Translating Evidence from Clonal Hematopoiesis to Cardiovascular Disease: A Systematic Review
Abstract
:1. Introduction
2. Age-Related Clonal Hematopoiesis and Cancer
3. CHIP Is Associated with Cardiovascular Diseases
4. Mechanisms by which CHIP Increases Cardiovascular Risk
4.1. TET2 and DNMT3A
4.2. JAK2 Mutations Promote Thrombotic Diseases and Increase Inflammation
4.3. CHIP, The Chronic Inflammatory State and Ageing: One Culprit for Different Pathologies?
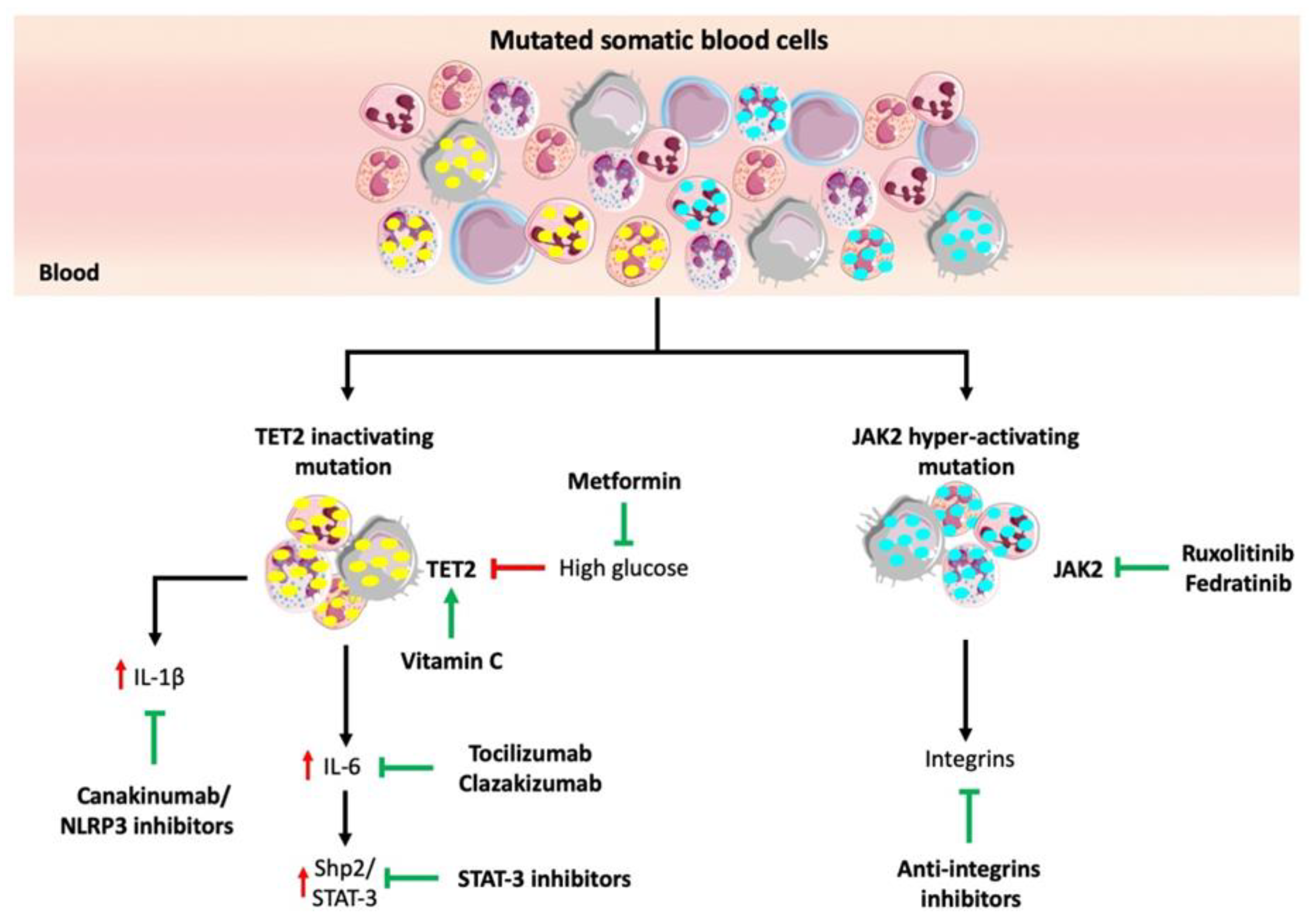
5. Potential Strategies for Targeting High-Risk CHIP Mutations
5.1. Immune Therapy
5.2. JAK2 Inhibitors
5.3. Vitamin C to Compensate for TET2 Function
5.4. Glucose-Lowering Drugs
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CH | clonal hematopoiesis |
| CHIP | clonal hematopoiesis of indeterminate potential |
| CVD | cardiovascular disease |
| DNMT3A | DNA methyltransferase 3A |
| TET2 | Tet methylcytosine dioxygenase 2 |
| HSPC | hematopoietic stem/progenitor cells |
| MDS | myelo-dysplastic syndromes |
| ASXL1 | additional sex combs-like transcriptional regulator 1 |
| AML | acute myeloid leukemia |
| TP53 | tumor protein p53 |
| JAK2 | janus kinase 2 |
| SF3B1 | splicing factor 3b subunit 1 |
| CBL | casitas B-lineage lymphoma gene |
| SRSF2 | serine and arginine rich splicing factor 2 |
| PPM1D | protein phosphatase, Mg2+/Mn2+ Dependent 1D |
| BCOR | BCL6 Corepressor |
| HF | heart failure |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| CD163 | Cluster Designation number 163, hemoglobin (Hb) scavenger receptor |
| CXCR4 | C-X-C chemokine receptor type 4 |
| CXCL12 | C-X-C chemokine ligand 12 |
| NOTCH1 | Notch homolog 1, translocation-associated |
| MPN | myeloproliferative neoplasms |
| NET | neutrophil extracellular traps |
| CRP | C-reactive protein |
| SHP2 | src homology region 2 (SH2)-containing protein tyrosine phosphatase 2 |
| STAT3 | signal transducer and activator of transcription 3 |
| CMN | chronic myeloproliferative neoplasia |
| CSC | cardiac stem/progenitors cells |
| SASP | senescence-associated secretory phenotype |
| ECM | extracellular matrix |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| hs-CRP | high sensitivity C-reactive protein |
References
- Jaiswal, S.; Ebert, B.L. Clonal hematopoiesis in human aging and disease. Science 2019, 366, eaan4673. [Google Scholar] [CrossRef]
- Nowell, P.C. The clonal evolution of tumor cell populations. Science 1976, 194, 3–28. [Google Scholar] [CrossRef]
- Genovese, G.; Kähler, A.K.; Handsaker, R.E.; Lindberg, J.; Rose, S.A.; Bakhoum, S.F.; Chambert, K.; Mick, E.; Neale, B.M.; Fromer, M.; et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 2014, 371, 2477–2487. [Google Scholar] [CrossRef] [Green Version]
- Stratton, M.R.; Campbell, P.J.; Futreal, P.A. The cancer genome. Nature 2009, 458, 719–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, J.N.; Young, N.S. Perspective Clonality in context: Hematopoietic clones in their marrow environment. Blood 2017, 130, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Biasco, L.; Pellin, D.; Scala, S.; Dionisio, F.; Basso-Ricci, L.; Leonardelli, L.; Scaramuzza, S.; Baricordi, C.; Ferrua, F.; Cicalese, M.P.; et al. In vivo tracking of human hematopoiesis reveals patterns of clonal dynamics during early and steady-state reconstitution phases. Cell Stem Cell 2016, 19, 107–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.G. On the nature of susceptibility to cancer. The presidential address. Cancer 1980, 46, 1307–1318. [Google Scholar] [CrossRef]
- Steensma, D.P.; Bejar, R.; Jaiswal, S.; Lindsley, R.C.; Sekeres, M.A.; Hasserjian, R.P.; Ebert, B.L. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015, 126, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steensma, D.P. Clinical consequences of clonal hematopoiesis of indeterminate potential. Blood Adv. 2018, 2, 3404–3410. [Google Scholar] [CrossRef]
