Utility of Minimally Invasive Technology for Inguinal Lymph Node Dissection in Penile Cancer
Abstract
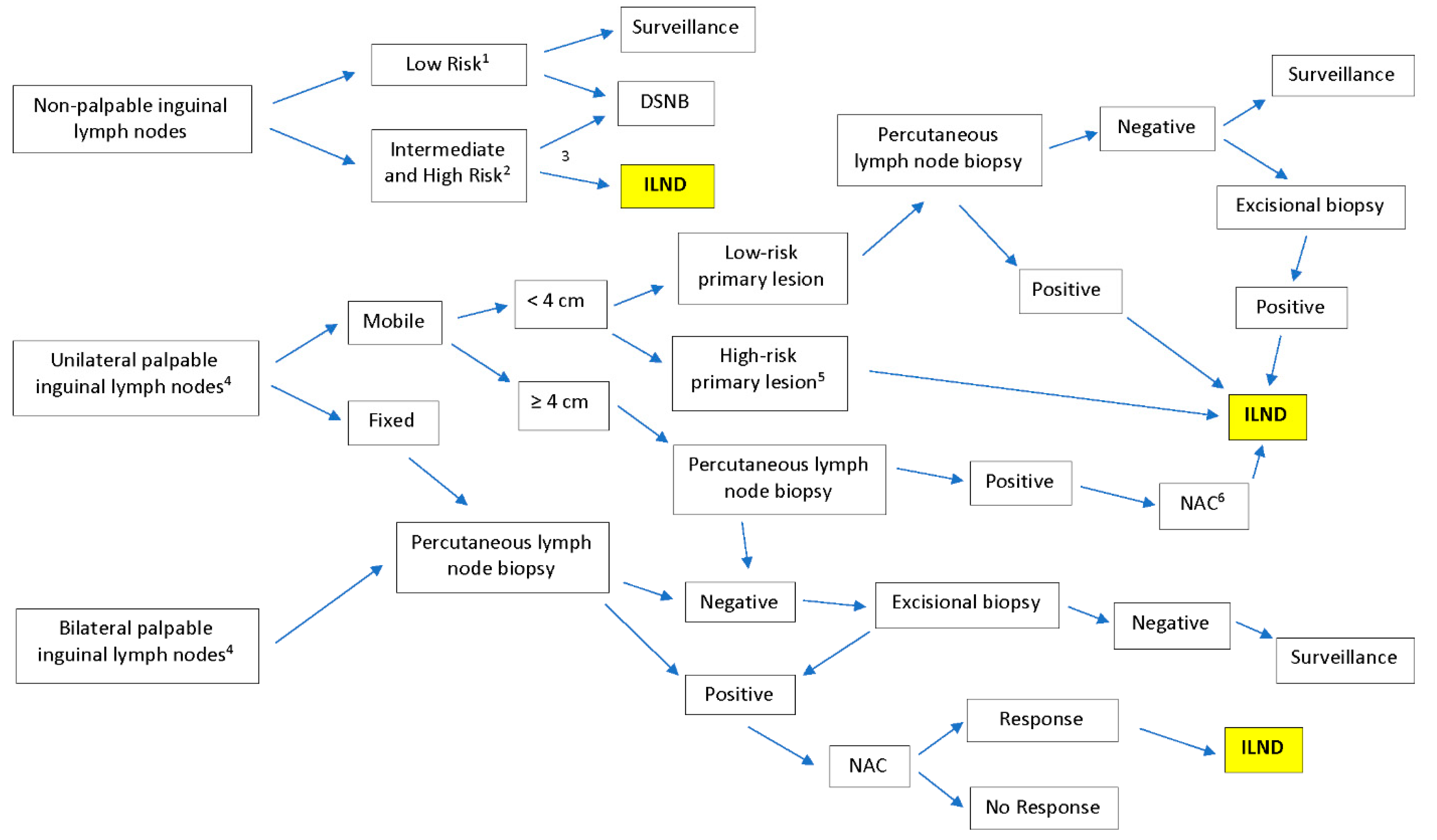
:1. Introduction
2. Materials and Methods
3. Results
3.1. Inguinal Lymph Node Dissection
3.2. Modifications of ILND Templates
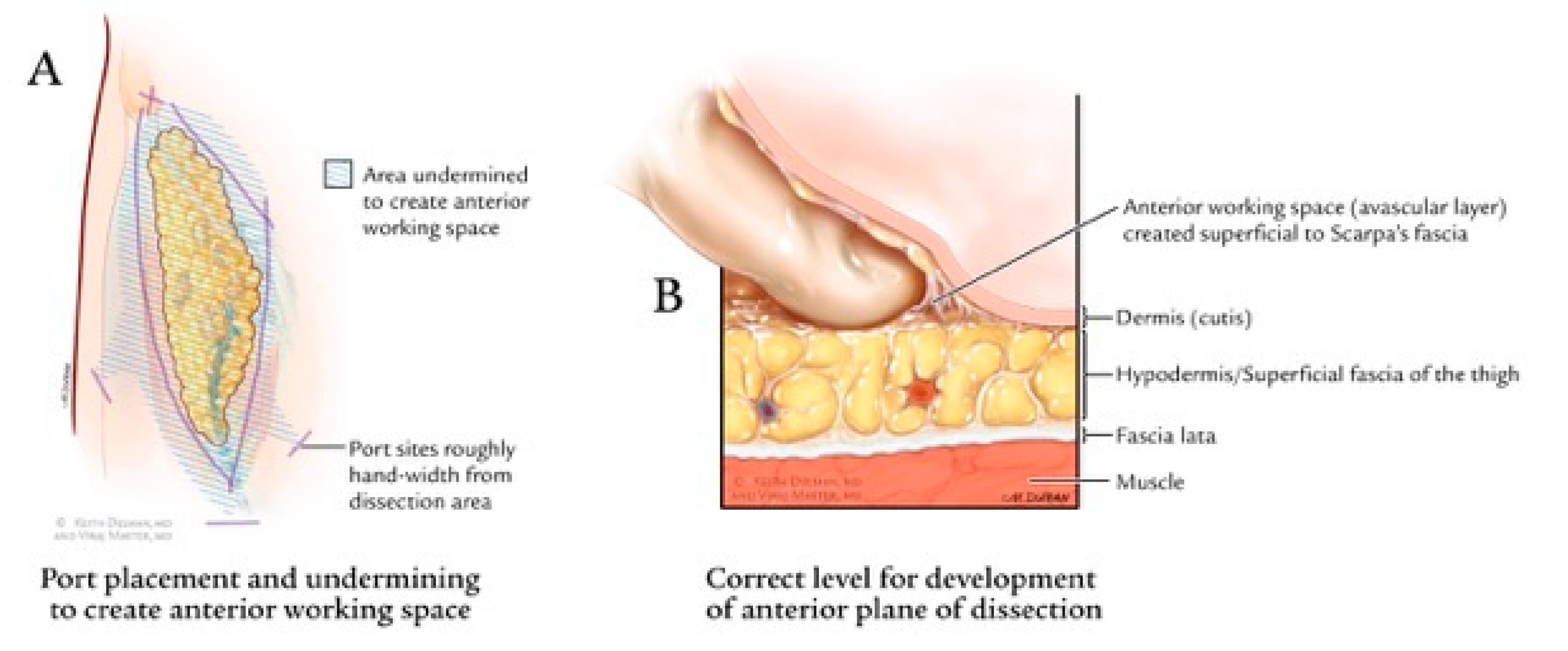
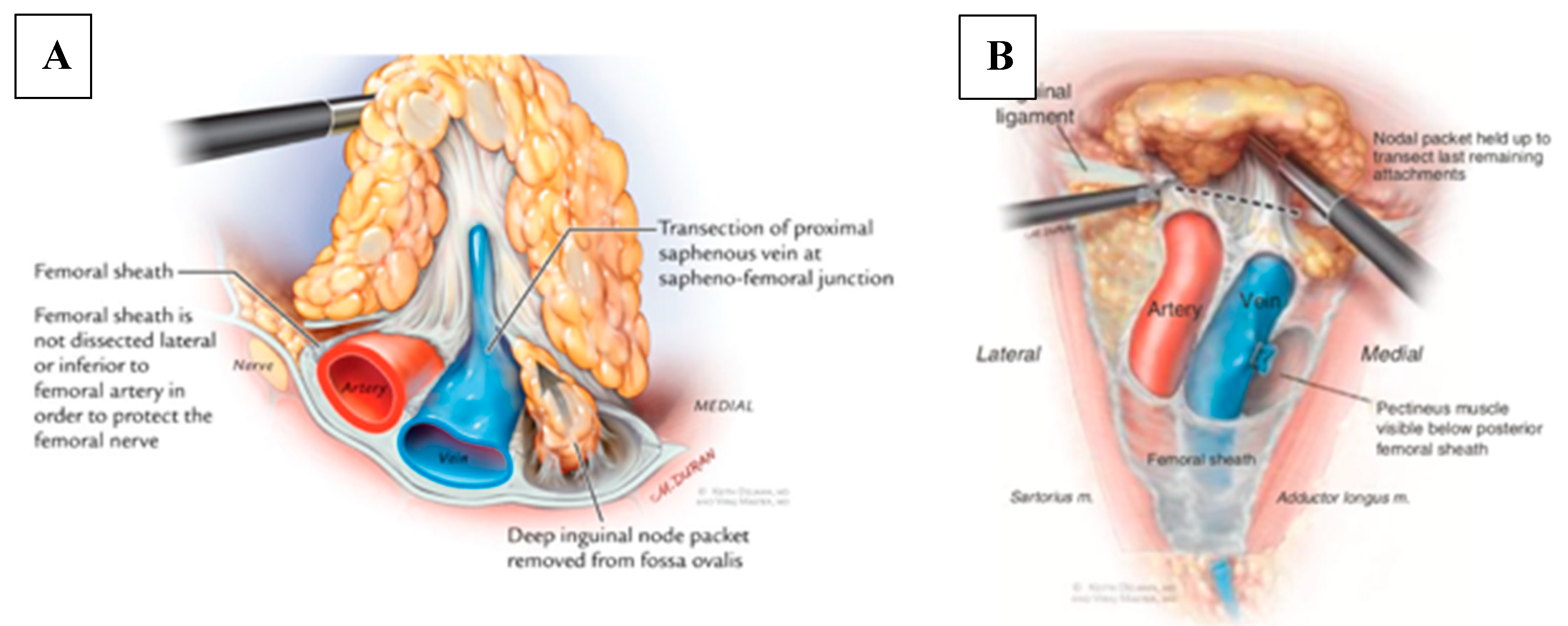
3.3. Minimally Invasive Approaches
3.4. Videoscopic Endoscopic Inguinal Lymphadenectomy (VEIL) Technique
3.5. Robotic Videoscopic Inguinal Lymphadenectomy (RVEIL) Technique
3.6. Post-Operative Care
3.7. Peri-Operative Outcomes and Complications
4. Oncological Outcomes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Author, (Year) | n of Patients (n of VEIL) | Histology | Blood Loss (mL) | Lymph Node Yield; Positive Nodes | Drain Removal in Days | Hospital Days | Post-Op Complications |
|---|---|---|---|---|---|---|---|
| VEIL Studies | |||||||
| Wang et al. (2017) [24] | 16 (19) | Penile cancer | Mean: 22.50; SD: 14.24 | Mean: 10.78 SD: 5.22; 11 positive nodes | Mean: 7.23; SD: 1.79 | Mean: 10.43; SD: 2.53 | Prolonged lymphedema 15.8% Wound infection 5.3% Skin necrosis 5.3% |
| Kumar and Sethia, (2017) [25] | 20 (33) | Penile cancer | n/a | Mean: 9.36; Mean Positive nodes: 1.24 | n/a | Mean: 2.5; Range: 0–14 | Wound complications 6% Prolonged lymphedema 3% Lymphocele 27% |
| Schwentner et al. (2013) [26] | 16 (28) | Penile: 14 patients Melanoma: 2 patients | n/a | Mean: 7.1; Range: 4–13; positive nodes Mean: 1.6 SD: 1.9 | n/a | n/a | Lymphatic complication 7.1% Post-operative hemorrhage 7.1% |
| Tobias-Machado et al. (2008) [29] | 15 (20) | Penile SCC | n/a | Mean: 10.8; Range: 7–16; 4 positive nodes | Mean: 4.9; Range: 3–12 | B/L VEIL Mean: 24 h; Range: 12–36 h VEIL/Open Mean: 6.4 days; Range: 5–10 days | Total: 20% Hematoma 5% Lymphocele 5% Lymphorrhea 5% Skin necrosis 5% |
| RVEIL Studies | |||||||
