Long-Term Outcomes and Risk Factors of Renal Failure Requiring Dialysis after Heart Transplantation: A Nationwide Cohort Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Sources of Data
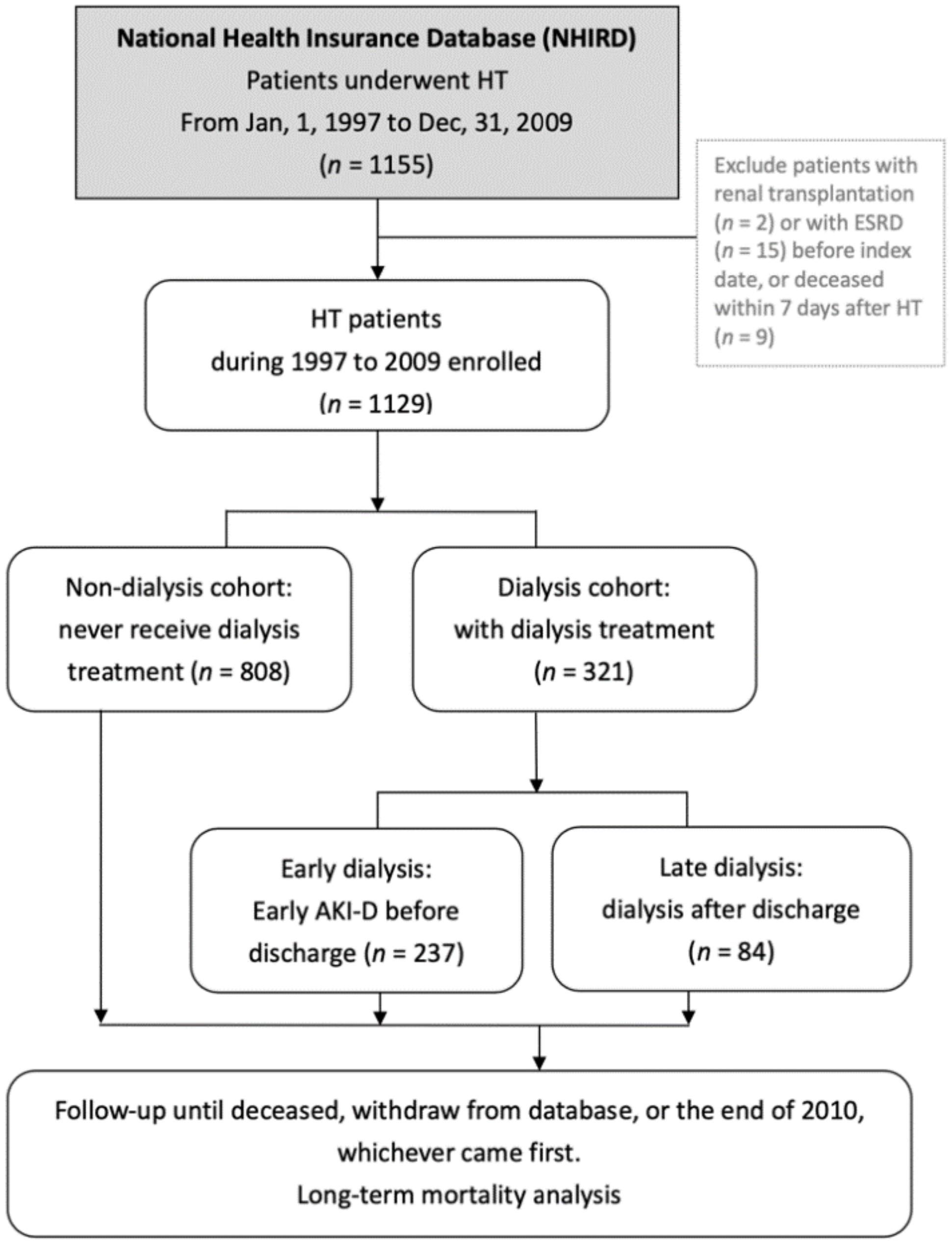
2.2. Study Subjects and Study Design
2.3. Subgroup Definitions
2.4. Statistical Analysis
3. Results
3.1. Incidence of Renal Failure Requiring Dialysis after HT
3.2. Mortality in Patients with or without Need for Dialysis after HT
3.3. Timing of Dialysis Delivery and the Consequences after HT
3.4. Risk Factors of Renal Failure Requiring Dialysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khush, K.K.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D., Jr.; Hsich, E.; Meiser, B.; Potena, L.; Robinson, A.; Rossano, J.W.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report-2019; focus theme: Donor and recipient size match. J. Heart Lung Transpl. 2019, 38, 1056–1066. [Google Scholar] [CrossRef]
- McCartney, S.L.; Patel, C.; del Rio, J.M. Long-term outcomes and management of the heart transplant recipient. Best Pr. Res. Clin. Anaesthesiol. 2017, 31, 237–248. [Google Scholar] [CrossRef]
- Nadkarni, G.N.; Chauhan, K.; Patel, A.; Saha, A.; Poojary, P.; Kamat, S.; Patel, S.; Ferrandino, R.; Konstantinidis, I.; Garimella, P.S.; et al. Temporal trends of dialysis requiring acute kidney injury after orthotopic cardiac and liver transplant hospitalizations. BMC Nephrol. 2017, 18, 244. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, A.; Haller, H.; Schmitt, R.; Schiffer, M.; Koenecke, C.; Strassburg, C.; Lehner, F.; Gottlieb, J.; Bara, C.; Becker, J.U.; et al. Biopsy-diagnosed renal disease in patients after transplantation of other organs and tissues. Am. J. Transpl. 2010, 10, 2017–2025. [Google Scholar] [CrossRef]
- Alam, A.; Badovinac, K.; Ivis, F.; Trpeski, L.; Cantarovich, M. The Outcome of Heart Transplant Recipients Following the Development of End-Stage Renal Disease: Analysis of the Canadian Organ Replacement Register (CORR). Am. J. Transpl. 2007, 7, 461–465. [Google Scholar] [CrossRef] [Green Version]
- Satchithananda, D.K.; Parameshwar, J.; Sharples, L.; Taylor, G.J.; McNeil, K.; Wallwork, J.; Large, S.R. The incidence of end-stage renal failure in 17 years of heart transplantation: A single center experience. J. Heart Lung Transplant. 2002, 21, 651–657. [Google Scholar] [CrossRef]
- Hamour, I.; Khaghani, A.; Kanagala, P.; Mitchell, A.; Banner, N. Current outcome of heart transplantation: A 10-year single centre perspective and review. QJM 2011, 104, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Thomas, H.L.; Banner, N.R.; Murphy, C.L.; Steenkamp, R.; Birch, R.; Fogarty, D.G.; Bonser, R.S. Incidence, Determinants, and Outcome of Chronic Kidney Disease after Adult Heart Transplantation in the United Kingdom. Transplantation 2012, 93, 1151–1157. [Google Scholar] [CrossRef]