- Heuser, M.; Thol, F.; Ganser, A. Clonal hematopoiesis of indeterminate potential. Dtsch. Aerzteblatt Online 2016, 113, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Zink, F.; Stacey, S.N.; Norddahl, G.L.; Frigge, M.L.; Magnusson, O.T.; Jonsdottir, I.; Thorgeirsson, T.E.; Sigurdsson, A.; Gudjonsson, S.A.; Gudmundsson, J.; et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood 2017, 130, 742–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvillo-Argüelles, O.; Jaiswal, S.; Shlush, L.I.; Moslehi, J.J.; Schimmer, A.; Barac, A.; Thavendiranathan, P. Connections between clonal hematopoiesis, cardiovascular disease, and cancer: A review. JAMA Cardiol. 2019, 4, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.J.; Steensma, D.P. New insights from studies of clonal hematopoiesis. Clin. Cancer Res. 2018, 24, 4633–4642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.J.; Shvartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal Hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef]
- Fuster, J.J.; Walsh, K. Somatic mutations and clonal hematopoiesis: Unexpected potential new drivers of age-related cardiovascular disease. Circ. Res. 2018, 122, 523–532. [Google Scholar] [CrossRef]
- Shlush, L.I.; Zandi, S.; Itzkovitz, S.; Schuh, A.C. Aging, clonal hematopoiesis and preleukemia: Not just bad luck? Int. J. Hematol. 2015, 102, 513–522. [Google Scholar] [CrossRef]
- Shlush, L.I. Age-related clonal hematopoiesis. Blood 2018, 131, 496–504. [Google Scholar] [CrossRef] [Green Version]
- Libby, P.; Sidlow, R.; Lin, A.E.; Gupta, D.; Jones, L.W.; Moslehi, J.; Zeiher, A.; Jaiswal, S.; Schulz, C.; Blankstein, R.; et al. Clonal hematopoiesis: Crossroads of aging, cardiovascular disease, and cancer: JACC review topic of the week. J. Am. Coll. Cardiol. 2019, 74, 567–577. [Google Scholar] [CrossRef]
- Xie, M.; Lu, C.; Wang, J.; McLellan, M.D.; Johnson, K.J.; Wendl, M.C.; McMichael, J.F.; Schmidt, H.K.; Yellapantula, V.; Miller, C.A.; et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 2014, 20, 1472–1478. [Google Scholar] [CrossRef]
- Sidlow, R.; Lin, A.E.; Gupta, D.; Bolton, K.L.; Steensma, D.P.; Levine, R.L.; Ebert, B.L.; Libby, P. The clinical challenge of clonal hematopoiesis, a newly recognized cardiovascular risk factor. JAMA Cardiol. 2020. [Google Scholar] [CrossRef]
- Coombs, C.C.; Zehir, A.; Devlin, S.M.; Kishtagari, A.; Syed, A.; Jonsson, P.; Hyman, D.M.; Solit, D.B.; Robson, M.E.; Baselga, J.; et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell 2017, 21, 374–382.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorsheimer, L.; Assmus, B.; Rasper, T.; Ortmann, C.A.; Ecke, A.; Abou-El-Ardat, K.; Schmid, T.; Brune, B.; Wagner, S.; Serve, H.; et al. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol. 2019, 4, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delhommeau, F.; Dupont, S.; Della Valle, V.; James, C.; Trannoy, S.; Masse, A.; Kosmider, O.; Le Couedic, J.P.; Robert, F.; Alberti, A.; et al. Mutation in TET2 in myeloid cancers. N. Engl. J. Med. 2009, 360, 2289–2301. [Google Scholar] [CrossRef] [PubMed]
- Follo, M.Y.; Finelli, C.; Mongiorgi, S.; Clissa, C.; Bosi, C.; Testoni, N.; Chiarini, F.; Ramazzotti, G.; Baccarani, M.; Martelli, A.M.; et al. Reduction of phosphoinositide-phospholipase C-beta1 methylation predicts the responsiveness to azacitidine in high-risk MDS. Proc. Natl. Acad. Sci. USA 2009, 106, 16811–16816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finelli, C.; Follo, M.Y.; Stanzani, M.; Parisi, S.; Clissa, C.; Mongiorgi, S.; Barraco, L.; Cocco, L. Clinical impact of hypomethylating agents in the treatment of myelodysplastic syndromes. Curr. Pharm. Des. 2016, 22, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.A.; Gore, S.D. DNA methyltransferase and histone deacetylase inhibitors in the treatment of myelodysplastic syndromes. Semin. Hematol. 2008, 45, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Inoue, D.; Kitaura, J.; Togami, K.; Nishimura, K.; Enomoto, Y.; Uchida, T.; Kagiyama, Y.; Kawabata, K.C.; Nakahara, F.; Izawa, K.; et al. Myelodysplastic syndromes are induced by histone methylation-altering ASXL1 mutations. J. Clin. Investig. 2013, 123, 4627–4640. [Google Scholar] [CrossRef]