| Singh et al. (2018) [21] | 51 (102) | Penile SCC | n/a | Median: 13; IQR: 11–14.5; 21 patients with positive nodes | Median: 12 | Median: 3 | Total major: 2% Edge necrosis 9.8% Flap necrosis 2% |
| Russel et al. (2017) [20] | 14 (27) | Penile cancer | Median: 50.0 IQR 15.0–50.0 | Median: 8; IQR: 6–12; 22% of limbs had positive superficial LN 20% of limbs had positive deep LN | Median: 36.0; IQR: 24–48.5 | Median: 1; Range: 1–2 | Total: 21% Lymphocele 4% Cellulitis 4% Abscess 4% |
| Alhawat et al. (2016) [19] | 3 (6) | 2 Penile cancer, 1 Urethral cancer | Mean: 66.66; Range: 50–80 | Mean: left 18, right 14.6; 19 positive nodes, 3 patients | Mean: 44.6; Range: 34–72 | 3 | Total: 33.3% Lymphocele 33.3% |
| Matin et al. (2013) [23] | 10 (20) | Penile cancer | Median: 100; Range: 10–200 | Left Mean: 9; Range: 5–21 Right Mean: 9; Range: 6–17 11 positive nodes (2 patients) | n/a | n/a | Total: 60% Open conversion 20% Skin necrosis 10% Wound breakdown 10% Cellulitis 20% Abscess 10% |
| Open ILND | |||||||
| Tsaur et al. (2015) [15] | 29 (57 limited inguinal lymph node dissections) | Penile cancer | n/a | Mean: 8.1; SD: 3.7 | n/a | Mean: 14.2; SD: 6.1 | Total rate: 54% 15 major and 16 minor complications Wound infection 22.8% Leg edema 15.8% Seroma/lymphocele 10.5% Hematoma 1.8% Paresthesia 1.8% DVT 1.8% |
| Gopman et al. (2014) [17] | 327 (374 modified inguinal lymph node dissections) | Penile cancer | n/a | Left Mean: 7; Range: 0–26 Mean positive: 1; Range: 0–9 Right Mean: 7; Range: 0–24 Mean positive: 1; Range: 0–10 | Mean: 11; Range: 0–61 | Mean: 9.6; Range: 0–62 | Total: 55.4% of patients Major: 34.3% Minor: 65.7% |
| Koifman et al. (2013) [18] | 170 (340) | Penile cancer | n/a | Per groin: Mean: 10.9; Range: 6–19 | n/a | Mean: 6.4; Range: 4–27 | Total: 10.3% 10 major and 25 minor complications Lymphedema 4.1% Seroma 1.2% Scrotal edema 0.9% Skin edge necrosis 0.9% Lymphocele 0.9% Wound infection 0.6% Wound abscess 0.6% DVT 0.6% |