- Arora, S.; Andreassen, A.; Simonsen, S.; Gude, E.; Dahl, C.; Skaardal, R.; Hoel, I.; Geiran, O.; Gullestad, L. Prognostic importance of renal function 1 year after heart transplantation for all-cause and cardiac mortality and development of allograft vasculopathy. Transplantation 2007, 84, 149–154. [Google Scholar] [CrossRef]
- Ojo, A.O.; Held, P.J.; Port, F.K.; Wolfe, R.A.; Leichtman, A.B.; Young, E.W.; Arndorfer, J.; Christensen, L.; Merion, R.M. Chronic renal failure after transplantation of a nonrenal organ. N. Engl. J. Med. 2003, 349, 931–940. [Google Scholar] [CrossRef]
- Villar, E.; Boissonnat, P.; Sebbag, L.; Hendawy, A.; Cahen, R.; Trolliet, P.; Labeeuw, M.; Ecochard, R.; Pouteil-Noble, C. Poor prognosis of heart transplant patients with end-stage renal failure. Nephrol. Dial. Transplant. 2007, 22, 1383–1389. [Google Scholar] [CrossRef] [Green Version]
- Fortrie, G.; Manintveld, O.C.; Caliskan, K.; Bekkers, J.A.; Betjes, M.G. Acute Kidney Injury as a Complication of Cardiac Transplantation: Incidence, Risk Factors, and Impact on 1-year Mortality and Renal Function. Transplantation 2016, 100, 1740–1749. [Google Scholar] [CrossRef]
- Rossi, A.P.; Vella, J.P. Acute Kidney Disease after Liver and Heart Transplantation. Transplantation 2016, 100, 506–514. [Google Scholar] [CrossRef]
- Jokinen, J.J.; Tikkanen, J.; Kukkonen, S.; Hämmäinen, P.; Lommi, J.; Sipponen, J.; Lemström, K.B. Natural course and risk factors for impaired renal function during the first year after heart transplantation. J. Heart Lung Transplant. 2010, 29, 633–640. [Google Scholar] [CrossRef]
- Escoresca Ortega, A.M.; Ruiz de Azua Lopez, Z.; Hinojosa Perez, R.; Ferrandiz Millon, C.M.; Diaz Martin, A.; Corcia Palomo, Y.; Lage Galle, E. Kidney failure after heart transplantation. Transpl. Proc. 2010, 42, 3193–3195. [Google Scholar] [CrossRef]
- Odim, J.; Wheat, J.; Laks, H.; Kobashigawa, J.; Gjertson, D.; Osugi, A.; Mukherjee, K.; Saleh, S. Peri-operative renal function and outcome after orthotopic heart transplantation. J. Heart Lung Transplant. 2006, 25, 162–166. [Google Scholar] [CrossRef]
- Fortrie, G.; Manintveld, O.C.; Constantinescu, A.A.; van de Woestijne, P.C.; Betjes, M.G.H. Renal function at 1 year after cardiac transplantation rather than acute kidney injury is highly associated with long-term patient survival and loss of renal function-a retrospective cohort study. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2017, 30, 788–798. [Google Scholar] [CrossRef] [Green Version]