- Gelsi-Boyer, V.; Brecqueville, M.; Devillier, R.; Murati, A.; Mozziconacci, M.J.; Birnbaum, D. Mutations in ASXL1 are associated with poor prognosis across the spectrum of malignant myeloid diseases. J. Hematol. Oncol. 2012, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Gelsi-Boyer, V.; Trouplin, V.; Adélaïde, J.; Bonansea, J.; Cervera, N.; Carbuccia, N.; Lagarde, A.; Prebet, T.; Nezri, M.; Sainty, D.; et al. Mutations of polycomb-associated gene ASXL1 in myelodysplastic syndromes and chronic myelomonocytic leukemia. Br. J. Haematol. 2009, 145, 788–800. [Google Scholar] [CrossRef]
- Shlush, L.I.; Zandi, S.; Mitchell, A.; Chen, W.H.; Brandwein, J.M.; Gupta, V.; Kennedy, J.A.; Schimmer, A.D.; Schuh, A.C.; Yee, K.W.; et al. Identification of pre-leukaemic hematopoietic stem cells in acute leukemia. Nature 2014, 506, 328–333. [Google Scholar] [CrossRef]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A.; et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boer, R.A.; Meijers, W.C.; van der Meer, P.; van Veldhuisen, D.J. Cancer and heart disease: Associations and relations. Eur. J. Heart Fail. 2019, 21, 1515–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P.; Kobold, S. Inflammation: A common contributor to cancer, aging, and cardiovascular diseases—Expanding the concept of cardio-oncology. Cardiovasc. Res. 2019, 115, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Totzeck, M.; Schuler, M.; Stuschke, M.; Heusch, G.; Rassaf, T. Cardio-oncology-strategies for management of cancer-therapy related cardiovascular disease. Int. J. Cardiol. 2019, 280, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared risk factors in cardiovascular disease and cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khot, U.N.; Khot, M.B.; Bajzer, C.T.; Sapp, S.K.; Ohman, E.M.; Brener, S.J.; Ellis, S.G.; Lincoff, A.M.; Topol, E.J. Prevalence of conventional risk factors in patients with coronary heart disease. J. Am. Med. Assoc. (JAMA) 2003, 290, 898–904. [Google Scholar] [CrossRef] [Green Version]
- North, B.J.; Sinclair, D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef]
- Oluwagbamigbe Fajemiroye, J.; da Cunha, L.C.; Saavedra-Rodríguez, R.; Rodrigues, K.L.; Naves, L.M.; Mourao, A.A.; da Silva, E.F.; Williams, N.E.E.; Martins, J.L.R.; Sousa, R.B.; et al. Aging-induced biological changes and cardiovascular diseases. BioMed Res. Int. 2018, 2018, 7156435. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, S.; Libby, P. Clonal hematopoiesis: Connecting ageing and inflammation in cardiovascular disease. Nat. Rev. Cardiol. 2019, 17, 137–144. [Google Scholar] [CrossRef]
- Mas-Peiro, S.; Hoffmann, J.; Fichtlscherer, S.; Dorsheimer, L.; Rieger, M.A.; Dimmeler, S.; Vasa-Nicotera, M.; Zeiher, A.M. Clonal hematopoiesis in patients with degenerative aortic valve stenosis undergoing transcatheter aortic valve implantation. Eur. Heart J. 2020, 41, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Libby, P.; Jaiswal, S.; Lin, A.E.; Ebert, B.L. CHIPping away at the pathogenesis of heart failure. JAMA Cardiol. 2019, 4, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Galli, D.; Manuguerra, D.; Monaco, R.; Manotti, L.; Goldoni, M.; Becchi, G.; Carubbi, C.; Vignali, G.; Cucurachi, N.; Gherli, T.; et al. Understanding the structural features of symptomatic calcific aortic valve stenosis: A broad-spectrum clinico-pathologic study in 236 consecutive surgical cases. Int. J. Cardiol. 2017, 228, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Abplanalp, W.T.; Mas-Peiro, S.; Cremer, S.; John, D.; Dimmeler, S.; Zeiher, A.M. Association of clonal hematopoiesis of indeterminate potential with inflammatory gene expression in patients with severe degenerative aortic valve stenosis or chronic postischemic heart failure. JAMA Cardiol. 