Diagnostic and Clinical Impact of 18F-FDG PET/CT in Staging and Restaging Soft-Tissue Sarcomas of the Extremities and Trunk: Mono-Institutional Retrospective Study of a Sarcoma Referral Center
Abstract
1. Introduction
2. Experimental Section
2.1. Patient Population
- Histology-demonstrated soft-tissue sarcomas of the extremities and trunk;
- 18F-FDG PET/CT performed at initial staging or for disease restaging (suspect of recurrent disease, doubtful conventional imaging findings and early post-surgical staging);
- Availability of the patient’s clinical information for at least 6 months after PET/CT imaging.
- Suspected local disease relapse (n = 97);
- Early post-surgical staging in patients at high risk for relapse or if a re-excision of the surgical scar was planned (n = 25);
- Characterization of suspected lung nodules at CT scan (n = 22);
- Suspected metastases in other sites (n = 30).
2.2. 18F-FDG PET/CT Imaging
2.3. Reference Standard
- -
- Lymph nodes: lesions showing a significant FDG uptake regardless of size that increased in number or size on follow-up imaging and/or showed an increase of 18F-FDG uptake on follow-up PET/CT were classified as “metastatic”;
- -
- Bone metastases: lytic/sclerotic bone lesions showing definite focal FDG uptake or areas of focal FDG uptake without a corresponding bone alteration that showed increased 18F-FDG uptake on follow-up PET/CT and/or that increased in number or size were reported as metastatic; the appearance of lytic/sclerotic changes in follow-up imaging was also considered a sign of malignancy. Non-FDG–avid lytic/sclerotic bone alterations showing no progression in number or size during follow-up were considered as “benign”;
- -
- Lung metastases: pulmonary nodules showing an obvious progression in number and/or size within 6 months were considered as “metastatic”. Lung nodules showing no progression for at least 6 months or that disappeared were considered as “benign”.
2.4. Changes in Patient Management
- Non-treatment to treatment;
- Migration to a different treatment regimen (e.g., from local surgery to systemic chemotherapy);
- Change in treatment within the same modality (e.g., extension of surgery, neoadjuvant vs. first-line chemotherapy);
- Treatment to non-treatment.
2.5. Statistical Analysis
3. Results
3.1. Local Relapse
3.2. Distant Metastases
3.3. Impact of 18F-FDG PET/CT Scan on Patient Management