- Boyle, J.M.; Moualla, S.; Arrigain, S.; Worley, S.; Bakri, M.H.; Starling, R.C.; Heyka, R.; Thakar, C.V. Risks and outcomes of acute kidney injury requiring dialysis after cardiac transplantation. Am. J. Kidney Dis. 2006, 48, 787–796. [Google Scholar] [CrossRef]
- Lee, H.Y.; Oh, B.H. Heart Transplantation in Asia. Circ. J. Off. J. Jpn. Circ. Soc. 2017, 81, 617–621. [Google Scholar] [CrossRef] [Green Version]
- Ho Chan, W.S. Taiwan’s healthcare report 2010. EPMA J. 2010, 1, 563–585. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.J.; Loh, E.W.; Lin, C.H.; Yu, T.M.; Chan, C.H.; Lan, T.H. Correlation of antidepressive agents and the mortality of end-stage renal disease. Nephrology 2012, 17, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, C.M.; Arons, R.R. The burden of acute renal failure in nonrenal solid organ transplantation. Transplantation 2004, 78, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gigorro, R.; Renes-Carreno, E.; Corres Peiretti, M.A.; Arribas Lopez, P.; Perez Vela, J.L.; Gutierrez Rodriguez, J.; Delgado, J.F.; Cortina Romero, J.M.; Montejo Gonzalez, J.C. Incidence, Risk Factors and Outcomes of Early Acute Kidney Injury after Heart Transplantation: An 18-year Experience. Transplantation 2018, 102, 1901–1908. [Google Scholar] [CrossRef] [PubMed]
- Gude, E.; Andreassen, A.K.; Arora, S.; Gullestad, L.; Grov, I.; Hartmann, A.; Leivestad, T.; Fiane, A.E.; Geiran, O.R.; Vardal, M.; et al. Acute renal failure early after heart transplantation: Risk factors and clinical consequences. Clin. Transpl. 2010, 24, E207–E213. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, L.; Rinaldi, M.; Pederzolli, C.; Pederzolli, N.; Goggi, C.; Mantovani, V.; Gavazzi, A.; Campana, C.; Vigano, M. Different results of cardiac transplantation in patients with ischemic and dilated cardiomyopathy. Eur. J. Cardiothorac. Surg. 1995, 9, 644–650. [Google Scholar] [CrossRef]
- He, S.J.; Liu, Q.; Li, H.Q.; Tian, F.; Chen, S.Y.; Weng, J.X. Role of statins in preventing cardiac surgery-associated acute kidney injury: An updated meta-analysis of randomized controlled trials. Clin. Risk Manag. 2018, 14, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Putzu, A.; Capelli, B.; Belletti, A.; Cassina, T.; Ferrari, E.; Gallo, M.; Casso, G.; Landoni, G. Perioperative statin therapy in cardiac surgery: A meta-analysis of randomized controlled trials. Crit. Care 2016, 20, 395. [Google Scholar] [CrossRef] [Green Version]
- Lubitz, S.A.; Pinney, S.; Wisnivesky, J.P.; Gass, A.; Baran, D.A. Statin therapy associated with a reduced risk of chronic renal failure after cardiac transplantation. J. Heart Lung Transplant. 2007, 26, 264–272. [Google Scholar] [CrossRef]
