Association between Proinflammatory Markers, Leukocyte–Endothelium Interactions, and Carotid Intima–Media Thickness in Type 2 Diabetes: Role of Glycemic Control
Abstract
:1. Introduction
2. Experimental Section
2.1. Human Subjects
2.2. Sample Collection
2.3. Laboratory Tests
2.4. Leukocyte Isolation
2.5. Soluble Cytokines and Adhesion Molecule Assay
2.6. Static Cytometry Measurements
2.7. PMN–Endothelium Interaction Assay
2.8. Assessment of Carotid Intima–Media Thickness (CIMT)
2.9. Statistical Analysis
3. Results
3.1. Anthropometric and Biochemical Parameters
3.2. Inflammation Markers
3.3. PMN–Endothelium Interactions
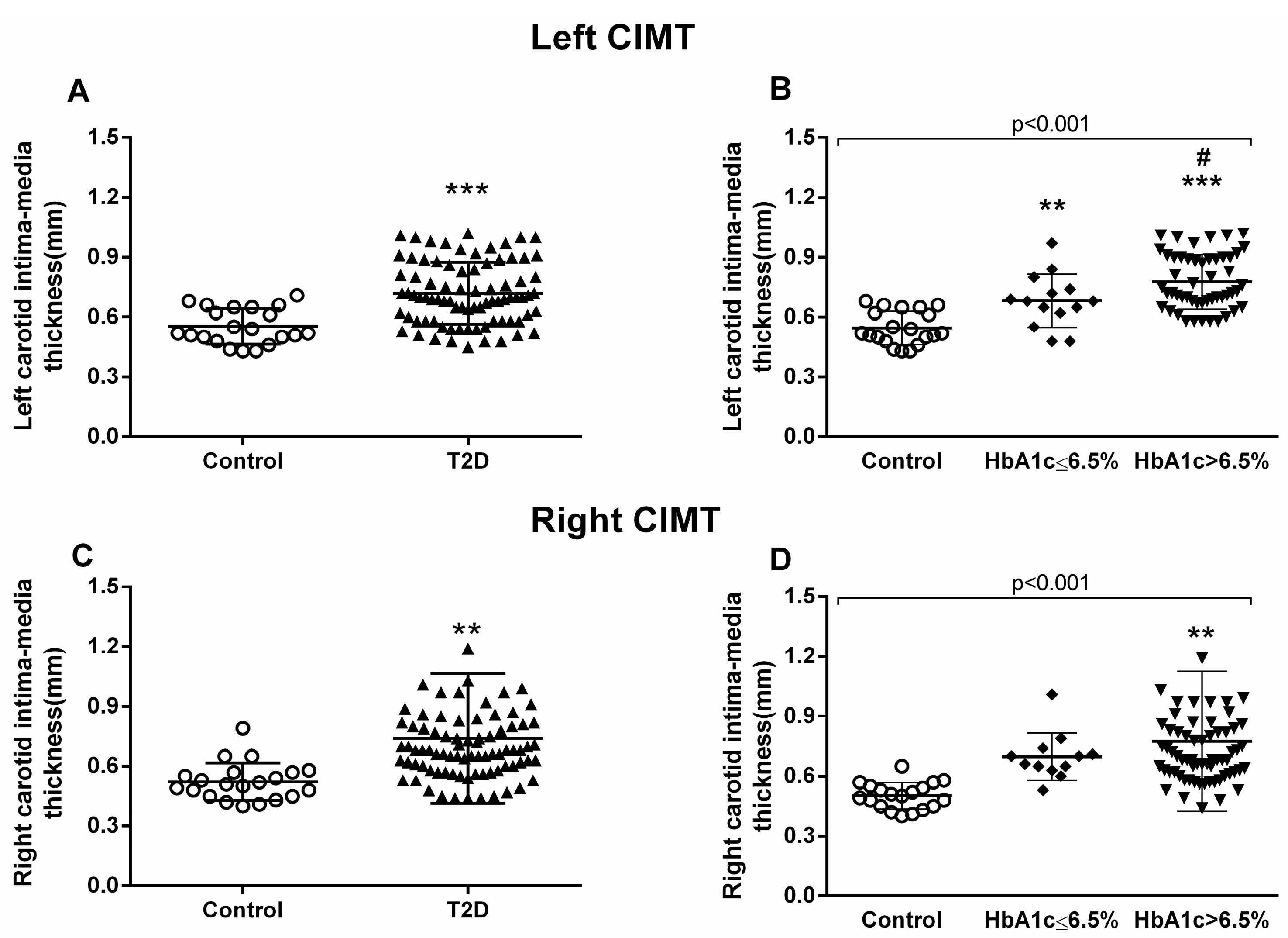
3.4. Carotid Intima–Media Thickness Measurements
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| AGE | Advanced glycation end products |
| AIP | Atherogenic index of plasma |
| CIMT | Carotid intima–media thickness |
| CT | Cholesterol |
| DAPI | 4′6′Diamidin-2-fenilindol |
| DBP | Diastolic blood pressure |
| HbA1c | Glycated hemoglobin |
| HBSS | Hanks’ Balanced Salt Solution |
| HDLc | High-density lipoprotein cholesterol |
| HOMA-IR | Homeostatic model assessment–insulin resistance |
| HUVEC | Human umbilical cord vein endothelial cell |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IL-6 | Interleukin 6 |
| IR | Insulin resistance |
| LDLc | Low-density lipoprotein cholesterol |
| PMN | Polymorphonuclear cells |
| ROS | Radical oxygen species |
| RPMI | Roswell Park Memorial Institute culture medium |
| SBP | Systolic blood pressure |
| TG | Triglycerides |
| TNFα | Tumor necrosis factor alpha |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VLDLc | Very low-density lipoprotein cholesterol |
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Pan, X.-F.; Chen, J.; Xia, L.; Cao, A.; Zhang, Y.; Wang, J.; Li, H.; Yang, K.; Guo, K.; et al. Combined lifestyle factors and risk of incident type 2 diabetes and prognosis among individuals with type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. Diabetologia 2020, 63, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Stirban, A.O.; Tschoepe, D. Cardiovascular complications in diabetes: Targets and interventions. Diabetes Care 2008, 31 (Suppl. 2), S215–S221. [Google Scholar] [CrossRef] [Green Version]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and Management of Type 2 Diabetes: Dietary Components and Nutritional Strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; E Grobbee, D.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41 (Suppl. 1), S55–S64. [Google Scholar] [CrossRef] [Green Version]
- Laiteerapong, N.; A Ham, S.; Gao, Y.; Moffet, H.H.; Liu, J.Y.; Huang, E.S.; Karter, A.J. The Legacy Effect in Type 2 Diabetes: Impact of Early Glycemic Control on Future Complications (The Diabetes & Aging Study). Diabetes Care 2019, 42, 416–426. [Google Scholar]
- Moodahadu, L.S.; Dhall, R.; Zargar, A.H.; Bangera, S.; Ramani, L.; Katipally, R. Tight Glycemic Control and Cardiovascular Effects in Type 2 Diabetic Patients. Heart Views 2014, 15, 111–120. [Google Scholar] [CrossRef]
- Hill, D.; Fisher, M. The effect of intensive glycaemic control on cardiovascular outcomes. Diabetes Obes. Metab. 2010, 12, 641–647. [Google Scholar] [CrossRef]