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.D.; Casali, P.G. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Serre, D.; Reichardt, P.; Martín-Broto, J.; Bauer, S. Options for treating different soft tissue sarcoma subtypes. Future Oncol. 2018, 14, 25–49. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Tepper, J.; Glatstein, E.; Costa, J.; Baker, A.; Brennam, M.; Demoss, E.V.; Seipp, C.; Sindelar, W.F.; Sugarbaker, P.; et al. The Treatment of Soft-tissue Sarcomas of the Extremities: Prospective Randomized Evaluations of (1) Limb-sparing surgery Plus Radiation Therapy Compared with Compared with amputation and (2) the Role of adjuvant Chemotherapy. Ann. Surg. 1982, 196, 305–315. [Google Scholar] [CrossRef]
- Duran-Moreno, J.; Kontogeorgakos, V.; Koumarianou, A. Soft tissue sarcomas of the upper extremities: Maximizing treatment opportunities and outcomes (Review). Oncol. Lett. 2019. [Google Scholar] [CrossRef]
- Sundby Hall, K.; Bruland, Ø.S.; Bjerkehagen, B.; Zaikova, O.; Engellau, J.; Hagberg, O.; Hansson, L.; Hagberg, H.; Ahlström, M.; Knobel, H.; et al. Adjuvant chemotherapy and postoperative radiotherapy in high-risk soft tissue sarcoma patients defined by biological risk factors—A Scandinavian Sarcoma Group study (SSG XX). Eur. J. Cancer 2018, 99, 78–85. [Google Scholar] [CrossRef]
- Casali, P.G.; Abecassis, N.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brodowicz, T.; Broto, J.M.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv51–iv67. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Ferrari, S.; Quagliuolo, V.; Broto, J.M.; Pousa, A.L.; Grignani, G.; Basso, U.; Blay, J.-Y.; Tendero, O.; Beveridge, R.D.; et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): An international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017, 18, 812–822. [Google Scholar] [CrossRef]
- Dossett, L.A.; Toloza, E.M.; Fontaine, J.; Robinson, L.A.; Reed, D.; Druta, M.; Letson, D.G.; Zager, J.S.; Gonzalez, R.J. Outcomes and clinical predictors of improved survival in a patients undergoing pulmonary metastasectomy for sarcoma. J. Surg. Oncol. 2015, 112, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, K.; Regler, J.; Baum, T.; Rechl, H.; Specht, K.; Haller, B.; von Eisenhart-Rothe, R.; Gradinger, R.; Rummeny, E.J.; Woertler, K. Local Staging of Soft-Tissue Sarcoma: Emphasis on Assessment of Neurovascular Encasement—Value of MR Imaging in 174 Confirmed Cases. Radiology 2015, 275, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; Kane, J.M.; et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2018, 16, 536–563. [Google Scholar] [CrossRef] [PubMed]