| Yao et al. (2009) [16] | 75 (150 modified inguinal lymph node dissections) | Penile cancer | n/a | n/a; 52% node positivity rate | n/a | n/a | Total: 37 complications Wound infection 1.4% Skin necrosis 4.7% Lymphedema 13.9% Lymphocele 2% DVT 0.7% |
| Kroon et al. (2005) [5] | 40 (n/A); 20 patients underwent resection upon discovery of palpable nodes during physical exam (delayed), 20 underwent DSNB prior to development of palpable nodes (early) | Penile cancer (T2–3) | n/a | n/a; node positivity per groin: Early Group Mean: 1.6; Range: 1–5 Delayed Group Mean: 2.1; Range: 1–6 | n/a | n/a | n/a |
References
- Koh, W.J.; Greer, B.E.; Abu-Rustum, N.R.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; Dizon, D.S.; et al. Vulvar cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J. Nat. Compr. Cancer Netw. 2017, 15, 92–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, P.E.; Spiess, P.E.; Agarwal, N.; Biagioli, M.C.; Eisenberger, M.A.; Greenberg, R.E.; Herr, H.W.; Inman, B.A.; Kuban, D.A.; Kuzel, T.M.; et al. Penile cancer. J. Nat. Compr. Cancer Netw. 2013, 11, 594–615. [Google Scholar] [CrossRef] [PubMed]
- Spiess, P.E. New treatment guidelines for penile cancer. J. Nat. Compr. Cancer Netw. 2013, 11, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Akduman, B.; Bouchot, O.; Palou, J.; Tobias-Machado, M. Prognostic factors in penile cancer. Urology 2010, 76, S66–S73. [Google Scholar] [CrossRef] [PubMed]
- Kroon, B.K.; Horenblas, S.; Lont, A.P.; Tanis, P.J.; Gallee, M.P.W.; Nieweg, O.E. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastasis. J. Urol. 2005, 173, 816–819. [Google Scholar] [CrossRef] [PubMed]
- McDougal, W.S. Preemptive lymphadenectomy markedly improves survival in patients with cancer of the penis who harbor occult metastases. J. Urol. 2005, 173, 681. [Google Scholar] [CrossRef] [PubMed]
- Spiess, P.E.; Hernandez, M.S.; Pettaway, C.A. Contemporary inguinal lymph node dissection: Minimizing complications. World J. Urol. 2008, 27, 205. [Google Scholar] [CrossRef]