2020, e202468. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.J.; Maclauchlan, S.; Zuriaga, M.A.; Polackal, M.N.; Ostriker, A.C.; Chakraborty, R.; Wu, C.L.; Sano, S.; Muralidharan, S.; Rius, C.; et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017, 355, 842–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, M.; Huang, Y.; Jankowska, A.M.; Pape, U.J.; Tahiliani, M.; Bandukwala, H.S.; An, J.; Lamperti, E.D.; Koh, K.P.; Ganetzky, R.; et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature 2010, 468, 839–843. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Cai, X.; Cai, C.-L.; Wang, J.; Zhang, W.; Petersen, B.E.; Yang, F.-C.; Xu, M. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood 2011, 118, 4509–4518. [Google Scholar] [CrossRef] [Green Version]
- Sano, S.; Oshima, K.; Wang, Y.; MacLauchlan, S.; Katanasaka, Y.; Sano, M.; Zuriaga, M.A.; Yoshiyama, M.; Goukassian, D.; Cooper, M.A.; et al. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J. Am. Coll. Cardiol. 2018, 71, 875–886. [Google Scholar] [CrossRef]
- Sano, S.; Oshima, K.; Wang, Y.; Katanasaka, Y.; Sano, M.; Walsh, K. CRISPR-Mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ. Res. 2018, 123, 335–341. [Google Scholar] [CrossRef]
- Wang, Y.; Sano, S.; Yura, Y.; Ke, Z.; Sano, M.; Oshima, K.; Ogawa, H.; Horitani, K.; Min, K.-D.; Miura-Yura, E.; et al. Tet2-mediated clonal hematopoiesis in nonconditioned mice accelerates age-associated cardiac dysfunction. JCI Insight 2020, 5, e135204. [Google Scholar] [CrossRef] [Green Version]
- Kaasinen, E.; Kuismin, O.; Rajamakj, K.; Ristolainen, H.; Aavikko, M.; Kondelin, J.; Saarinen, S.; Berta, D.G.; Katainen, R.; Hirvonen, E.A.M.; et al. Impact of constitutional TET2 haploinsufficiency on molecular and clinical phenotype in humans. Nat. Commun. 2019, 10, 1252. [Google Scholar] [CrossRef]
- Döring, Y.; Van Der Vorst, E.P.C.; Duchene, J.; Jansen, J.; Gencer, S.; Bidzhekov, K.; Atzler, D.; Santovito, D.; Rader, D.J.; Saleheen, D.; et al. CXCL12 Derived from endothelial cells promotes atherosclerosis to drive coronary artery disease. Circulation 2019, 139, 1338–1340. [Google Scholar] [CrossRef] [PubMed]
- Schunkert, H.; König, I.R.; Kathiresan, S.; Reilly, M.P.; Assimes, T.L.; Holm, H.; Preuss, M.; Stewart, A.F.R.; Barbalic, M.; Gieger, C.; et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 2011, 43, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.N.; Li, M.; William, D.; Khera, A.V.; DerOhannessian, S.; Qu, L.; Ferguson, J.F.; McLaughlin, C.; Shaikh, L.H.; Shah, R.; et al. The novel atherosclerosis locus at 10q11 regulates plasma CXCL12 levels. Eur. Heart J. 2011, 32, 963–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, K.; Lee, J.; Chrysanthou, S.; Zhao, Y.; Josephs, K.; Sato, H.; Teruya-Feldestein, J.; Zheng, D.; Dawlaty, M.M.; Ito, K. Non-catalytic roles of Tet2 are essential to regulate hematopoietic stem and progenitor cell homeostasis. Cell Rep. 2019, 28, 2480–2490.e4. [Google Scholar] [CrossRef]
- Pan, F.; Wingo, T.S.; Zhao, Z.; Gao, R.; Makishima, H.; Qu, G.; Lin, L.; Yu, M.; Ortega, J.M.; Wang, J.; et al. Tet2 loss leads to hypermutagenicity in hematopoietic stem/progenitor cells. Nat. Commun. 2017, 8, 15102. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Lan, Y.; Schwartz-Orbach, L.; Korol, K.; Tahiliani, M.; Evans, T.; Goll, M.G. Overlapping requirements for Tet2 and Tet3 in normal development and hematopoietic stem cell emergence. Cell Rep. 2015, 12, 1133–1143. [Google Scholar] [CrossRef] [Green Version]
- Vieceli Dalla Sega, F.; Fortini, F.; Aquila, G.; Campo, G.; Vaccarezza, M.; Rizzo, P. Notch signaling regulates immune responses in atherosclerosis. Front. Immunol. 2019, 10, 1130. [Google Scholar] [CrossRef] [Green Version]
- Fortini, F.; Vieceli Dalla Sega, F.; Caliceti, C.; Lambertini, E.; Pannuti, A.; Peiffer, D.S.; Balla, C.; Rizzo, P. Estrogen-mediated protection against coronary heart disease: The role of the Notch pathway. J. Steroid Biochem. Mol. Biol. 2019, 189, 87–100. [Google Scholar] [CrossRef]