- Schuetze, S.M.; Rubin, B.P.; Vernon, C.; Hawkins, D.S.; Bruckner, J.D.; Conrad, E.U.; Eary, J.F. Use of positron emission tomography in localized extremity soft tissue sarcoma treated with neoadjuvant chemotherapy. Cancer 2005, 103, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Sheikhbahaei, S.; Marcus, C.; Hafezi-Nejad, N.; Taghipour, M.; Subramaniam, R.M. Value of FDG PET/CT in Patient Management and Outcome of Skeletal and Soft Tissue Sarcomas. PET Clin. 2015, 10, 375–393. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.; Buck, A.K.; Benz, M.R.; Rudert, M.; Beer, A.J.; Mansour, A.; Pomykala, K.L.; Haller, B.; Juenger, H.; Scheidhauer, K.; et al. F-Fluorodeoxyglucose positron emission tomography/computed tomography for the detection of recurrent bone and soft tissue sarcoma: PET-CT in Recurrent Sarcoma. Cancer 2013, 119, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, U.; Yamaguchi, U.; Seki, K.; Terauchi, T.; Arai, Y.; Hasegawa, T. Glut-1 expression and enhanced glucose metabolism are associated with tumour grade in bone and soft tissue sarcomas: A prospective evaluation by [18F]fluorodeoxyglucose positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Gerth, H.U.; Juergens, K.U.; Dirksen, U.; Gerss, J.; Schober, O.; Franzius, C. Significant Benefit of Multimodal Imaging: PET/CT Compared with PET Alone in Staging and Follow-up of Patients with Ewing Tumors. J. Nucl. Med. 2007, 48, 1932–1939. [Google Scholar] [CrossRef]
- Macpherson, R.E.; Pratap, S.; Tyrrell, H.; Khonsari, M.; Wilson, S.; Gibbons, M.; Whitwell, D.; Giele, H.; Critchley, P.; Cogswell, L.; et al. Retrospective audit of 957 consecutive 18F-FDG PET–CT scans compared to CT and MRI in 493 patients with different histological subtypes of bone and soft tissue sarcoma. Clin. Sarcoma Res. 2018, 8, 9. [Google Scholar] [CrossRef]
- Benz, M.R.; Czernin, J.; Allen-Auerbach, M.S.; Tap, W.D.; Dry, S.M.; Elashoff, D.; Chow, K.; Evilevitch, V.; Eckardt, J.J.; Phelps, M.E.; et al. FDG-PET/CT Imaging Predicts Histopathologic Treatment Responses after the Initial Cycle of Neoadjuvant Chemotherapy in High-Grade Soft-Tissue Sarcomas. Clin. Cancer Res. 2009, 15, 2856–2863. [Google Scholar] [CrossRef]
