoran, T.B.; Sessler, D.I.; Mills, G.H.; Cata, J.P.; Painter, T.; et al. Anaesthetic depth and complications after major surgery: An international, randomised controlled trial. Lancet 2019, 394, 1907–1914. [Google Scholar] [CrossRef]
- Bannister, C.F.; Brosius, K.K.; Sigl, J.C.; Meyer, B.J.; Sebel, P.S. The effect of bispectral index monitoring on anesthetic use and recovery in children anesthetized with sevoflurane in nitrous oxide. Anesth. Analg. 2001, 92, 877–881. [Google Scholar] [CrossRef]
- Denman, W.T.; Swanson, E.L.; Rosow, D.; Ezbicki, K.; Connors, P.D.; Rosow, C.E. Pediatric evaluation of the bispectral index (BIS) monitor and correlation of BIS with end-tidal sevoflurane concentration in infants and children. Anesth. Analg. 2000, 90, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Messieha, Z.S.; Ananda, R.C.; Hoffman, W.E.; Punwani, I.C.; Koenig, H.M. Bispectral Index System (BIS) monitoring reduces time to discharge in children requiring intramuscular sedation and general anesthesia for outpatient dental rehabilitation. Pediatr. Dent. 2004, 26, 256–260. [Google Scholar] [PubMed]
- Liu, W.H.; Thorp, T.A.; Graham, S.G.; Aitkenhead, A.R. Incidence of awareness with recall during general anaesthesia. Anaesthesia 1991, 46, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Blussé van Oud-Alblas, H.J.; van Dijk, M.; Liu, C.; Tibboel, D.; Klein, J.; Weber, F. Intraoperative awareness during paediatric anaesthesia. Br. J. Anaesth. 2009, 102, 104–110. [Google Scholar] [CrossRef]
- Malviya, S.; Galinkin, J.L.; Bannister, C.F.; Burke, C.; Zuk, J.; Popenhagen, M.; Brown, S.; Voepel-Lewis, T. The incidence of intraoperative awareness in children: Childhood awareness and recall evaluation. Anesth. Analg. 2009, 109, 1421–1427. [Google Scholar] [CrossRef]
- Lin, E.P.; Lee, J.R.; Lee, C.S.; Deng, M.; Loepke, A.W. Do anesthetics harm the developing human brain? An integrative analysis of animal and human studies. Neurotoxicol. Teratol. 2017, 60, 117–128. [Google Scholar] [CrossRef]
- Davidson, A.J.; Disma, N.; de Graaff, J.C.; Withington, D.E.; Dorris, L.; Bell, G.; Stargatt, R.; Bellinger, D.C.; Schuster, T.; Arnup, S.J.; et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): An international multicentre, randomised controlled trial. Lancet 2016, 387, 239–250. [Google Scholar] [CrossRef]
- Disma, N.; O’Leary, J.D.; Loepke, A.W.; Brambrink, A.M.; Becke, K.; Clausen, N.G.; De Graaff, J.C.; Liu, F.; Hansen, T.G.; McCann, M.E.; et al. Anesthesia and the developing brain: A way forward for laboratory and clinical research. Paediatr. Anaesth. 2018, 28, 758–763. [Google Scholar] [CrossRef]
- McCann, M.E.; de Graaff, J.C.; Dorris, L.; Disma, N.; Withington, D.; Bell, G.; Grobler, A.; Stargatt, R.; Hunt, R.W.; Sheppard, S.J.; et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): An international, multicentre, randomised, controlled equivalence trial. Lancet 2019, 393, 664–677. [Google Scholar] [CrossRef]
