Predicting the Role of DNA Polymerase β Alone or with KRAS Mutations in Advanced NSCLC Patients Receiving Platinum-Based Chemotherapy
Abstract
:1. Introduction
2. Material and Methods
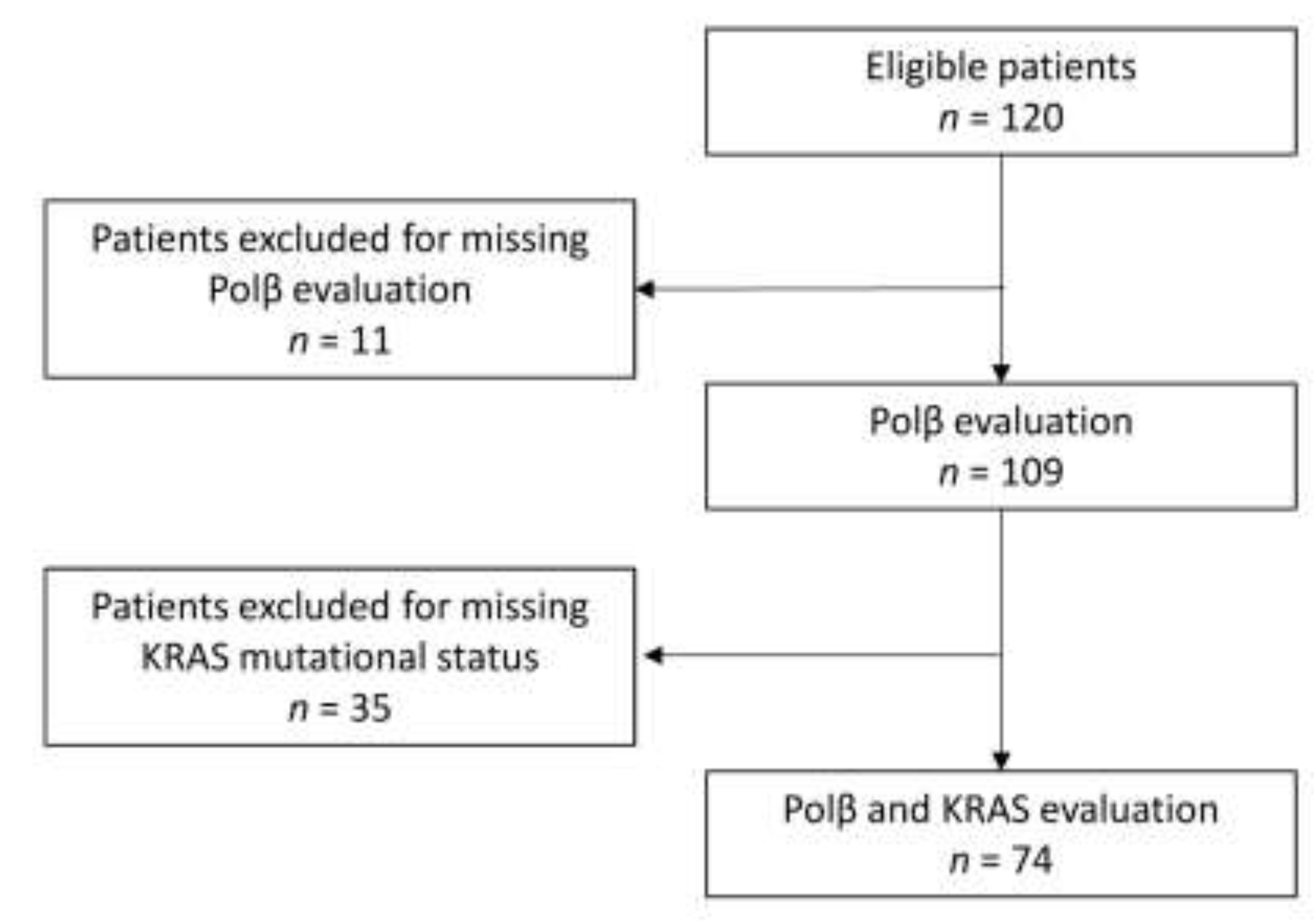
2.1. Study Population and Samples
2.2. Mutational Analysis
2.3. Immunohistochemical Analysis (IHC)
2.4. Outcomes
2.5. Statistical Methods
3. Results
3.1. Progression-Free Survival
3.2. Overall Survival
3.3. Overall Response Rate
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2018, 54, 407–419. [Google Scholar] [PubMed]
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Dómine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.V. Developing combination strategies using PD-1 checkpoint inhibitors to treat cancer. Semin. Immunopathol. 2019, 41, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piva, S.; Ganzinelli, M.; Garassino, M.C.; Caiola, E.; Farina, G.; Broggini, M.; Marabese, M. Across the universe of K-RAS mutations in non-small-cell-lung cancer. Curr. Pharm. Des. 2014, 20, 3933–3943. [Google Scholar] [CrossRef] [PubMed]
- Marabese, M.; Ganzinelli, M.; Garassino, M.C.; Shepherd, F.A.; Piva, S.; Caiola, E.; Macerelli, M.; Bettini, A.; Lauricella, C.; Floriani, I.; et al. KRAS mutations affect prognosis of non-small-cell lung cancer patients treated with first-line platinum containing chemotherapy. Oncotarget 2015, 6, 34014–34022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deans, A.J.; West, S.C. DNA interstrand crosslink repair and cancer. Nat. Rev. Cancer 2011, 11, 467–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, B.; Saadat, N.; Myung, B.; Shekhar, M.P. Crosstalk between translesion synthesis, Fanconi anemia network, and homologous recombination repair pathways in interstrand DNA crosslink repair and development of chemoresistance. Mutat. Res.-Rev. Mutat. Res. 2015, 763, 258–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.; Lippard, S.J. Direct cellular responses to platinum-induced DNA damage. Chem. Rev. 2007, 107, 1387–1407. [Google Scholar] [CrossRef] [PubMed]
- Caiola, E.; Salles, D.; Frapolli, R.; Lupi, M.; Rotella, G.; Ronchi, A.; Garassino, M.C.; Mattschas, N.; Colavecchio, S.; Broggini, M.; et al. Base excision repair-mediated resistance to cisplatin in KRAS(G12C) mutant NSCLC cells. Oncotarget 2015, 6, 30072–30087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garassino, M.C.; Martelli, O.; Broggini, M.; Farina, G.; Veronese, S.M.; Rulli, E.; Bianchi, F.; Bettini, A.; Longo, F.; Moscetti, L.; et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): A randomised controlled trial. Lancet Oncol. 2013, 14, 981–988. [Google Scholar] [CrossRef]