- Dimitrakopoulou-Strauss, A.; Strauss, L.G.; Egerer, G.; Vasamiliette, J.; Mechtersheimer, G.; Schmitt, T.; Lehner, B.; Haberkorn, U.; Stroebel, P.; Kasper, B. Impact of Dynamic 18F-FDG PET on the Early Prediction of Therapy Outcome in Patients with High-Risk Soft-Tissue Sarcomas After Neoadjuvant Chemotherapy: A Feasibility Study. J. Nucl. Med. 2010, 51, 551–558. [Google Scholar] [CrossRef]
- Vlenterie, M.; Oyen, W.J.; Steeghs, N.; Desar, I.M.E.; Verheijen, R.B.; Koenen, A.M.; Grootjans, W.; De Geus-Oei, L.-F.; Van Erp, N.P.; Van Der Graaf, W.T. Early Metabolic Response as a Predictor of Treatment Outcome in Patients With Metastatic Soft Tissue Sarcomas. Anticancer Res. 2019, 39, 1309–1316. [Google Scholar] [CrossRef]
- Kubo, T.; Furuta, T.; Johan, M.P.; Ochi, M. Prognostic significance of 18F-FDG PET at diagnosis in patients with soft tissue sarcoma and bone sarcoma; systematic review and meta-analysis. Eur. J. Cancer 2016, 58, 104–111. [Google Scholar] [CrossRef]
- Hagi, T.; Nakamura, T.; Sugino, Y.; Matsubara, T.; Asanuma, K.; Sudo, A. Is FDG-PET/CT Useful for Diagnosing Pulmonary Metastasis in Patients with Soft Tissue Sarcoma? Anticancer Res. 2018, 38, 3635–3639. [Google Scholar] [CrossRef] [PubMed]
- Fuglø, H.M.; Jørgensen, S.M.; Loft, A.; Hovgaard, D.; Petersen, M.M. The diagnostic and prognostic value of 18F-FDG PET/CT in the initial assessment of high-grade bone and soft tissue sarcoma. A retrospective study of 89 patients. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Völker, T.; Denecke, T.; Steffen, I.; Misch, D.; Schönberger, S.; Plotkin, M.; Ruf, J.; Furth, C.; Stöver, B.; Hautzel, H.; et al. Positron Emission Tomography for Staging of Pediatric Sarcoma Patients: Results of a Prospective Multicenter Trial. J. Clin. Oncol. 2007, 25, 5435–5441. [Google Scholar] [CrossRef] [PubMed]
- Keung, E.Z.; Chiang, Y.-J.; Voss, R.K.; Cormier, J.N.; Torres, K.E.; Hunt, K.K.; Feig, B.W.; Roland, C.L. Defining the incidence and clinical significance of lymph node metastasis in soft tissue sarcoma. Eur. J. Surg. Oncol. 2018, 44, 170–177. [Google Scholar] [CrossRef]
- Elmanzalawy, A.; Vali, R.; Chavhan, G.B.; Gupta, A.A.; Omarkhail, Y.; Amirabadi, A.; Shammas, A. The impact of 18F-FDG PET on initial staging and therapy planning of pediatric soft-tissue sarcoma patients. Pediatr. Radiol. 2020, 50, 252–260. [Google Scholar] [CrossRef]

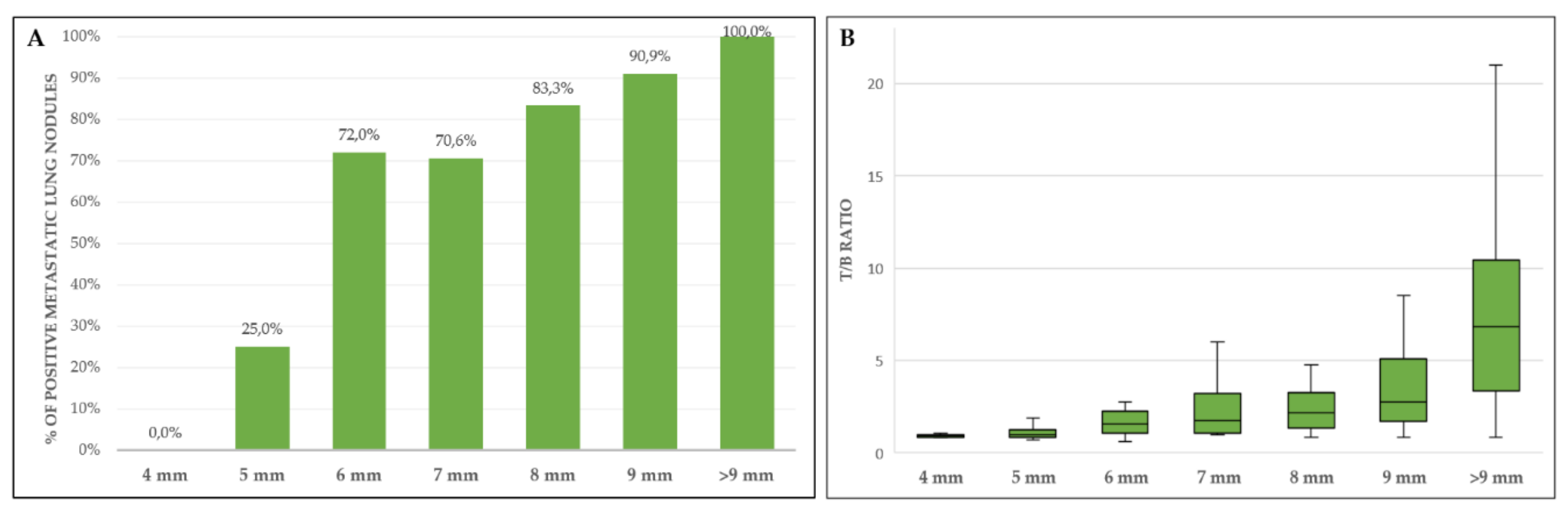