- Aquila, G.; Kostina, A.; Vieceli Dalla Sega, F.; Shlyakhto, E.; Kostareva, A.; Marracino, L.; Ferrari, R.; Rizzo, P.; Malaschicheva, A. The notch pathway: A novel therapeutic target for cardiovascular diseases? Expert Opin. Ther. Targets 2019, 23, 695–710. [Google Scholar] [CrossRef]
- Rosti, V.; Villani, L.; Riboni, R.; Poletto, V.; Bonetti, E.; Tozzi, L.; Bergamaschi, G.; Catarsi, P.; Dallera, E.; Novara, F.; et al. Spleen endothelial cells from patients with myelofibrosis harbor the JAK2V617F mutation. Blood 2013, 121, 360–368. [Google Scholar] [CrossRef]
- Sozer, S.; Fiel, M.I.; Schiano, T.; Xu, M.; Mascarenhas, J.; Hoffman, R. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood 2009, 113, 5246–5249. [Google Scholar] [CrossRef] [PubMed]
- Teofili, L.; Martini, M.; Iachininoto, M.G.; Capodimonti, S.; Nuzzolo, E.R.; Torti, L.; Cenci, T.; Larocca, L.M.; Leone, G. Endothelial progenitor cells are clonal and exhibit the JAK2 V617F mutation in a subset of thrombotic patients with Ph-negative myeloproliferative neoplasms. Blood 2011, 117, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, S.L.; Roh, M.E.; Cosgrove, M.E.; Sangkhae, V.; Fox, N.E.; Chen, J.; Lopez, J.A.; Kaushansky, K.; Hitchcock, I.S. JAK2V 617 F-positive endothelial cells contribute to clotting abnormalities in myeloproliferative neoplasms. Proc. Natl. Acad. Sci. USA 2014, 111, 2295–2300. [Google Scholar] [CrossRef] [Green Version]
- Hobbs, C.M.; Manning, H.; Bennett, C.; Vasquez, L.; Severin, S.; Brain, L.; Mazharian, A.; Guerrero, J.A.; Li, J.; Soranzo, N.; et al. JAK2V617F leads to intrinsic changes in platelet formation and reactivity in a knock-in mouse model of essential thrombocythemia. Blood 2013, 122, 3787–3797. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, H.; Cumano, A.; Müller, M.; Wu, H.; Huffstatd, U.; Pfeffer, K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell 1998, 93, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Wolach, O.; Sellar, R.S.; Martinod, K.; Cherpokova, D.; McConkey, M.; Chappell, R.J.; Silver, A.J.; Adams, D.; Castellano, C.A.; Schneider, R.K.; et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci. Transl. Med. 2018, 10, eaan8292. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Liu, W.; Fidler, T.; Wang, Y.; Tang, Y.; Woods, B.; Welch, C.; Cai, B.; Silvestre-Roig, C.; Ai, D.; et al. Macrophage inflammation, erythrophagocytosis, and accelerated atherosclerosis in Jak2 V617F Mice. Circ. Res. 2018, 123, e35–e47. [Google Scholar] [CrossRef]
- Sano, S.; Wang, Y.; Yura, Y.; Sano, M.; Oshima, K.; Yang, Y.; Katanasaka, Y.; Min, K.-D.; Matsuura, S.; Ravid, K.; et al. JAK2 V617F-Mediated clonal hematopoiesis accelerates pathological remodeling in murine heart failure. JACC Transl. Sci. 2019, 4, 684–697. [Google Scholar]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, H.; Rosenberg, Y.; Iturriaga, E.; et al. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef]
- Abegunde, S.O.; Buckstein, R.; Wells, R.A.; Rauh, M.J. An inflammatory environment containing TNFα favors Tet2-mutant clonal hematopoiesis. Exp. Hematol. 2018, 59, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.K.; Izukawa, T.; Young, S.; Rosen, G.; Jamali, M.; Zhang, L.; Johnson, D.; Bain, E.; Hilland, J.; Ferrone, C.K.; et al. Comorbid and inflammatory characteristics of genetic subtypes of clonal hematopoiesis. Blood Adv. 2019, 3, 2482–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yura, Y.; Sano, S.; Walsh, K. Clonal hematopoiesis: A new step linking inflammation to heart failure. JACC Basic Transl. Sci. 2020, 5, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Dorsheimer, L.; Assmus, B.; Rasper, T.; Ortmann, C.A.; Abou-El-Hardat, K.; Kiefer, K.C.; Hoffmann, J.; Seeger, F.; Bonig, H.; Dimmeler, S.; et al. Hematopoietic alterations in chronic heart failure patients by somatic mutations leading to clonal hematopoiesis. Haematologica 2020, 105, e328–e332. [Google Scholar] [CrossRef] [Green Version]