- Wills, A.; Obermair, A. A review of complications associated with the surgical treatment of vulvar cancer. Gynecol. Oncol. 2013, 131, 467–479. [Google Scholar] [CrossRef]
- Chang, S.B.; Askew, R.L.; Xing, Y.; Weaver, S.; Gershenwald, J.E.; Lee, J.E.; Royal, R.; Lucci, A.; Ross, M.I.; Cormier, J.N. Prospective assessment of postoperative complications and associated costs following inguinal lymph node dissection (ILND) in melanoma patients. Ann. Surg. Oncol. 2010, 17, 2764–2772. [Google Scholar] [CrossRef]
- Woldu, S.L.; Ci, B.; Hutchinson, R.C.; Krabbe, L.-M.; Singla, N.; Passoni, N.M.; Clinton, T.N.; Raj, G.V.; Miller, D.S.; Sagalowsky, A.I.; et al. Usage and survival implications of surgical staging of inguinal lymph nodes in intermediate- to high-risk, clinical localized penile cancer: A propensity-score matched analysis. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 159.e7–159.e17. [Google Scholar] [CrossRef]
- Pettaway, C.A.; Pisters, L.L.; Dinney, C.P.N.; Jularbal, F.; Swanson, D.A.; von Eschenbach, A.C.; Ayala, A. Sentinel lymph node dissection for penile carcinoma: The M.D. Anderson Cancer Center experience. J. Urol. 1995, 154, 1999–2003. [Google Scholar] [CrossRef]
- Crawshaw, J.W.; Hadway, P.; Hoffland, D.; Bassingham, S.; Corbishley, C.M.; Smith, Y.; Pilcher, J.; Allan, R.; Watkin, N.A.; Heenan, S.D. Sentinel lymph node biopsy using dynamic lymphoscintigraphy combined with ultrasound-guided fine needle aspiration in penile carcinoma. BJR 2009, 8248, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Galfano, A. Should the dynamic sentinel node biopsy (DSNB) be considered the gold standard in the evaluation of lymph node status in patients with penile carcinoma? Eur. Urol. 2007, 1, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Catalona, W.J. Modified inguinal lymphadenectomy for carcinoma of the penis with preservation of saphenous veins: Technique and preliminary results. J. Urol. 1988, 140, 306–310. [Google Scholar] [CrossRef]