- Punthakee, Z.; Miller, M.E.; Simmons, D.L.; Riddle, M.C.; Ismail-Beigi, F.; Brillon, D.J.; Bergenstal, R.M.; Savage, P.J.; Hramiak, I.; Largay, J.F.; et al. Durable change in glycaemic control following intensive management of type 2 diabetes in the ACCORD clinical trial. Diabetologia 2014, 57, 2030–2037. [Google Scholar] [CrossRef] [Green Version]
- Boussageon, R.; Bejan-Angoulvant, T.; Saadatian-Elahi, M.; Lafont, S.; Bergeonneau, C.; Kassaï, B.; Erpeldinger, S.; Wright, J.M.; Gueyffier, F.; Cornu, C. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: Meta-analysis of randomised controlled trials. BMJ 2011, 343, d4169. [Google Scholar] [CrossRef] [Green Version]
- ADVANCE Collaborative Group; Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot, L.; Woodward, M.; Marre, M.; Cooper, M.; Glasziou, P.; et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar] [CrossRef] [Green Version]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef]
- Cruz, N.G.; Sousa, L.P.; Sousa, M.O.; Pietrani, N.T.; Fernandes, A.P.; Gomes, K.B. The linkage between inflammation and Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2013, 99, 85–92. [Google Scholar] [CrossRef]
- Valentine, W.; Palmer, A.; Nicklasson, L.; Cobden, D.; Roze, S. Improving life expectancy and decreasing the incidence of complications associated with type 2 diabetes: A modelling study of HbA1c targets. Int. J. Clin. Pract. 2006, 60, 1138–1145. [Google Scholar] [CrossRef]
- Lainampetch, J.; Panprathip, P.; Phosat, C.; Chumpathat, N.; Prangthip, P.; Soonthornworasiri, N.; Puduang, S.; Wechjakwen, N.; Kwanbunjan, K. Association of Tumor Necrosis Factor Alpha, Interleukin 6, and C-Reactive Protein with the Risk of Developing Type 2 Diabetes: A Retrospective Cohort Study of Rural Thais. J. Diabetes Res. 2019, 2019, 9051929. [Google Scholar] [CrossRef] [Green Version]
- Fadaei, R.; Bagheri, N.; Heidarian, E.; Nouri, A.; Hesari, Z.; Moradi, N.; Ahmadi, A.; Ahmadi, R. Serum levels of IL-32 in patients with type 2 diabetes mellitus and its relationship with TNF-α and IL-6. Cytokine 2020, 125, 154832. [Google Scholar] [CrossRef]
- Donath, M.Y.; Dinarello, C.A.; Mandrup-Poulsen, T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat. Rev. Immunol. 2019, 19, 734–746. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; De Marañon, A.M.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Llabata, V.; Torres, I.R.; et al. Relationship between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [Green Version]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.-Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 2011, 208, 519–533. [Google Scholar] [CrossRef]
- Ungvari, Z.; Orosz, Z.; Labinskyy, N.; Rivera, A.; Xiangmin, Z.; Smith, K.; Csiszar, A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H37–H47. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Xia, L.; Goldberg, H.; Lee, K.W.; Quaggin, S.E.; Fantus, I.G. Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. J. Biol. Chem. 2013, 288, 6835–6848. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Yang, B.; Fritsche, K.L.; Beversdorf, D.Q.; Gu, Z.; Lee, J.C.; Folk, W.R.; Greenlief, C.M.; Sun, G.Y. Yin-Yang Mechanisms Regulating Lipid Peroxidation of Docosahexaenoic Acid and Arachidonic Acid in the Central Nervous System. Front. Neurol. 2019, 10, 642. [Google Scholar] [CrossRef]