- Hesse, S.; Kreuzer, M.; Hight, D.; Gaskell, A.; Devari, P.; Singh, D.; Taylor, N.B.; Whalin, M.K.; Lee, S.; Sleigh, J.W.; et al. Association of electroencephalogram trajectories during emergence from anaesthesia with delirium in the postanaesthesia care unit: An early sign of postoperative complications. Br. J. Anaesth. 2019, 122, 622–634. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Wang, K. Bispectral Index Monitoring During Anesthesia Promotes Early Postoperative Recovery of Cognitive Function and Reduces Acute Delirium in Elderly Patients with Colon Carcinoma: A Prospective Controlled Study using the Attention Network Test. Med. Sci. Monit. 2018, 24, 7785–7793. [Google Scholar] [CrossRef] [PubMed]
- Radtke, F.M.; Franck, M.; Lendner, J.; Krüger, S.; Wernecke, K.D.; Spies, C.D. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br. J. Anaesth. 2013, 110 (Suppl. S1), i98–i105. [Google Scholar] [CrossRef] [PubMed]
- Avidan, M.S.; Jacobsohn, E.; Glick, D.; Burnside, B.A.; Zhang, L.; Villafranca, A.; Karl, L.; Kamal, S.; Torres, B.; O’Connor, M.; et al. Prevention of intraoperative awareness in a high-risk surgical population. N. Engl. J. Med. 2011, 365, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Pavlin, J.D.; Souter, K.J.; Hong, J.Y.; Freund, P.R.; Bowdle, T.A.; Bower, J.O. Effects of bispectral index monitoring on recovery from surgical anesthesia in 1580 inpatients from an academic medical center. Anesthesiology 2005, 102, 566–573. [Google Scholar] [CrossRef]
- Mashour, G.A.; Shanks, A.; Tremper, K.K.; Kheterpal, S.; Turner, C.R.; Ramachandran, S.K.; Picton, P.; Schueller, C.; Morris, M.; Vandervest, J.C.; et al. Prevention of intraoperative awareness with explicit recall in an unselected surgical population: A randomized comparative effectiveness trial. Anesthesiology 2012, 117, 717–725. [Google Scholar] [CrossRef]
- Fritz, B.A.; Rao, P.; Mashour, G.A.; Abdallah, A.B.; Burnside, B.A.; Jacobsohn, E.; Zhang, L.; Avidan, M.S. Postoperative recovery with bispectral index versus anesthetic concentration-guided protocols. Anesthesiology 2013, 118, 1113–1122. [Google Scholar] [CrossRef]
- Leslie, K.; Myles, P.S.; Forbes, A.; Chan, M.T. The effect of bispectral index monitoring on long-term survival in the B-aware trial. Anesth. Analg. 2010, 110, 816–822. [Google Scholar] [CrossRef]
| Prone Position (Contraindicates BIS Application) | 36 |
| Planned procedure length too short | 25 |
| Research staff unavailable | 19 |
| Extended recovery time required | 18 |
| History of seizures | 15 |
| Developmental delay- unable to obtain assent | 10 |
| Adhesive allergy (concern with BIS adhesive) | 1 |
| Other (scheduling issues, anesthesia staff or family decline participation, behavioral concerns regarding patient) | 65 |
| Total number that were screened and excluded | 189 |