- Spence, J.D.; Dresser, G.K. Overcoming Challenges with Statin Therapy. J. Am. Heart Assoc. 2016, 5, e002497. [Google Scholar] [CrossRef] [Green Version]
- Herlitz, H.; Lindelöw, B. Renal failure following cardiac transplantation. Nephrol. Dial. Transplant. 2000, 15, 311–314. [Google Scholar] [CrossRef] [Green Version]
- Andreassen, A.K.; Andersson, B.; Gustafsson, F.; Eiskjaer, H.; Radegran, G.; Gude, E.; Jansson, K.; Solbu, D.; Sigurdardottir, V.; Arora, S.; et al. Everolimus initiation and early calcineurin inhibitor withdrawal in heart transplant recipients: A randomized trial. Am. J. Transpl. 2014, 14, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, A.; Osorio-Jaramillo, E.; Aliabadi-Zuckermann, A.Z. mTOR Inhibition and Clinical Transplantation: Heart. Transplantation 2018, 102, S27–S29. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, A.K.; Andersson, B.; Gustafsson, F.; Eiskjaer, H.; Radegran, G.; Gude, E.; Jansson, K.; Solbu, D.; Karason, K.; Arora, S.; et al. Everolimus Initiation with Early Calcineurin Inhibitor Withdrawal in de novo Heart Transplant Recipients: Three-Year Results from the Randomized SCHEDULE Study. Am. J. Transpl. 2016, 16, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Jennings, D.L.; Lange, N.; Shullo, M.; Latif, F.; Restaino, S.; Topkara, V.K.; Takeda, K.; Takayama, H.; Naka, Y.; Farr, M.; et al. Outcomes associated with mammalian target of rapamycin (mTOR) inhibitors in heart transplant recipients: A meta-analysis. Int. J. Cardiol. 2018, 265, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Feingold, B.; Zheng, J.; Law, Y.M.; Morrow, W.R.; Hoffman, T.M.; Schechtman, K.B.; Dipchand, A.I.; Canter, C.E. Risk factors for late renal dysfunction after pediatric heart transplantation: A multi-institutional study. Pediatric Transplant. 2011, 15, 699–705. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.C.; Hwang, S.J.; Wen, C.P.; Chang, H.Y.; Chen, T.; Shiu, R.S.; Horng, S.S.; Chang, Y.K.; Yang, W.C. High prevalence and low awareness of CKD in Taiwan: A study on the relationship between serum creatinine and awareness from a nationally representative survey. Am. J. Kidney Dis. 2006, 48, 727–738. [Google Scholar] [CrossRef] [Green Version]
- Whaley-Connell, A.; Sowers, J.R.; McCullough, P.A.; Roberts, T.; McFarlane, S.I.; Chen, S.C.; Li, S.; Wang, C.; Collins, A.J.; Bakris, G.L. Diabetes mellitus and CKD awareness: The Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES). Am. J. Kidney Dis. 2009, 53, S11–S21. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.D.; McCulloch, C.E.; Banerjee, T.; Pavkov, M.E.; Burrows, N.R.; Gillespie, B.W.; Saran, R.; Shlipak, M.G.; Powe, N.R.; Tuot, D.S. CKD Awareness among US Adults by Future Risk of Kidney Failure. Am. J. Kidney Dis. 2020, 76, 174–183. [Google Scholar] [CrossRef]