- Fischer, R.; Maier, O. Interrelation of Oxidative Stress and Inflammation in Neurodegenerative Disease: Role of TNF. Oxid. Med. Cell. Longev. 2015, 2015, 610813. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Zhuang, X.; Huang, Z.; Zou, J.; Yang, D.; Hu, X.; Du, Z.; Wang, L.; Liao, X. Klotho protects the heart from hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated inflammation both in vitro and in vivo. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 238–251. [Google Scholar] [CrossRef]
- Luo, B.; Li, B.; Wang, W.; Liu, X.; Xia, Y.; Zhang, C.; Zhang, M.; Zhang, Y.; An, F. NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS ONE 2014, 9, e104771. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Chen, X.; Zong, B.; Yuan, H.; Wang, Z.; Wei, Y.; Wang, X.; Liu, G.; Zhang, J.; Li, S.; et al. Gypenosides improve diabetic cardiomyopathy by inhibiting ROS-mediated NLRP3 inflammasome activation. J. Cell. Mol. Med. 2018, 22, 4437–4448. [Google Scholar] [CrossRef] [Green Version]
- Le Rossignol, S.; Ketheesan, N.; Haleagrahara, N. Redox-sensitive transcription factors play a significant role in the development of rheumatoid arthritis. Int. Rev. Immunol. 2018, 37, 129–143. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, K.A.; Papavassiliou, A.G. Redox and NF-κB signaling in osteoarthritis. Free Radic. Biol. Med. 2019, 132, 90–100. [Google Scholar] [CrossRef]
- Pradhan, A.; Bagchi, A.; De, S.; Mitra, S.; Mukherjee, S.; Ghosh, P.; Ghosh, A.; Chatterjee, M. Role of redox imbalance and cytokines in mediating oxidative damage and disease progression of patients with rheumatoid arthritis. Free Radic. Res. 2019, 53, 768–779. [Google Scholar] [CrossRef]
- Duecker, R.; Baer, P.C.; Eickmeier, O.; Strecker, M.; Kurz, J.; Schaible, A.; Henrich, D.; Zielen, S.; Schubert, R. Oxidative stress-driven pulmonary inflammation and fibrosis in a mouse model of human ataxia-telangiectasia. Redox Biol. 2018, 14, 645–655. [Google Scholar] [CrossRef]
- Nandi, A.; Bishayi, B. CCR-2 neutralization augments murine fresh BMC activation by Staphylococcus aureus via two distinct mechanisms: At the level of ROS production and cytokine response. Innate Immun. 2017, 23, 345–372. [Google Scholar] [CrossRef] [Green Version]
- Diehl, A.M. Cytokine regulation of liver injury and repair. Immunol. Rev. 2000, 174, 160–171. [Google Scholar] [CrossRef]
- Han, S.; Cai, W.; Yang, X.; Jia, Y.; Zheng, Z.; Wang, H.; Li, J.; Li, Y.; Gao, J.; Fan, L.; et al. ROS-Mediated NLRP3 Inflammasome Activity Is Essential for Burn-Induced Acute Lung Injury. Mediat. Inflamm. 2015, 2015, 720457. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.-T.; Yang, C.-M. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem. Pharmacol. 2012, 84, 581–590. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef] [Green Version]
- Ouedraogo, R.; Gong, Y.; Berzins, B.; Wu, X.; Mahadev, K.; Hough, K.; Chan, L.; Goldstein, B.J.; Scalia, R. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J. Clin. Investig. 2007, 117, 1718–1726. [Google Scholar] [CrossRef]
- Yan, Y.; Li, S.; Liu, Y.; Bazzano, L.; He, J.; Mi, J.; Chen, W. Temporal relationship between inflammation and insulin resistance and their joint effect on hyperglycemia: The Bogalusa Heart Study. Cardiovasc. Diabetol. 2019, 18, 109. [Google Scholar] [CrossRef] [Green Version]
- Galkina, E.; Ley, K. Immune and Inflammatory Mechanisms of Atherosclerosis. Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef] [Green Version]