| Statistic | BIS Visible (N = 27) | BIS Blind (N = 27) | Overall (N = 54) | |
|---|---|---|---|---|
| Gender | ||||
| Female | n (%) | 20 (74.1) | 12 (44.4) | 32 (59.3) |
| Male | 7 (25.9) | 15 (55.6) | 22 (40.7) | |
| Age (years) | n | 27 | 27 | 54 |
| Mean (SD) | 9.0 (3.42) | 9.9 (3.01) | 9.5 (3.23) | |
| Median | 11.0 | 11.0 | 11.0 | |
| Min, Max | 2.0, 12.0 | 2.0, 13.0 | 2.0, 13.0 | |
| Weight (kg) | n | 27 | 27 | 54 |
| Mean (SD) | 36.9 (18.54) | 45.9 (18.33) | 41.4 (18.82) | |
| Median | 34.4 | 47.4 | 40.1 | |
| Min, Max | 14.2, 93.1 | 13.4, 82.1 | 13.4, 93.1 | |
| Height (cm) | n | 27 | 27 | 54 |
| Mean (SD) | 134.5 (24.56) | 144.0 (19.97) | 139.3 (22.68) | |
| Median | 139.0 | 148.6 | 143.0 | |
| Min, Max | 91.5, 169.0 | 90.3, 170.0 | 90.3, 170.0 |
| BIS Blind | BIS Visible |
|---|---|
|
|
| Statistic | BIS Visible (N = 27) | BIS Blind (N = 27) | Overall (N = 54) | |
|---|---|---|---|---|
| BIS Score | n | 27 | 25 | 52 |
| Mean (SD) | 43.7 (7.59) | 42.2 (7.84) | 43.0 (7.67) | |
| Median | 43.8 | 41.5 | 43.1 | |
| Min, Max | 26.9, 62.7 | 26.4, 55.8 | 26.4, 62.7 | |
| p-value * | 0.4935 | |||
| End-tidal Sevoflurane Concentration (%) | n | 27 | 27 | 54 |
| Mean (SD) | 1.8 (0.67) | 1.8 (0.64) | 1.8 (0.65) | |
| Median | 2.0 | 1.9 | 1.9 | |
| Min, Max | 0.3, 2.9 | 0.1, 3.0 | 0.1, 3.0 | |
| p-value * | 0.8365 | |||
| Duration of time in recovery (mins) [1] | n | 26 | 27 | 53 |
| Mean (SD) | 54.1 (43.36) | 43.3 (33.97) | 48.6 (38.87) | |
| Median | 46.0 | 39.0 | 40.0 | |
| Min, Max | 7.0, 222.0 | −37.0 ^, 154.0 | −37.0 ^, 222.0 | |
| p-value * | 0.3174 | |||
| Duration of anesthesia (mins) [2] | n | 27 | 27 | 54 |
| Mean (SD) | 86.9 (36.52) | 96.3 (44.08) | 91.6 (40.38) | |
| Median | 81.0 | 81.0 | 81.0 | |
| Min, Max | 35.0, 192.0 | 45.0, 208.0 | 35.0, 208.0 | |
| p-value * | 0.3952 | |||
| Time to Aldrete score of 9 (mins) | n | 3 | 1 | 4 |
| Mean (SD) | 119.7 (37.23) | 56.0 (NC) | 103.8 (44.02) | |
| Median | 105.0 | 56.0 | 98.5 | |
| Min, Max | 92.0, 162.0 | 56.0, 56.0 | 56.0, 162.0 | |
| p-value * | 0.2768 | |||
| PAED Score | n | 11 | 10 | 21 |
| Mean (SD) | 5.3 (5.02) | 3.3 (6.13) | 4.3 (5.53) | |
| Median | 5.0 | 1.0 | 3.0 | |
| Min, Max | 0.0, 12.0 | 0.0, 20.0 | 0, 20.0 | |
| p-value * | 0.4280 | |||
| FLACC Pain Assessment Score | n | 26 | 8 | 34 |
| Mean (SD) | 0.8 (1.54) | 1.4 (2.88) | 1.0 (1.90) | |
| Median | 0.0 | 0.0 | 0.0 | |
| Min, Max | 0, 5 | 0, 8 | 0, 8 | |
| p-value * | 0.6310 | |||
| NRS Generalized Pain Score | n | 15 | 20 | 35 |
| Mean (SD) | 2.6 (2.90) | 3.6 (2.30) | 3.2 (2.62) | |
| Median | 2.0 | 4.0 | 3.0 | |
| Min, Max | 0, 8 | 0, 8 | 0, 8 | |
| p-value * | 0.0867 | |||
| Wong Generalized Pain Score | n | 11 | 9 | 20 |
| Mean (SD) | 3.3 (2.57) | 3.8 (2.73) | 3.5 (2.59) | |
| Median | 2.0 | 4.0 | 4.0 | |
| Min, Max | 0, 8 | 0, 8 | 0, 8 | |
| p-value * | 0.6758 | |||
| Activity—FLACC | n (%) | |||
| Lying quietly, normal position, moves easily (0) | 24 (77.4) | 7 (22.6) | 31 (91.2) | |