- Minutolo, R.; de Nicola, L.; Mazzaglia, G.; Postorino, M.; Cricelli, C.; Mantovani, L.G.; Conte, G.; Cianciaruso, B. Detection and awareness of moderate to advanced CKD by primary care practitioners: A cross-sectional study from Italy. Am. J. Kidney Dis. 2008, 52, 444–453. [Google Scholar] [CrossRef]
- Chen, J.W.; Lin, C.H.; Hsu, R.B. Incidence, risk factor, and prognosis of end-stage renal disease after heart transplantation in Chinese recipients. J. Formos. Med. Assoc. Taiwan Yi Zhi 2014, 113, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Ko, W.J.; Chou, N.K.; Hsu, R.B.; Chen, Y.S.; Wang, S.S.; Chu, S.H.; Lai, M.Y. Hepatitis B virus infection in heart transplant recipients in a hepatitis B endemic area. J. Heart Lung Transpl. 2001, 20, 865–875. [Google Scholar] [CrossRef]
- De Santo, L.S.; Romano, G.; Amarelli, C.; Maiello, C.; Baldascino, F.; Bancone, C.; Grimaldi, F.; Nappi, G. Implications of acute kidney injury after heart transplantation: What a surgeon should know. Eur. J. Cardiothorac. Surg. 2011, 40, 1355–1361. [Google Scholar] [CrossRef] [PubMed]


| Total (n = 1129) | Nondialysis (n = 808) | Dialysis (n = 321) | |||||
|---|---|---|---|---|---|---|---|
| Variables | n | (%) | n | (%) | n | (%) | pa |
| Sex, men | 792 | (70.2) | 537 | (66.5) | 255 | (79.4) | <0.001 |
| Age, years | 0.088 | ||||||
| Mean ± SD | 45.5 ± 16.8 | 45.3 ± 17.0 | 46.1 ± 16.3 | ||||
| <18 | 97 | (8.6) | 69 | (8.5) | 28 | (8.7) | |
| 18–39 | 272 | (24.1) | 211 | (26.1) | 61 | (19.0) | |
| 40–59 | 539 | (47.7) | 373 | (46.2) | 166 | (51.7) | |
| ≥60 | 221 | (19.6) | 155 | (19.2) | 66 | (20.6) | |
| Comorbidities | |||||||
| HBV | 48 | (4.3) | 41 | (5.1) | 7 | (2.2) | 0.030 |
| HCV | 17 | (1.5) | 13 | (1.6) | 4 | (1.2) | 0.652 |
| Cirrhosis | 186 | (16.5) | 122 | (15.1) | 64 | (19.9) | 0.048 |
| DM | 279 | (24.7) | 166 | (20.5) | 113 | (35.2) | <0.001 |
| CKD | 58 | (5.1) | 22 | (2.7) | 36 | (11.2) | <0.001 |
| Hypertension | 449 | (39.8) | 296 | (36.6) | 153 | (47.7) | <0.001 |
| Hyperlipidemia | 271 | (24.0) | 184 | (22.8) | 87 | (27.1) | 0.124 |
| AKI | 52 | (4.6) | 21 | (2.6) | 31 | (9.7) | <0.001 |
| Primary diagnosis for HT | <0.001 | ||||||
| CHD | 73 | (6.5) | 62 | (7.7) | 11 | (3.4) | |
| Cardiomyopathy | 452 | (40.0) | 291 | (36.0) | 161 | (50.2) | |
| CAD | 278 | (24.6) | 142 | (17.6) | 136 | (42.4) | |
| Others | 326 | (28.9) | 313 | (38.7) | 13 | (4.0) | |
| Statin | 305 | (27.0) | 196 | (24.3) | 109 | (34.0) | <0.001 |
| Immunosuppressants Calcineurin inhibitors | <0.001 | ||||||
| No | 393 | (34.8) | 371 | (45.9) | 22 | (6.9) | |
| Cyclosporin | 618 | (54.7) | 358 | (44.3) | 260 | (81.0) | |