- Legein, B.; Temmerman, L.; Biessen, E.A.L.; Lutgens, E. Inflammation and immune system interactions in atherosclerosis. Cell. Mol. Life Sci. 2013, 70, 3847–3869. [Google Scholar] [CrossRef]
- Kavousi, M.; Elias-Smale, S.; Rutten, J.H.; Leening, M.J.G.; Vliegenthart, R.; Verwoert, G.C.; Krestin, G.P.; Oudkerk, M.; De Maat, M.P.; Leebeek, F.W.; et al. Evaluation of newer risk markers for coronary heart disease risk classification: A cohort study. Ann. Intern. Med. 2012, 156, 438–444. [Google Scholar] [CrossRef]
- Lau, K.K.; Wong, Y.-K.; Chan, Y.-H.; Yiu, K.-H.; Teo, K.C.; Li, L.S.-W.; Ho, S.-L.; Chan, K.H.; Siu, C.-W.; Tse, H.-F. Prognostic implications of surrogate markers of atherosclerosis in low to intermediate risk patients with type 2 diabetes. Cardiovasc. Diabetol. 2012, 11, 101. [Google Scholar] [CrossRef] [Green Version]
- Ortega, E.; Gilabert, R.; Núñez, I.; Cofán, M.; Sala-Vila, A.; De Groot, E.; Ros, E. White blood cell count is associated with carotid and femoral atherosclerosis. Atherosclerosis 2012, 221, 275–281. [Google Scholar] [CrossRef]
- Blüher, M.; Unger, R.; Rassoul, F.; Richter, V.; Paschke, R. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or Type II diabetes. Diabetologia 2002, 45, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Jude, E.B.; Douglas, J.T.; Anderson, S.G.; Young, M.J.; Boulton, A.J.M. Circulating cellular adhesion molecules ICAM-1, VCAM-1, P- and E-selectin in the prediction of cardiovascular disease in diabetes mellitus. Eur. J. Intern. Med. 2002, 13, 185–189. [Google Scholar] [CrossRef]
- Pankow, J.S.; Decker, P.A.; Berardi, C.; Hanson, N.Q.; Sale, M.; Tang, W.; Kanaya, A.M.; Larson, N.B.; Tsai, M.; Wassel, C.L.; et al. Circulating cellular adhesion molecules and risk of diabetes: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabet. Med. 2016, 33, 985–991. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Cai, X.; Liu, J.; Yang, B.; Zügel, M.; Steinacker, J.M.; Sun, Z.; Schumann, U. Association between circulating cell adhesion molecules and risk of type 2 diabetes: A meta-analysis. Atherosclerosis 2019, 287, 147–154. [Google Scholar] [CrossRef]
- Nguyen, P.A.; Won, J.S.; Rahman, M.K.; Bae, E.J.; Cho, M.K. Modulation of Sirt1/NF-κB interaction of evogliptin is attributed to inhibition of vascular inflammatory response leading to attenuation of atherosclerotic plaque formation. Biochem. Pharmacol. 2019, 168, 452–464. [Google Scholar] [CrossRef]
- Sardu, C.; De Lucia, C.; Wallner, M.; Santulli, G. Diabetes Mellitus and Its Cardiovascular Complications: New Insights into an Old Disease. J. Diabetes Res. 2019, 2019, 1905194. [Google Scholar] [CrossRef] [Green Version]
- Rangel, É.B.; Rodrigues, C.O.; de Sá, J.R. Micro- and Macrovascular Complications in Diabetes Mellitus: Preclinical and Clinical Studies. J Diabetes Res. 2019, 2019, 2161085. [Google Scholar] [CrossRef] [Green Version]
- Golden, S.H.; Folsom, A.R.; Coresh, J.; Sharrett, A.R.; Szklo, M.; Brancati, F. Risk factor groupings related to insulin resistance and their synergistic effects on subclinical atherosclerosis: The atherosclerosis risk in communities study. Diabetes 2002, 51, 3069–3076. [Google Scholar] [CrossRef] [Green Version]