| Squirming, shifting back and forth, tense (1) | 1 (33.3) | 2 (66.7) | 3 (8.8) | |
| Consolability—FLACC | n (%) | |||
| Content, relaxed (0) | 21 (77.8) | 6 (22.2) | 27 (79.4) | |
| Difficult to console or comfort (2) | 0 | 1 (100.0) | 1 (2.9) | |
| Reassured by occasional touching, hugging, distractible (1) | 5 (83.3) | 1 (16.7) | 6 (17.7) | |
| Cry—FLACC | n (%) | |||
| Crying steadily, screams or sobs, frequent complaints (2) | 0 | 1 (100.0) | 1 (2.9) | |
| Moans or whimpers; occasional complaint (1) | 5 (16.7) | 1 (83.3) | 6 (17.7) | |
| No cry (awake or asleep) (0) | 21 (77.8) | 6 (22.2) | 27 (79.4) | |
| Facial—FLACC | n (%) | |||
| Frequent to constant quivering chin, clenched jaw (2) | 0 | 1 (100.0) | 1 (2.9) | |
| No particular expression or smile (0) | 19 (76.0) | 6 (24.0) | 25 (73.5) | |
| Occasional grimace or frown, withdrawn, disinterested (1) | 7 (87.5) | 1 (12.5) | 8 (23.5) | |
| Legs—FLACC | n (%) | |||
| Normal position or relaxed (0) | 23 (76.7) | 7 (23.3) | 30 (88.2) | |
| Uneasy, restless, tense (1) | 3 (75.0) | 1 (25.5) | 4 (11.8) |
| Parameter Statistic | BIS Visible (N = 27) | BIS Blind (N = 27) | ||
|---|---|---|---|---|
| Age (2–6) | Age (7–13) | Age (2–6) | Age (7–13) | |
| BIS Score | ||||
| n | 6 | 21 | 4 | 21 |
| Mean (SD) | 42.9 (6.44) | 43.9 (8.02) | 39.5 (10.67) | 42.7 (7.41) |
| Median | 41.1 | 44.5 | 39.7 | 41.5 |
| Min, Max | 34.9, 53.1 | 26.95, 62.72 | 26.4, 52.2 | 31.3, 55.8 |
| End-tidal Sevoflurane Concentration | ||||
| n | 6 | 21 | 4 | 23 |
| Mean (SD) | 2.1 (0.65) | 1.7 (0.67) | 2.4 (0.47) | 1.7 (0.61) |
| Median | 2.2 | 2.0 | 2.3 | 1.7 |
| Min, Max | 1.3, 2.9 | 0.3, 2.7 | 1.9, 3.0 | 0.1, 2.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sullivan, C.A.; Egbuta, C.; Park, R.S.; Lukovits, K.; Cavanaugh, D.; Mason, K.P. The Use of Bispectral Index Monitoring Does Not Change Intraoperative Exposure to Volatile Anesthetics in Children. J. Clin. Med. 2020, 9, 2437. https://doi.org/10.3390/jcm9082437
Sullivan CA, Egbuta C, Park RS, Lukovits K, Cavanaugh D, Mason KP. The Use of Bispectral Index Monitoring Does Not Change Intraoperative Exposure to Volatile Anesthetics in Children. Journal of Clinical Medicine. 2020; 9(8):2437. https://doi.org/10.3390/jcm9082437
Chicago/Turabian StyleSullivan, Cornelius A., Chinyere Egbuta, Raymond S. Park, Karina Lukovits, David Cavanaugh, and Keira P. Mason. 2020. "The Use of Bispectral Index Monitoring Does Not Change Intraoperative Exposure to Volatile Anesthetics in Children" Journal of Clinical Medicine 9, no. 8: 2437. https://doi.org/10.3390/jcm9082437
APA StyleSullivan, C. A., Egbuta, C., Park, R. S., Lukovits, K., Cavanaugh, D., & Mason, K. P. (2020). The Use of Bispectral Index Monitoring Does Not Change Intraoperative Exposure to Volatile Anesthetics in Children. Journal of Clinical Medicine, 9(8), 2437. https://doi.org/10.3390/jcm9082437