- Tsaur, I.; Biegel, C.; Gust, K.; Huesch, T.; Borgmann, H.; Brandt, M.P.J.K.; Kurosch, M.; Reiter, M.; Bartsch, G.; Schilling, D.; et al. Feasibility, complications and oncologic results of a limited inguinal lymph node dissection in the management of penile cancer. Int. Braz. J. Urol. 2015, 41, 486–495. [Google Scholar] [CrossRef]
- Yao, K.; Tu, H.; Li, Y.-H.; Qin, Z.-K.; Liu, Z.-W.; Zhou, F.-J.; Han, H. Modified technique of radical inguinal lymphadenectomy for penile carcinoma: Morbidity and outcome. J. Urol. 2010, 184, 546–552. [Google Scholar] [CrossRef]
- Gopman, J.M.; Djajadiningrat, R.S.; Baumgarten, A.S.; Espiritu, P.N.; Horenblas, S.; Zhu, Y.; Protzel, C.; Pow-Sang, J.M.; Kim, T.; Sexton, W.J.; et al. Predicting postoperative complications of inguinal lymph node dissection for penile cancer in an international multicentre cohort. BJU Int. 2015, 116, 196–201. [Google Scholar] [CrossRef]
- Koifman, L.; Hampl, D.; Koifman, N.; Vides, A.J.; Ornellas, A.A. Radical open inguinal lymphadenectomy for penile carcinoma: Surgical technique, early complications and late outcomes. J. Urol. 2013, 190, 2086–2092. [Google Scholar] [CrossRef]
- Ahlawat, R.; Khera, R.; Gautam, G.; Kumar, A. Robot-assisted simultaneous bilateral radical inguinal lymphadenectomy along with robotic bilateral pelvic lymphadenectomy: A feasibility study. J. Laparoendosc. Adv. Surg. Tech. 2016, 26, 845–849. [Google Scholar] [CrossRef]
- Russell, C.M.; Salami, S.; Niemann, A.; Weizer, A.Z.; Tomlins, S.; Morgan, T.M.; Montgomery, J.S. Minimally invasive inguinal lymphadenectomy in the management of penile carcinoma. Urology 2017, 106, 113–118. [Google Scholar] [CrossRef]
- Singh, A.; Jaipuria, J.; Goel, A.; Shah, S.; Bhardwaj, R.; Baidya, S.; Jain, J.; Jain, C.; Rawal, S. Comparing outcomes of robotic and open inguinal lymph node dissection in patients with carcinoma of the penis. J. Urol. 2018, 199, 1518–1525. [Google Scholar] [CrossRef]