- Tabatabaei-Malazy, O.; Fakhrzadeh, H.; Sharifi, F.; Mirarefin, M.; Arzaghi, S.M.; Badamchizadeh, Z.; Alizadeh-Khoei, M.; Larijani, B. Effect of metabolic control on oxidative stress, subclinical atherosclerosis and peripheral artery disease in diabetic patients. J. Diabetes Metab. Disord. 2015, 14, 84. [Google Scholar] [CrossRef] [Green Version]
- Alharby, H.; Abdelati, T.; Rizk, M.; Youssef, E.; Moghazy, K.; Gaber, N.; Yafei, S. Association of lipid peroxidation and interleukin-6 with carotid atherosclerosis in type 2 diabetes. Cardiovasc. Endocrinol. Metab. 2019, 8, 73–76. [Google Scholar] [CrossRef]
- Bauer, M.; Caviezel, S.; Teynor, A.; Erbel, R.; Mahabadi, A.A.; Schmidt-Trucksäss, A. Carotid intima-media thickness as a biomarker of subclinical atherosclerosis. Swiss Med. Wkly. 2012, 142, w13705. [Google Scholar] [CrossRef]
- Abd El Dayem, S.M.; Battah, A.A.; El Bohy, A.E.M. Assessment of Increase in Aortic and Carotid Intimal Medial Thickness in Type 1 Diabetic Patients. Open Access Maced. J. Med. Sci. 2016, 4, 630. [Google Scholar] [CrossRef] [Green Version]
- Pignoli, P.; Tremoli, E.; Poli, A.; Oreste, P.; Paoletti, R. Intimal plus medial thickness of the arterial wall: A direct measurement with ultrasound imaging. Circulation 1986, 74, 1399–1406. [Google Scholar] [CrossRef] [Green Version]
- Santos, I.S.; Bittencourt, M.S.; Goulart, A.C.; Schmidt, M.I.; Diniz, M.D.F.H.S.; Lotufo, P.A.; Benseñor, I.M. Insulin resistance is associated with carotid intima-media thickness in non-diabetic subjects. A cross-sectional analysis of the ELSA-Brasil cohort baseline. Atherosclerosis 2017, 260, 34–40. [Google Scholar] [CrossRef]
- Jeevarethinam, A.; Venuraju, S.; Dumo, A.; Ruano, S.; Mehta, V.S.; Rosenthal, M.; Nair, D.; Cohen, M.; Darko, D.; Lahiri, A.; et al. Relationship between carotid atherosclerosis and coronary artery calcification in asymptomatic diabetic patients: A prospective multicenter study. Clin. Cardiol. 2017, 40, 752–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Leary, D.H.; Polak, J.F.; Kronmal, R.A.; Manolio, T.A.; Burke, G.L.; Wolfson, S.K. Carotid-Artery Intima and Media Thickness as a Risk Factor for Myocardial Infarction and Stroke in Older Adults. N. Engl. J. Med. 1999, 340, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W.; Price, J.F.; Robertson, C.; Bots, M.L.; Polak, J.F.; Poppert, H.; Kavousi, M.; Dorr, M.; Stensland, E.; Ducimetiere, P.; et al. Carotid intima-media thickness progression and risk of vascular events in people with diabetes: Results from the PROG-IMT collaboration. Diabetes Care 2015, 38, 1921–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenjin, A.; Nagai, Y.; Yuji, S.; Ishii, S.; Kato, H.; Ohta, A.; Tanaka, Y. Short-term change of carotid intima-media thickness after treatment of hyperglycemia in patients with diabetes: A cross-sectional study. BMC Res. Notes 2016, 9, 281. [Google Scholar] [CrossRef] [Green Version]
- Asemi, Z.; Raygan, F.; Bahmani, F.; Rezavandi, Z.; Talari, H.R.; Rafiee, M.; Poladchang, S.; Mofrad, M.D.; Taheri, S.; Mohammadi, A.A.; et al. The effects of vitamin D, K and calcium co-supplementation on carotid intima-media thickness and metabolic status in overweight type 2 diabetic patients with CHD. Br. J. Nutr. 2016, 116, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Herder, M.; Arntzen, K.; Johnsen, S.; Mathiesen, E. The metabolic syndrome and progression of carotid atherosclerosis over 13 years. The Tromsø Study. Cardiovasc. Diabetol. 2012, 11, 77. [Google Scholar] [CrossRef] [Green Version]