- Ferrucci, L.; Corsi, A.; Lauretani, F.; Bandinelli, S.; Bartali, B.; Taub, D.D.; Guralnik, J.M.; Longo, D.L. The origins of age-related proinflammatory state. Blood 2005, 105, 2294–2299. [Google Scholar] [CrossRef] [Green Version]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Bick, A.G.; Pirruccello, J.P.; Griffin, G.K.; Gupta, N.; Gabriel, S.; Saleheen, D.; Libby, P.; Kathiresan, S.; Natarajan, P. Genetic interleukin 6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation 2020, 141, 124–131. [Google Scholar] [CrossRef]
- Bick, A.G.; Weinstock, J.S.; Nandakumar, S.K.; Fulco, C.P.; Leventhal, M.J.; Bao, E.L.; Nasser, J.; Zekavat, S.M.; Szeto, M.D.; Laurie, C.; et al. Inherited causes of clonal hematopoiesis of indeterminate potential in TOPMed whole genomes. bioRxiv 2019, 782748. [Google Scholar] [CrossRef] [Green Version]
- Busque, L.; Sun, M.; Buscariet, M.; Ayachi, S.; Feroz Zada, Y.; Provost, S.; Bourgoin, V.; Mollica, L.; Meisel, M.; Hinterleitner, E.R.; et al. High-sensitivity C-reactive protein is associated with clonal hematopoiesis of indeterminate potential. Blood Adv. 2020, 4, 2430–2438. [Google Scholar] [CrossRef]
- Buscarlet, M.; Provost, S.; Feroz Zada, Y.; Bourgoin, V.; Mollica, L.; Dube, M.-P.; Busque, L. Lineage restriction analyses in CHIP indicate myeloid bias for TET2 and multipotent stem cell origin for DNMT3A. Blood 2018, 132, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Arends, C.M.; Galan-Sousa, J.; Hoyer, K.; Chan, W.; Jager, M.; Yoshida, K.; Seemann, R.; Noerenberg, D.; Waldhueter, N.; Fleischer-Notten, H.; et al. Hematopoietic lineage distribution and evolutionary dynamics of clonal hematopoiesis. Leukemia 2018, 32, 1908–1919. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Kotzin, J.J.; Ramdas, B.; Chen, S.; Nelanuthala, S.; Palam, L.R.; Pandey, R.; Mali, R.S.; Liu, Y.; Kelley, M.R.; et al. Inhibition of inflammatory signaling in Tet2 mutant preleukemic cells mitigates stress-induced abnormalities and clonal hematopoiesis. Cell Stem Cell 2018, 23, 833–849.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.M.; Moreland, L.W. Interleukin-6 inhibition for treatment of rheumatoid arthritis: A review of tocilizumab therapy. Drug Des. Dev. Ther. 2010, 4, 263–278. [Google Scholar] [CrossRef] [Green Version]
- Eskandary, F.; Dürr, M.; Budde, K.; Doberer, K.; Reindl-Schwaighofer, R.; Waiser, J.; Wahrmann, M.; Regele, H.; Spittler, A.; Lachmann, N.; et al. Clazakizumab in late antibody-mediated rejection: Study protocol of a randomized controlled pilot trial. Trials 2019, 20, 37. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Jia, J.; Yu, Z.; Duanmu, Z.; He, H.; Chen, S.; Qu, C. Inhibition of JAK2/STAT3/SOCS3 signaling attenuates atherosclerosis in rabbit. BMC Cardiovasc. Disord. 2020, 20, 133. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Liu, W.; Wang, W.; Fidler, T.; Woods, B.; Levine, R.L.; Tall, A.R.; Wang, N. Inhibition of JAK2 suppresses myelopoiesis and atherosclerosis in Apoe-/- mice. Cardiovasc. Drugs Ther. 2020, 34, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Edelmann, B.; Gupta, N.; Schnoeder, T.M.; Oelschlegel, A.M.; Shahzad, K.; Goldschmidt, J.; Philipsen, L.; Weinert, S.; Ghosh, A.; Saafeld, F.C.; et al. JAK2-V617F promotes venous thrombosis through β1/β2 integrin activation. J. Clin. Investig. 2018, 128, 4359–4371. [Google Scholar] [CrossRef]
- Cimmino, L.; Dolgalev, I.; Wang, Y.; Yoshimi, A.; Martin, G.H.; Wang, J.; Ng, V.; Xia, B.; Witkowski, M.T.; Mitchell-Flack, M.; et al. Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cell 2017, 170, 1079–1095. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Zhu, H.; Huang, J.; Zhu, Y.; Hong, M.; Zhu, H.; Zhang, J.; Li, S.; Yang, L.; Lian, Y.; et al. The synergy of Vitamin C with decitabine activates TET2 in leukemic cells and significantly improves overall survival in elderly patients with acute myeloid leukemia. Leuk. Res. 2018, 66, 1–7. [Google Scholar] [CrossRef]