- Marabese, M.; Marchini, S.; A Sabatino, M.; Polato, F.; Vikhanskaya, F.; Marrazzo, E.; Riccardi, E.; Scanziani, E.; Broggini, M. Effects of inducible overexpression of DNp73α on cancer cell growth and response to treatment in vitro and in vivo. Cell Death Differ. 2005, 12, 805–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepherd, F.A.; Domerg, C.; Hainaut, P.; Jänne, P.A.; Pignon, J.-P.; Graziano, S.; Douillard, J.-Y.; Brambilla, E.; Le Chevalier, T.; Seymour, L.; et al. Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early-stage resected non–small-cell lung cancer in four trials of adjuvant chemotherapy. J. Clin. Oncol. 2013, 31, 2173–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, Z.; Aird, K.M.; Bitler, B.G.; Nicodemus, J.P.; Beeharry, N.; Xia, B.; Yen, T.J.; Zhang, R. Oncogenic RAS regulates BRIP1 expression to induce dissociation of BRCA1 from Chromatin, Inhibit DNA repair, and promote senescence. Dev. Cell 2011, 21, 1077–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsunoda, T.; Takashima, Y.; Fujimoto, T.; Koyanagi, M.; Yoshida, Y.; Doi, K.; Tanaka, Y.; Kuroki, M.; Sasazuki, T.; Shirasawa, S. Three-dimensionally specific inhibition of DNA repair-related genes by activated KRAS in colon crypt model. Neoplasia 2010, 12, 397–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahnel, P.S.; Enders, B.; Sasca, D.; Roos, W.P.; Kaina, B.; Bullinger, L.; Theobald, M.; Kindler, T. Targeting components of the alternative NHEJ pathway sensitizes KRAS mutant leukemic cells to chemotherapy. Blood 2014, 123, 2355–2366. [Google Scholar] [CrossRef] [PubMed]
- Bergoglio, V.; Canitrot, Y.; Hogarth, L.; Minto, L.; Howell, S.B.; Cazaux, C.; Hoffmann, J.-S. Enhanced expression and activity of DNA polymerase beta in human ovarian tumor cells: Impact on sensitivity towards antitumor agents. Oncogene 2001, 20, 6181–6187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwatsuki, M.; Mimori, K.; Yokobori, T.; Tanaka, F.; Tahara, K.; Inoue, H.; Baba, H.; Mori, M. A platinum agent resistance gene, POLB, is a prognostic indicator in colorectal cancer. J. Surg. Oncol. 2009, 100, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Weller, M.C.; Repmann, S.; Moch, H.; Jiricny, J. Synthetic lethality between BRCA1 deficiency and poly(ADP-ribose) polymerase inhibition is modulated by processing of endogenous oxidative DNA damage. Nucleic Acids Res. 2019, 47, 9132–9143. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| n (%) | p | p | |||