| Metastatic Site (Total Patients/Lesions Analyzed) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) |
|---|---|---|---|---|---|
| Lung (all nodules, ≥4 mm) | |||||
| PET/CT (patients n = 80) | 86.0 (73.3–94.2) | 96.7 (82.8–99.9) | 97.7 (86.2–99.7) | 80.6 (67.5–89.2) | 90.0 (81.2–95.6) |
| PET/CT (nodules n = 237) | 74.1 (67.3–80.1) | 97.7 (87.9–99.9) | 99.3 (95.4–99.9) | 46.2 (40.3–52.3) | 78.5 (72.7–83.5) |
| Lung (nodules ≥6 mm) | |||||
| PET/CT (patients n = 70) | 89.6 (77.3–96.5) | 95.5 (77.2–99.9) | 97.7 (86.3–99.7) | 80.8 (64.6–90.6) | 91.4 (82.3–96.8) |
| PET/CT (nodules n = 181) | 88.4 (82.3–93.0) | 96.2 (80.4–99.9) | 99.3 (95.2–99.9) | 58.1 (47.2–68.3) | 89.5 (84.1–93.6) |
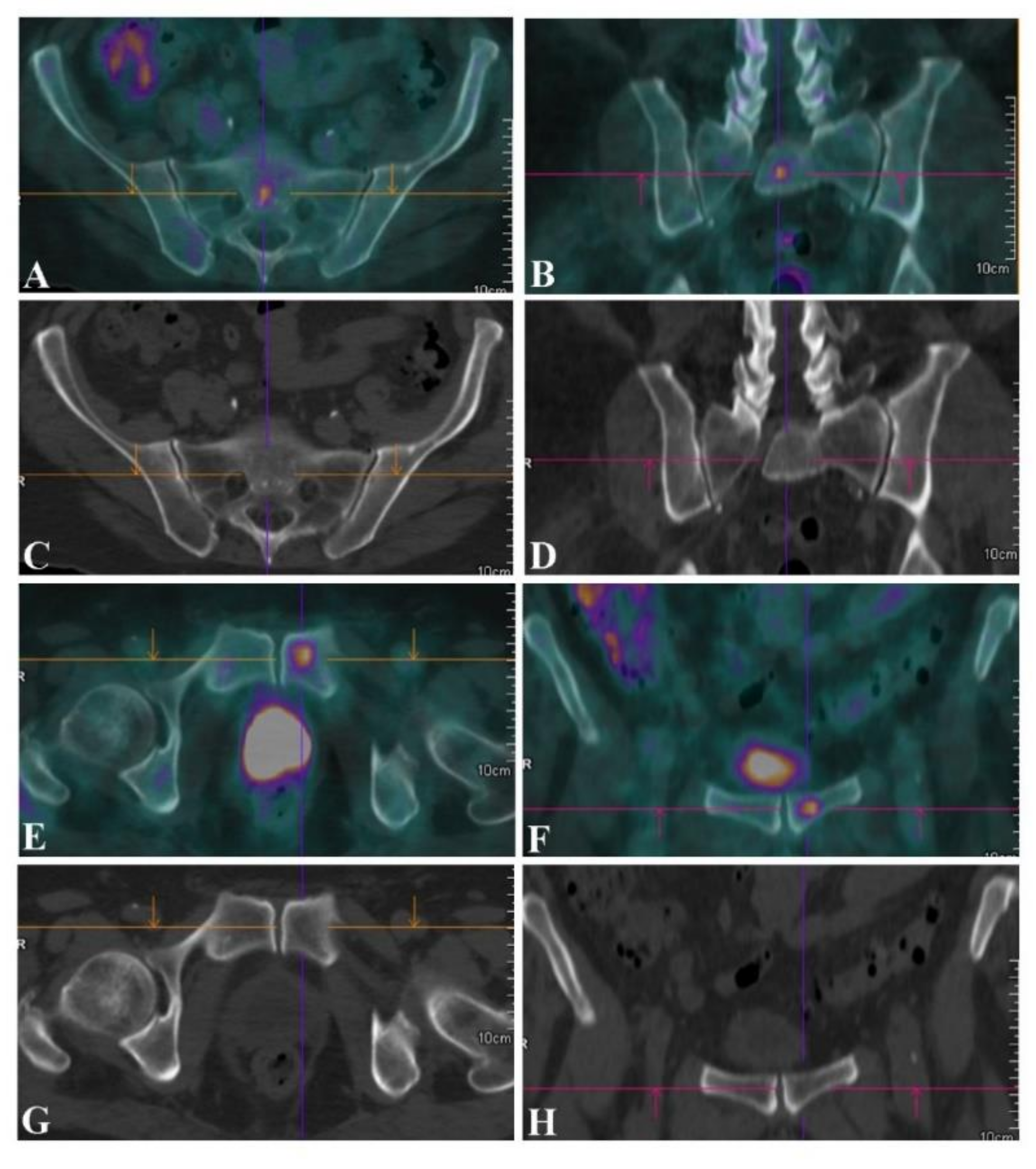
| Bone | |||||
| PET/CT (patients n = 27) | 100 (86.8–100) | 100 (2.5–100) | 100 | 100 | 100 (87.2–100) |
| CT alone | 69.2 (48.2–85.7) | – | 94.7 (93.3–95.9) | – | 66.7 (46.0–83.5) |
| PET/CT (lesions n = 81) | 100 (95.5–100) | 100 (2.5–100) | 100 | 100 | 100 (95.6–100) |
| CT alone | 48.8 (37.4–60.2) | – | 97.5 (96.9–98.0) | – | 48.2 (36.9–59.5) |
| Lymph nodes | |||||
| PET/CT (patients n = 35) | 96.0 (79.7–99.9) | 50.0 (18.7–81.3) | 82.8 (72.0–90.0) | 83.3 (39.9–97.4) | 82.9 (66.4–93.4) |
| CT alone | 56.0 (34.9–75.6) | 10.0 (0.3–44.5) | 60.9 (50.9–70.0) | 8.3 (1.3–38.1) | 42.9 (26.3–60.7) |
| Staging | Restaging | |
|---|---|---|
| FDG-positive occult/suspected disease sites | ||
| Lung | 1 | 6 |
| Bone | 6 | 2 |
| Lymph nodes | 5 * | 3 |
| Others (pleura, soft tissues, abdomen) | 1 | 5 |
| Multiple sites | 2 | 5 |
| Local tumor extension/skip metastases | 3 | 2 |
| Local relapse | – | 6 |
| Unconfirmed suspected disease sites (FDG negative) | ||
| Lung | 5 | 7 |
| Bone | – | 1 |
| Lymph nodes | 2 | 5 ** |
| Others (pleura, soft tissues, abdomen) | 3 | 2 # |
| Local tumor extension/skip metastases | – | 0 |
| Local relapse | – | 8 |
| Total | 28 | 52 |
| Management Plan (Staging) | Management Plan (Restaging) | ||
|---|---|---|---|
| Before PET/CT | After PET/CT | Before PET/CT | After PET/CT |
| Surgery n = 56 | Surgery n = 44 Surgery + CHT n = 1 CHT n = 10 PIA+surgery n = 1 | Surgery n = 82 | Surgery n = 59 Surgery + RT n = 1 CHT n = 9 RT/CHT n = 1 NACT + surgery n = 1 FUP/W&S n = 11 |
| Surgery + CHT n = 15 | Surgery + CHT n = 15 | Surgery + CHT n = 5 | Surgery + CHT n = 5 |
| Surgery + RT n = 23 | Surgery + RT n = 18 Surgery + CHT n = 2 Surgery + RT/CHT n = 2 CHT n = 1 | Surgery + RT n = 14 | Surgery + RT n = 11 CHT n = 1 RT n = 2 |