| Tacrolimus | 118 | (10.5) | 79 | (9.8) | 39 | (12.2) | |
| mTOR inhibitors | 0.003 | ||||||
| No | 767 | (67.9) | 570 | (70.5) | 197 | (61.4) | |
| Rapamycin | 362 | (32.1) | 238 | (29.5) | 124 | (38.6) | |
| Antimetabolites | <0.001 | ||||||
| No | 454 | (40.2) | 415 | (51.4) | 39 | (12.2) | |
| MMF | 484 | (42.9) | 328 | (40.6) | 156 | (48.6) | |
| Azathioprine | 191 | (16.9) | 65 | (8.0) | 126 | (39.3) | |
| Nondialysis Cohort | Dialysis Cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Events | FT † | Rate | Events | FT | Rate | HR ‖ | (95% CI) | p |
| Outcome = Mortality | |||||||||
| Overall | 311 | 4253 | 7.31 | 220 | 1361 | 16.16 | 3.44 | (2.73–4.33) | <0.001 |
| 1y | 111 | 747 | 14.86 | 116 | 235 | 49.36 | 5.89 | (4.09–4.48) | <0.001 |
| 3y | 208 | 1916 | 10.86 | 152 | 587 | 25.89 | 4.03 | (3.03–5.34) | <0.001 |
| 5y | 251 | 2773 | 9.05 | 170 | 852 | 19.95 | 3.68 | (2.83–4.78) | <0.001 |
| Overall | Dialysis Cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | n | (%) | Events | FT † | Rate | HR ‖‖ | (95% CI) | p | HR ‖‖ | (95% CI) | p |
| Outcome = Mortality | |||||||||||
| Dialysis timing †† | |||||||||||
| Nondialysis | 808 | (71.6) | 311 | 4253 | 7.31 | 1.00 | (reference) | ||||
| Early | 237 | (21.0) | 151 | 972 | 15.53 | 3.58 | (2.74–4.67) | <0.001 | 1.00 | (reference) | |
| Late | 84 | (7.4) | 69 | 389 | 17.74 | 3.27 | (2.44–4.36) | <0.001 | 1.23 | (0.88–1.71) | 0.220 |
| Variables | OR | (95% CI) | p-Value |
|---|---|---|---|
| Demographics | |||
| Age (years) | |||
| <18 | 1.49 | (0.81–2.71) | 0.203 |
| 18–39 | 0.80 | (0.54–1.18) | 0.264 |
| 40–59 | 1.00 | (reference) | |
| ≥60 | 1.17 | (0.80–1.73) | 0.418 |
| Sex | |||
| Female | 1.00 | (reference) | |
| Male | 1.27 | (0.89–1.81) | 0.185 |
| Comorbidities (yes vs. no) | |||
| HBV | 0.32 | (0.14–0.76) | 0.010 |
| HCV | 0.67 | (0.21–2.21) | 0.515 |
| Cirrhosis | 1.11 | (0.76–1.63) | 0.577 |
| DM | 1.50 | (1.07–2.11) | 0.019 |
| CKD | 2.65 | (1.46–4.79) | 0.001 |
| Hypertension | 0.95 | (0.69–1.30) | 0.746 |
| Hyperlipidemia | |||
| without statin | 0.63 | (0.38–1.07) | 0.086 |
| with statin | 0.71 | (0.47–1.06) | 0.090 |
| AKI | 2.36 | (1.27–4.41) | 0.007 |
| CHD | 1.19 | (0.68–2.07) | 0.536 |
| Cardiomyopathy | 0.76 | (0.53–1.10) | 0.144 |
| CAD | 10.78 | (6.33–18.4) | <0.001 |
| Variables | OR | (95% CI) | p-Value |
|---|---|---|---|
| Demographics | |||
| Age (years) | |||
| <18 | 1.45 | (0.73–2.90) | 0.293 |
| 18–39 | 0.88 | (0.58–1.35) | 0.564 |
| 40–59 | 1.00 | (reference) | |
| ≥60 | 1.01 | (0.64–1.58) | 0.978 |
| Sex | |||
| Female | 1.00 | (reference) | |
| Male | 1.39 | (0.93–2.08) | 0.112 |
| Comorbidities (yes vs. no) | |||
| HBV | 0.37 | (0.15–0.93) | 0.034 |