|---|---|---|---|---|---|
| Polβ Continuous | Polβ pos vs. neg | ||||
| Age of diagnosis | Median(Q1–Q3) | 66.8 (60.0–71.4) | 0.448 * | 0.788 † | |
| Missing | 3 | ||||
| Gender | Male | 70 | 65.4 | 0.717 † | 0.366 ** |
| Female | 37 | 34.6 | |||
| Missing | 2 | ||||
| ECOG-PS | 0 | 78 | 81.3 | 0.157 † | 0.443 ** |
| 1 | 17 | 17.7 | |||
| 2 | 1 | 1 | |||
| Missing | 13 | ||||
| Smoking | Never | 21 | 20 | 0.618 † | 1.000 ** |
| Former smokers | 42 | 40 | |||
| Smokers | 42 | 40 | |||
| Missing | 4 | ||||
| Stage at diagnosis | IIIB | 28 | 26.2 | 0.038 † | 0.507 ** |
| IV | 79 | 73.8 | |||
| Missing | 2 | ||||
| Histotype | Adenocarcinoma | 90 | 82.6 | 0.291 † | 0.184 ** |
| Squamous | 17 | 15.6 | |||
| Other | 2 | 1.8 | |||
| Platinum-based therapy | Cisplatin | 33 | 34.7 | 0.726 † | 0.486 ** |
| Carboplatin | 62 | 65.3 | |||
| Missing | 14 | ||||
| Immunotherapy | No | 62 | 58.5 | 0.248 † | 0.352 ** |
| Yes | 44 | 41.5 | |||
| Missing | 3 | ||||
| Polβ | Median(Q1-Q3) | 160.0 (60.0–200.0) | - | - | |
| negative | 13 | 11.9 | - | - | |
| positive | 96 | 88.1 | |||
| KRAS | Mutated | 35 | 47.3 | 0.053 † | 0.125 ** |
| Wild-Type | 39 | 52.7 | |||
| Missing | 35 | ||||
| Polβ Continuous | Polβ pos vs. neg | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Polβ (10-unit increment) | 0.99 (0.97–1.02) | 0.579 | - | - | |
| Polβ | |||||
| Positive | - | - | reference | ||
| Negative | - | - | 1.07 (0.49–2.38) | 0.857 | |
| Age at metastasis diagnosis (5 years increment) | 0.82 (0.72–0.94) | 0.005 | 0.82 (0.72–0.95) | 0.006 | |
| Histology | |||||
| Adenocarcinoma | reference | reference | |||
| Squamous | 1.10 (0.60–2.03) | 0.755 | 1.12 (0.60–2.10) | 0.728 | |
| Nos or other | 2.26 (0.27–18.6) | 0.449 | 2.34 (0.28–19.5) | 0.432 | |
| Smoke | |||||
| Never | reference | reference | |||
| Previous | 1.25 (0.69–2.26) | 0.463 | 1.27 (0.70–2.29) | 0.434 | |
| Current | 0.79 (0.40–1.56) | 0.495 | 0.81 (0.41–1.59) | 0.543 | |
| ECOG-PS | 1.51 (0.78–2.94) | 0.223 | 1.51 (0.76–3.00) | 0.244 | |
| Therapy | |||||
| Cisplatin | reference | reference | |||
| Carboplatin | 1.73 (1.02–2.92) | 0.041 | 1.69 (1.01–2.85) | 0.046 | |
| Polβ Continuous | Polβ pos vs. neg | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Polβ (10-unit increment) | 0.99 (0.96–1.01) | 0.390 | - | - |