- Escribano-López, I.; De Marañon, A.M.; Iannantuoni, F.; López-Domènech, S.; Abad-Jiménez, Z.; Díaz-Pozo, P.; Sola, E.; Apostolova, N.; Rocha, M.; Víctor, V.M. The Mitochondrial Antioxidant SS-31 Modulates Oxidative Stress, Endoplasmic Reticulum Stress, and Autophagy in Type 2 Diabetes. J. Clin. Med. 2019, 8, 1322. [Google Scholar] [CrossRef] [Green Version]
- González, F.; Rote, N.S.; Minium, J.; Kirwan, J.P. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, A.; Tilley, D.G. The Role of Leukocytes in Diabetic Cardiomyopathy. Front. Physiol. 2018, 9, 1547. [Google Scholar] [CrossRef] [PubMed]
- Mijares, A.H.; Rocha, M.; Rovira-Llopis, S.; Bañuls, C.; Bellod, L.; De Pablo, C.; Alvarez, A.; Roldán, I.; Sola-Izquierdo, E.; Victor, V.M. Human Leukocyte/Endothelial Cell Interactions and Mitochondrial Dysfunction in Type 2 Diabetic Patients and Their Association with Silent Myocardial Ischemia. Diabetes Care 2013, 36, 1695–1702. [Google Scholar] [CrossRef] [Green Version]
- Alcántar-Fernández, J.; González-Maciel, A.; Reynoso-Robles, R.; Andrade, M.E.P.; Hernández-Vázquez, A.D.J.; Velázquez-Arellano, A.; Miranda-Ríos, J. High-glucose diets induce mitochondrial dysfunction in Caenorhabditis elegans. PLoS ONE 2019, 14, e0226652. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Weng, S.-W.; Chang, Y.-H.; Su, B.Y.-J.; Chang, C.-M.; Tsai, C.-J.; Shen, F.-C.; Chuang, J.-H.; Lin, T.-K.; Liou, C.-W.; et al. The Causal Role of Mitochondrial Dynamics in Regulating Insulin Resistance in Diabetes: Link through Mitochondrial Reactive Oxygen Species. Oxid. Med. Cell. Longev. 2018, 2018, 7514383. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H. Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? J. Cell. Biochem. 2017, 118, 3577–3585. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar]
- Diao, F.-Y.; Xu, M.; Hu, Y.; Li, J.; Xu, Z.; Lin, M.; Wang, L.; Zhou, Y.; Zhou, Z.; Liu, J.; et al. The molecular characteristics of polycystic ovary syndrome (PCOS) ovary defined by human ovary cDNA microarray. J. Mol. Endocrinol. 2004, 33, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Dupré-Crochet, S.; Erard, M.; Nüβe, O. ROS production in phagocytes: Why, when, and where? J. Leukoc. Biol. 2013, 94, 657–670. [Google Scholar] [CrossRef]
- Rao, K.M.K. MAP kinase activation in macrophages. J. Leukoc. Biol. 2001, 69, 3–10. [Google Scholar]
- Okazaki, S.; Sakaguchi, M.; Miwa, K.; Furukado, S.; Yamagami, H.; Yagita, Y.; Mochizuki, H.; Kitagawa, K. Association of interleukin-6 with the progression of carotid atherosclerosis: A 9-year follow-up study. Stroke 2014, 45, 2924–2929. [Google Scholar] [CrossRef] [Green Version]
- Skoog, T.; Dichtl, W.; Boquist, S.; Skoglund-Andersson, C.; Karpe, F.; Tang, R.; Bond, M.; De Faire, U.; Nilsson, J.; Eriksson, P.; et al. Plasma tumour necrosis factor-alpha and early carotid atherosclerosis in healthy middle-aged men. Eur. Heart J. 2002, 23, 376–383. [Google Scholar] [CrossRef]
- Ijsselmuiden, A.J.; Musters, R.J.; De Ruiter, G.; Van Heerebeek, L.; Alderse-Baas, F.; Van Schilfgaarde, M.; Leyte, A.; Tangelder, G.-J.; Laarman, G.J.; Paulus, W.J. Circulating white blood cells and platelets amplify oxidative stress in heart failure. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 811–820. [Google Scholar] [CrossRef]