- Das, A.B.; Kakadia, P.M.; Wojcik, D.; Pemberton, L.; Browett, P.J.; Bohlander, S.K.; Vissers, M.C.M. Clinical remission following ascorbate treatment in a case of acute myeloid leukemia with mutations in TET2 and WT1. Blood Cancer J. 2019, 9, 1234567890. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; LeRoith, D. Obesity and diabetes: The increased risk of cancer and cancer-related mortality. Physiol. Rev. 2015, 95, 727–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Hu, D.; Chen, H.; Shi, G.; Fetahu, I.S.; Wu, F.; Rabidou, K.; Fang, R.; Tan, L.; Xu, S.; et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature 2018, 559, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Lewis-McDougall, F.C.; Ruchaya, P.J.; Domenjo-Vila, E.; Shin Teoh, T.; Prata, L.; Cottle, B.J.; Clark, J.E.; Punjabi, P.P.; Awad, W.; Torella, D.; et al. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell 2019, 18, e12931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cianflone, E.; Torella, M.; Chimenti, C.; De Angelis, A.; Beltrami, A.P.; Urbanek, K.; Rota, M.; Torella, D. Adult cardiac stem cell aging: A reversible stochastic phenomenon? Oxidative Med. Cell. Longev. 2019, 2019, 5813147. [Google Scholar] [CrossRef] [Green Version]
- Cianflone, E.; Torella, M.; Biamonte, F.; De Angelis, A.; Urbanek, K.; Costanzo, F.S.; Rota, M.; Ellison-Hughes, G.M.; Torella, D. Targeting cardiac stem cell senescence to treat cardiac aging and disease. Cells 2020, 9, 1558. [Google Scholar] [CrossRef]
- Kim, D.H.; Bang, E.; Arulkumar, R.; Ha, S.; Chung, K.W.; Park, M.H.; Choi, Y.J.; Yu, B.P.; Chung, H.Y. Senoinflammation: A major mediator underlying age-related metabolic dysregulation. Exp. Gerontol. 2020, 134, 110891. [Google Scholar] [CrossRef]

| Clone | Cells that share identical genomes within a cellular population. |
| Clonality | Historically refers to myeloid and lymphoid neoplasms; today denotes a uniform population of cells that could be either wild-type or mutant and eventually malignant. |
| Driver mutation | Driver mutations confer a growth advantage to the cells carrying them, and are positively selected during the evolution of cancer. They occur in a particular set of genes called “cancer genes”, and are often the primary causative agent of cancer. |
| Passenger mutation | Passenger mutations are inert somatic mutations. They are unable to confer clonal growth advantages, and therefore do not lead to cancer development. Nevertheless, passenger mutations are usually found in cancer cells’ genomes because when a driver mutation occurs, even a passenger mutation is carried together with the clonal expansion. |
| Clonal hematopoiesis of indeterminate potential (CHIP) | Any clonal expansion of hematopoietic cells in a non-hematologic patient. It is characterized by
|
| EPIDEMIOLOGICAL/ CLINICAL STUDIES | |||
|---|---|---|---|
| STUDY/REFERENCE | C.H.I.P. STATUS | STUDY COHORT | MAIN FINDINGS |
| Jaiswal et al., 2014 | Variants in 160 genes associated with hematological neoplasms. | 17,182 subjects without hematological alterations. | CHIP is associated with increase in the risk of incident CHD and ischemic stroke. TET2, DNMT3A and ASXL1 mutations individually associated with CHD and ischemic stroke. |
| Jaywalk et al., 2017 | Variants in 74 genes associated with hematological neoplasms. | 4726 subjects with CHD and 3529 controls. | CHIP is associated with increased risk of CHD and early-onset MI. TET2, DNMT3, ASXL1 and JAK2 mutations individually associated with CHD and early-onset MI. |
| Dorsheimer et al., 2019 | Variants in 56 genes associated with hematological neoplasms. | 200 patients with HF following MI. | DNMT3A and TET2 carriers have increased death or HF re-hospitalization during a median follow-up of 4.4 years. |
| Mas-Peiro et al., 2019 | Variants in TET2 and DNMT3A. | 279 patients undergoing TAVI for severe aortic valve stenosis. | Patients with CHIP have increased all-cause mortality following successful TAVI during median follow-up of 9 months. |