| Polβ | ||||
| Positive | - | - | reference | |
| Negative | - | - | 1.43 (0.57–3.57) | 0.439 |
| Age at metastasis diagnosis (5 years increments) | 0.87 (0.75–1.01) | 0.066 | 0.87 (0.75–1.01) | 0.065 |
| Histology | ||||
| Adenocarcinoma | reference | reference | ||
| Squamous | 0.94 (0.46–1.95) | 0.877 | 0.98 (0.47–2.06) | 0.960 |
| Nos or other | 7.67 (0.83–70.6) | 0.072 | 8.86 (0.94–83.3) | 0.056 |
| Smoke | ||||
| Never | reference | reference | ||
| Previous | 2.65 (1.15–6.12) | 0.022 | 2.76 (1.21–6.30) | 0.016 |
| Current | 1.58 (0.61–4.11) | 0.350 | 1.63 (0.63–4.26) | 0.316 |
| ECOG-PS | 1.18 (0.48–2.90) | 0.724 | 1.12 (0.44–2.87) | 0.812 |
| Therapy | ||||
| Cisplatin | reference | reference | ||
| Carboplatin | 1.74 (0.94–3.21) | 0.075 | 1.70 (0.93–3.12) | 0.084 |
| Immunotherapy | ||||
| No | reference | reference | ||
| Yes | 0.57 (0.32–1.03) | 0.063 | 0.55 (0.31–0.98) | 0.041 |
| Polβ neg n = 12 | Polβ pos n = 64 | Chi-Squared Test | Logistic Regression Model | |
|---|---|---|---|---|
| CR + PR −n (%) | 4 (33.3) | 23 (35.9) | Chi = 0.03 | OR = 1.002 |
| 95% CI | 34.9–90.1 | 51.1–75.7 | Df = 1 | 95%CI = 0.997–1.006 |
| SD + PD −n (%) | 8 (66.7) | 41 (64.1) | p = 0.864 | p = 0.505 |
| 95% CI | 9.9–65.1 | 24.3–48.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvisi, M.F.; Ganzinelli, M.; Linardou, H.; Caiola, E.; Lo Russo, G.; Cecere, F.L.; Bettini, A.C.; Psyrri, A.; Milella, M.; Rulli, E.; et al. Predicting the Role of DNA Polymerase β Alone or with KRAS Mutations in Advanced NSCLC Patients Receiving Platinum-Based Chemotherapy. J. Clin. Med. 2020, 9, 2438. https://doi.org/10.3390/jcm9082438
Alvisi MF, Ganzinelli M, Linardou H, Caiola E, Lo Russo G, Cecere FL, Bettini AC, Psyrri A, Milella M, Rulli E, et al. Predicting the Role of DNA Polymerase β Alone or with KRAS Mutations in Advanced NSCLC Patients Receiving Platinum-Based Chemotherapy. Journal of Clinical Medicine. 2020; 9(8):2438. https://doi.org/10.3390/jcm9082438
Chicago/Turabian StyleAlvisi, Maria Francesca, Monica Ganzinelli, Helena Linardou, Elisa Caiola, Giuseppe Lo Russo, Fabiana Letizia Cecere, Anna Cecilia Bettini, Amanda Psyrri, Michele Milella, Eliana Rulli, and et al. 2020. "Predicting the Role of DNA Polymerase β Alone or with KRAS Mutations in Advanced NSCLC Patients Receiving Platinum-Based Chemotherapy" Journal of Clinical Medicine 9, no. 8: 2438. https://doi.org/10.3390/jcm9082438