- Bárány, T.; Simon, A.; Szabó, G.; Benkő, R.; Mezei, Z.; Molnár, L.; Becker, D.; Merkely, B.; Zima, E.; Horváth, E.M. Oxidative Stress-Related Parthanatos of Circulating Mononuclear Leukocytes in Heart Failure. Oxid. Med. Cell. Longev. 2017, 2017, 1249614. [Google Scholar] [CrossRef] [Green Version]
- Tiyerili, V.; Camara, B.; Becher, M.U.; Schrickel, J.W.; Lütjohann, D.; Mollenhauer, M.; Baldus, S.; Nickenig, G.; Andrié, R.P. Neutrophil-derived myeloperoxidase promotes atherogenesis and neointima formation in mice. Int. J. Cardiol. 2016, 204, 29–36. [Google Scholar] [CrossRef]
- Victor, V.M.; Rocha, M.; Sola, E.; Banuls, C.; Garcia-Malpartida, K.; Hernandez-Mijares, A. Oxidative Stress, Endothelial Dysfunction and Atherosclerosis. Curr. Pharm. Des. 2009, 15, 2988–3002. [Google Scholar] [CrossRef]
- Pettersson, U.S.; Christoffersson, G.; Massena, S.; Ahl, D.; Jansson, L.; Henriksnäs, J.; Phillipson, M. Increased Recruitment but Impaired Function of Leukocytes during Inflammation in Mouse Models of Type 1 and Type 2 Diabetes. PLoS ONE 2011, 6, e22480. [Google Scholar] [CrossRef]
- Novak, V.; Zhao, P.; Manor, B.; Sejdić, E.; Alsop, D.; Abduljalil, A.; Roberson, P.K.; Munshi, M.; Novak, P. Adhesion molecules, altered vasoreactivity, and brain atrophy in type 2 diabetes. Diabetes Care 2011, 34, 2438–2441. [Google Scholar] [CrossRef] [Green Version]
- Giugliano, D.; Marfella, R.; Coppola, L.; Verrazzo, G.; Acampora, R.; Giunta, R.; Nappo, F.; Lucarelli, C.; D’Onofrio, F. Vascular Effects of Acute Hyperglycemia in Humans Are Reversed by l-Arginine. Circulation 1997, 95, 1783–1790. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Bir, S.C.; Kevil, C.G. Endothelial dysfunction and diabetes: Effects on angiogenesis, vascular remodeling, and wound healing. Int. J. Vasc. Med. 2012, 2012, 918267. [Google Scholar] [CrossRef] [Green Version]
- Nezu, T.; Hosomi, N.; Aoki, S.; Matsumoto, M. Carotid Intima-Media Thickness for Atherosclerosis. J. Atheroscler. Thromb. 2016, 23, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Liu, Y.; Zhang, Y.; Chen, Y.; Yang, Z.-F.; Zhu, Y.; Xu, S. Gender difference in carotid intima-media thickness in type 2 diabetic patients: A 4-year follow-up study. Cardiovasc. Diabetol. 2012, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Yang, Y.; Cao, T.; Li, Z. Differences in left and right carotid intima-media thickness and the associated risk factors. Clin. Radiol. 2011, 66, 393–398. [Google Scholar] [CrossRef]
- Hedna, V.S.; Bodhit, A.N.; Ansari, S.; Falchook, A.D.; Stead, L.; Heilman, K.M.; Waters, M.F. Hemispheric Differences in Ischemic Stroke: Is Left-Hemisphere Stroke More Common? J. Clin. Neurol. 2013, 9, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Hernaández, S.A.R.; Kroon, A.A.; Van Boxtel, M.P.; Mess, W.H.; Lodder, J.; Jolles, J.; De Leeuw, P.W. Is there a side predilection for cerebrovascular disease? Hypertension 2003, 42, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Selwaness, M.; Bouwhuijsen, Q.V.D.; Van Onkelen, R.S.; Hofman, A.; Franco, O.H.; Van Der Lugt, A.; Wentzel, J.J.; Vernooij, M.W. Atherosclerotic plaque in the left carotid artery is more vulnerable than in the right. Stroke 2014, 45, 3226–3230. [Google Scholar] [CrossRef] [Green Version]