| HCV | 0.72 | (0.19–2.74) | 0.632 |
| Cirrhosis | 1.20 | (0.79–1.81) | 0.402 |
| DM | 1.33 | (0.91–1.96) | 0.142 |
| CKD | 2.84 | (1.54–5.24) | <0.001 |
| HTN | 0.79 | (0.56–1.13) | 0.203 |
| Hyperlipidemia | |||
| without statin | 0.65 | (0.36–1.18) | 0.157 |
| with statin | 0.63 | (0.40–0.99) | 0.045 |
| AKI | 3.00 | (1.56–5.78) | 0.001 |
| CHD | 1.31 | (0.71–2.42) | 0.383 |
| Cardiomyopathy | 0.56 | (0.38–0.83) | 0.004 |
| CAD | 17.29 | (9.17–32.6) | <0.001 |
| Variables | OR | (95% CI) | p-Value |
|---|---|---|---|
| Demographics | |||
| Age (years) | |||
| <18 | 0.78 | (0.25–2.43) | 0.674 |
| 18–39 | 0.51 | (0.22–1.18) | 0.114 |
| 40–59 | 1.00 | (reference) | |
| ≥60 | 1.30 | (0.65–2.56) | 0.458 |
| Sex | |||
| Female | 1.00 | (reference) | |
| Male | 0.64 | (0.32–1.30) | 0.216 |
| Comorbidities (yes vs. no) | |||
| HBV | 1.51 | (0.16–14.3) | 0.720 |
| HCV | 0.71 | (0.07–7.46) | 0.773 |
| Cirrhosis | 0.64 | (0.30–1.37) | 0.250 |
| DM | 1.17 | (0.62–2.23) | 0.625 |
| CKD | 0.58 | (0.20–1.64) | 0.300 |
| HTN | 1.69 | (0.91–3.13) | 0.096 |
| Hyperlipidemia | |||
| without statin | 0.82 | (0.29–2.34) | 0.714 |
| with statin | 1.42 | (0.67–3.00) | 0.362 |
| AKI | 0.62 | (0.20–1.92) | 0.406 |
| Maintenance immunosuppressive drugs Calcineurin inhibitors | |||
| Cyclosporin | 1.00 | (reference) | |
| Tacrolimus | 1.06 | (0.38–2.95) | 0.907 |
| None | 0.67 | (0.21–2.18) | 0.511 |
| mTOR inhibitors | |||
| Sirolimus (Rapamycin) | 0.17 | (0.08–0.36) | <0.001 |
| Antimetabolites | |||
| Azathioprine | 1.00 | (reference) | |
| MMF | 0.26 | (0.13–0.51) | <0.001 |
| None | 0.66 | (0.25–1.76) | 0.409 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-J.; Lin, C.-H.; Wei, H.-J.; Wu, M.-J. Long-Term Outcomes and Risk Factors of Renal Failure Requiring Dialysis after Heart Transplantation: A Nationwide Cohort Study. J. Clin. Med. 2020, 9, 2455. https://doi.org/10.3390/jcm9082455
Wang T-J, Lin C-H, Wei H-J, Wu M-J. Long-Term Outcomes and Risk Factors of Renal Failure Requiring Dialysis after Heart Transplantation: A Nationwide Cohort Study. Journal of Clinical Medicine. 2020; 9(8):2455. https://doi.org/10.3390/jcm9082455
Chicago/Turabian StyleWang, Tsai-Jung, Ching-Heng Lin, Hao-Ji Wei, and Ming-Ju Wu. 2020. "Long-Term Outcomes and Risk Factors of Renal Failure Requiring Dialysis after Heart Transplantation: A Nationwide Cohort Study" Journal of Clinical Medicine 9, no. 8: 2455. https://doi.org/10.3390/jcm9082455
APA StyleWang, T.-J., Lin, C.-H., Wei, H.-J., & Wu, M.-J. (2020). Long-Term Outcomes and Risk Factors of Renal Failure Requiring Dialysis after Heart Transplantation: A Nationwide Cohort Study. Journal of Clinical Medicine, 9(8), 2455. https://doi.org/10.3390/jcm9082455