- Herrel, A.L.; Butterworth, R.M.; Jafri, S.M.; Ying, C.; Delman, A.K.; Kooby, D.A.; Ogan, E.K.; Canter, D.J.; Master, V.A. Bilateral endoscopic inguinofemoral lymphadenectomy using simultaneous carbon dioxide insufflation: An initial report of a novel approach. Can. J. Urol. 2012, 19, 6306–6309. [Google Scholar] [PubMed]
- Matin, S.; Cormier, J.N.; Ward, J.; Pisters, L.L.; Wood, C.G.; Dinney, C.P.N.; Royal, R.E.; Huang, X.; Pettaway, C.A. Phase 1 prospective evaluation of the oncological adequacy of robotic assisted video-endoscopic inguinal lymphadenectomy in patients with penile carcinoma. BJU Int. 2013, 111, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Du, P.; Tang, X.; An, C.; Zhang, N.; Yang, Y. Comparison of efficiency of video endoscopy and open inguinal lymph node dissection. Anticancer Res. 2017, 37, 4623–4628. [Google Scholar]
- Kumar, V.; Sethia, K.K. Prospective study comparing video-endoscopic radical inguinal lymph node dissection (VEILND) with open radical ILND (OILND) for penile cancer over an 8-year period. BJU Int. 2017, 119, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Schwentner, C.; Todenhöfer, T.; Seibold, J.; Alloussi, S.H.; Mischinger, J.; Aufderklamm, S.; Stenzl, A.; Gakis, G. Endoscopic inguinofemoral lymphadenectomy—Extended follow-up. J. Endourol. 2012, 27, 497–503. [Google Scholar] [CrossRef]
- Gkegkes, I.D.; Minis, E.E.; Iavazzo, C. Robotic-assisted inguinal lymphadenectomy: A systematic review. J. Robot. Surg. 2019, 13, 1–8. [Google Scholar] [CrossRef]
- Kharadjian, T.B.; Matin, S.F.; Pettaway, C.A. Early experience of robotic-assisted inguinal lymphadenectomy: Review of surgical outcomes relative to alternative approaches. Curr. Urol. Rep. 2014, 15, 412. [Google Scholar] [CrossRef]
- Tobias-Machado, M.; Tavares, A.; Ornellas, A.A.; Molina, W.R.; Juliano, R.V.; Wroclawski, E.R. Video endoscopic inguinal lymphadenectomy: A new minimally invasive procedure for radical management of inguinal nodes in patients with penile squamous cell carcinoma. J. Urol. 2007, 177, 953–958. [Google Scholar] [CrossRef]