| Bick et al., 2020 | Variants in TET2 and DNMT3A. | 35,416 subjects from UK Biobank without prevalent CVD. | CHIP is associated with increased risk of CVD. CHIP carriers with protective IL-6R variants have decreased CVD risk. |
| Abplanalp et al., 2020 | Variants in TET2 and DNMT3A. | 8 patients with severe aortic valve stenosis and 6 patients with HF. | CHIP carriers’ monocytes display a pro-inflammatory expression profile. |
| Wolach et al., 2018 | JAK2VF variant | 10,893 subjects without myeloid disorders. | JAK2VF mutations associated with increased risk of venous thrombosis. |
| ANIMAL MODEL STUDIES | |||
| STUDY | C.H.I.P. GENES | ANIMAL MODELS | MAIN FINDINGS |
| Fuster et al., 2017 | Tet2 | Competitive BM transplant with Tet2 -/- cells in irradiated ldlr -/- mice. HF/HC-induced atherosclerosis. | Tet2 deficiency increases atherosclerotic plaque size and total number of macrophages in the intima of the vascular wall. Tet2-deficient macrophages show increase in NLRP3 inflammasome-mediated IL-1β secretion. |
| Jaiswal et al., 2017 | Tet2 | Irradiated ldlr -/- mice transplanted with Tet2 -/+ or Tet2 -/- BM cells. HF/HC-induced atherosclerosis. | Tet2 deficiency increases atherosclerotic lesions in the aortic root and aorta. Tet2-deficient macrophages express more pro-inflammatory chemokines and cytokines. |
| Sano et al., 2018 | Tet2 | Competitive BM transplant with Tet2 -/- cells in irradiated mice or conditional myeloid-restricted inactivation of Tet2. HF alternatively induced by TAC or LAD ligation. | Tet2 deficiency worsens cardiac remodeling and function, and increases IL-1β expression. |
| Wang et al., 2020 | Tet2 | Non-preconditioned mice transplanted with Tet2 -/+ or Tet2 -/- BM cells. | Tet2 deficiency causes age-related hypertrophy and fibrosis. Donor-derived macrophages in the heart have increased inflammatory features. |
| Sano et al., 2018 | Tet2 and Dnmt3a | Irradiated mice transplanted with Tet2 -/- or Dnmt3a -/- CRISPR-edited HSPCs. Angiotensin-II-induced HF. | Tet2/Dnmt3a mutations cause increased cardiac hypertrophy and fibrosis, and reduction in cardiac function. |
| Wang et al., 2018 | Jak2 | Irradiated ldlr -/- mice transplanted with Jak2VF-expressing BM cells. HF/HC-induced atherosclerosis. | Jak2VF mutation increases early and advanced atherosclerosis, promoting neutrophil infiltration and plaque instability. Jak2VF macrophages show increased pro-inflammatory cytokines and chemokines. |
| Sano et al., 2019 | Jak2 | Irradiated mice transplanted with HSPCs expressing Jak2VF. HF alternatively induced by TAC or LAD ligation. | Jak2VF mutation causes HF associated with increased expression of IL-6 and IL-1β. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papa, V.; Marracino, L.; Fortini, F.; Rizzo, P.; Campo, G.; Vaccarezza, M.; Vieceli Dalla Sega, F. Translating Evidence from Clonal Hematopoiesis to Cardiovascular Disease: A Systematic Review. J. Clin. Med. 2020, 9, 2480. https://doi.org/10.3390/jcm9082480
Papa V, Marracino L, Fortini F, Rizzo P, Campo G, Vaccarezza M, Vieceli Dalla Sega F. Translating Evidence from Clonal Hematopoiesis to Cardiovascular Disease: A Systematic Review. Journal of Clinical Medicine. 2020; 9(8):2480. https://doi.org/10.3390/jcm9082480
Chicago/Turabian StylePapa, Veronica, Luisa Marracino, Francesca Fortini, Paola Rizzo, Gianluca Campo, Mauro Vaccarezza, and Francesco Vieceli Dalla Sega. 2020. "Translating Evidence from Clonal Hematopoiesis to Cardiovascular Disease: A Systematic Review" Journal of Clinical Medicine 9, no. 8: 2480. https://doi.org/10.3390/jcm9082480
APA StylePapa, V., Marracino, L., Fortini, F., Rizzo, P., Campo, G., Vaccarezza, M., & Vieceli Dalla Sega, F. (2020). Translating Evidence from Clonal Hematopoiesis to Cardiovascular Disease: A Systematic Review. Journal of Clinical Medicine, 9(8), 2480. https://doi.org/10.3390/jcm9082480