- Einarson, T.R.; Hunchuck, J.; Hemels, M. Relationship between blood glucose and carotid intima media thickness: A meta-analysis. Cardiovasc. Diabetol. 2010, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Pillai, P.R.; Tiwari, D.; Jatav, O.P.; Rai, H. Lipid profile and carotid artery intima-media thickness in diabetic and non-diabetic ischaemic stroke. Int. J. Adv. Med. 2017, 4, 471. [Google Scholar] [CrossRef] [Green Version]





| Control | T2D | ||
|---|---|---|---|
| HbA1c ≤ 6.5% | HbA1c > 6.5% | ||
| N | 108 | 57 | 104 |
| Age (Years) | 57 ± 11 | 58 ± 8 | 60 ± 9 |
| %Women | 62.2% | 43.93% | 56.11% |
| Weight (kg) | 68.51 ± 15.18 | 85.02 ± 16,07 * | 83.59 ± 15.84 * |
| BMI (kg/cm2) | 24.18 ± 4.11 | 31.18 ± 4.23 * | 30.43 ± 5.13 * |
| SBP (mmHg) | 119.43 ± 18.18 | 139.82 ± 14.23 * | 138.38 ± 17.05 * |
| DBP (mmHg) | 72.35 ± 10.94 | 82.03 ± 10.97 * | 78.25 ± 9.35 * |
| Waist (cm) | 79.81 ± 12.62 | 106.39 ± 11.46 * | 103.99 ± 13.35 * |
| Hip (cm) | 99.09 ± 7.21 | 108.71 ± 9.53 * | 108.48 ± 12.62 * |
| Waist–Hip ratio | 0.80 ± 0.09 | 0.97 ± 0.08 * | 0.95 ± 0.08 * |
| Glucose (mg/dL) | 88.08 ± 10.75 | 112.37 ± 22.59 * | 160.51 ± 55.06 *‡ |
| HOMA | 1.69 ± 1.19 | 3.72 ± 2.00 * | 6.57 ± 4.45 *‡ |
| HbA1c (%) | 5.18 ± 0,26 | 5.94 ± 0.30 * | 7.85 ± 1.30 * |
| Total cholesterol (mg/dL) | 185.43 ± 35.32 | 173.67 ± 34.31 | 167.42 ± 37.67 * |
| HDL-c (mg/dL) | 56.04 ± 13.61 | 45.10 ± 11.83 * | 42.94 ± 10.46 * |
| LDL-c (mg/dL) | 111.38 ± 28.72 | 102.62 ± 31.33 | 95.17 ± 31.09 * |
| VLDL (mg/dL) | 26.01 ± 10.81 | 28.63 ± 19.54 * | 28.91 ± 22.04 * |
| Cholesterol/HDL | 3.46 ± 0.94 | 4.07 ± 1.18 * | 4.06 ± 1.14 * |
| Triglycerides (mg/dL) | 87.62 (55.50;103.00) | 130.29 (90.5; 169.00) * | 150.75 (92.00; 162.63) * |
| Non-HDL Cholesterol (mg/dL) | 129.39 ± 33.28 | 129.37 ± 32.51 | 124.48 ± 36.57 |
| AIP (TG/HDL-c) | 0.10 (−0.06; 0.33) | 0.47 (0.23; 0.63) * | 0.47 (0.29; 0.68) * |
| APO A1 (mg/dL) | 164.02 ± 32.28 | 151.45 ± 27.21 * | 142.72 ± 22.87 *† |
| APO B (mg/dL) | 90.78 ± 26.60 | 90.33 ± 25.82 | 94.18 ± 25.27 |
| APOB/APOA1 | 0.57 ± 0.20 | 0.64 ± 0.24 | 0.67 ± 0.19 * |
| hsCRP (mg/L) | 0.75 (0.36; 1.83) | 2.64 (1.61; 7.07) † | 2.87 (1.31; 6.59) † |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Marañón, A.M.; Iannantuoni, F.; Abad-Jiménez, Z.; Canet, F.; Díaz-Pozo, P.; López-Domènech, S.; Roldán-Torres, I.; Morillas, C.; Rocha, M.; Víctor, V.M. Association between Proinflammatory Markers, Leukocyte–Endothelium Interactions, and Carotid Intima–Media Thickness in Type 2 Diabetes: Role of Glycemic Control. J. Clin. Med. 2020, 9, 2522. https://doi.org/10.3390/jcm9082522
de Marañón AM, Iannantuoni F, Abad-Jiménez Z, Canet F, Díaz-Pozo P, López-Domènech S, Roldán-Torres I, Morillas C, Rocha M, Víctor VM. Association between Proinflammatory Markers, Leukocyte–Endothelium Interactions, and Carotid Intima–Media Thickness in Type 2 Diabetes: Role of Glycemic Control. Journal of Clinical Medicine. 2020; 9(8):2522. https://doi.org/10.3390/jcm9082522
Chicago/Turabian Stylede Marañón, Aranzazu Martinez, Francesca Iannantuoni, Zaida Abad-Jiménez, Francisco Canet, Pedro Díaz-Pozo, Sandra López-Domènech, Ildefonso Roldán-Torres, Carlos Morillas, Milagros Rocha, and Víctor M. Víctor. 2020. "Association between Proinflammatory Markers, Leukocyte–Endothelium Interactions, and Carotid Intima–Media Thickness in Type 2 Diabetes: Role of Glycemic Control" Journal of Clinical Medicine 9, no. 8: 2522. https://doi.org/10.3390/jcm9082522