- Johnson, T.V.; Hsiao, W.; Delman, K.A.; Jani, A.B.; Brawley, O.W.; Master, V.A. Extensive inguinal lymphadenectomy improves overall 5-year survival in penile cancer patients. Cancer 2010, 116, 2960–2966. [Google Scholar] [CrossRef]
- Leijte, J.A.P.; Kirrander, P.; Antonini, N.; Windahl, T.; Horenblas, S. Recurrence patterns of squamous cell carcinoma of the penis: Recommendations for follow-up based on a two-centre analysis of 700 patients. Eur. Urol. 2008, 54, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Graafland, N.M.; Moonen, L.M.; Van Boven, H.H.; Van Werkhoven, E.; Kerst, J.M.; Horenblas, S. Inguinal recurrence following therapeutic lymphadenectomy for node positive penile carcinoma: Outcome and implications for management. J. Urol. 2011, 185, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, H.; Cui, Y.; Liu, P.; Zhou, X.; Liu, L.; Chen, H.; Chen, J.; Zu, X. Comparison of clinical feasibility and oncological outcomes between video endoscopic and open inguinal lymphadenectomy for penile cancer: A systematic review and meta-analysis. Medicine 2019, 98, e15862. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabavizadeh, R.; Petrinec, B.; Necchi, A.; Tsaur, I.; Albersen, M.; Master, V. Utility of Minimally Invasive Technology for Inguinal Lymph Node Dissection in Penile Cancer. J. Clin. Med. 2020, 9, 2501. https://doi.org/10.3390/jcm9082501
Nabavizadeh R, Petrinec B, Necchi A, Tsaur I, Albersen M, Master V. Utility of Minimally Invasive Technology for Inguinal Lymph Node Dissection in Penile Cancer. Journal of Clinical Medicine. 2020; 9(8):2501. https://doi.org/10.3390/jcm9082501
Chicago/Turabian StyleNabavizadeh, Reza, Benjamin Petrinec, Andrea Necchi, Igor Tsaur, Maarten Albersen, and Viraj Master. 2020. "Utility of Minimally Invasive Technology for Inguinal Lymph Node Dissection in Penile Cancer" Journal of Clinical Medicine 9, no. 8: 2501. https://doi.org/10.3390/jcm9082501