| Surgery + RT/CHT n = 9 | Surgery + RT/CHT n = 9 | Surgery + RT/CHT n = 4 | Surgery + RT/CHT n = 4 |
| CHT n = 11 | Cht n = 3 Surgery n = 5 Surgery+CHT n = 1 Surgery+RT/CHT n = 1 NACT + surgery n = 1 | CHT n = 21 | CHT n = 13 Surgery n = 1 Surgery+RT n = 1 FUP/W&S n = 5 RT n = 1 |
| RT/CHT n = 3 | RT/CHT n = 3 | RT/CHT n = 2 | RT/CHT n = 2 |
| RT/CHT + Surgery n = 6 | RT/CHT + Surgery n = 5 CHT n = 1 | ||
| NACT+surgery n = 45 | NACT+ surgery n = 41 CHT n = 4 | NACT+surgery n = 9 | NACT+surgery n = 7 CHT n = 2 |
| RT n = 1 | RT n = 1 | RT n = 6 | RT n = 5 CHT n = 1 |
| RT + surgery n = 2 | RT + surgery n = 2 | RT + surgery n = 1 | RT + surgery n = 1 |
| FUP/W&S n = 30 | FUP/W&S n = 7 Surgery n = 11 Surgery + CHT n = 1 Surgery + RT n = 1 CHT n = 8 RT/CHT n = 1 NACT + Surgery n = 1 | ||
| Staging (n = 34) | Restaging (n = 68) | |
|---|---|---|
| Non-treatment to treatment | – | 23 |
| Change in treatment strategy | 31 | 22 |
| Change in treatment within the same modality * | 3 | 7 |
| Treatment to non-treatment | – | 16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annovazzi, A.; Rea, S.; Zoccali, C.; Sciuto, R.; Baldi, J.; Anelli, V.; Petrongari, M.G.; Pescarmona, E.; Biagini, R.; Ferraresi, V. Diagnostic and Clinical Impact of 18F-FDG PET/CT in Staging and Restaging Soft-Tissue Sarcomas of the Extremities and Trunk: Mono-Institutional Retrospective Study of a Sarcoma Referral Center. J. Clin. Med. 2020, 9, 2549. https://doi.org/10.3390/jcm9082549
Annovazzi A, Rea S, Zoccali C, Sciuto R, Baldi J, Anelli V, Petrongari MG, Pescarmona E, Biagini R, Ferraresi V. Diagnostic and Clinical Impact of 18F-FDG PET/CT in Staging and Restaging Soft-Tissue Sarcomas of the Extremities and Trunk: Mono-Institutional Retrospective Study of a Sarcoma Referral Center. Journal of Clinical Medicine. 2020; 9(8):2549. https://doi.org/10.3390/jcm9082549
Chicago/Turabian StyleAnnovazzi, Alessio, Sandra Rea, Carmine Zoccali, Rosa Sciuto, Jacopo Baldi, Vincenzo Anelli, Maria G. Petrongari, Edoardo Pescarmona, Roberto Biagini, and Virginia Ferraresi. 2020. "Diagnostic and Clinical Impact of 18F-FDG PET/CT in Staging and Restaging Soft-Tissue Sarcomas of the Extremities and Trunk: Mono-Institutional Retrospective Study of a Sarcoma Referral Center" Journal of Clinical Medicine 9, no. 8: 2549. https://doi.org/10.3390/jcm9082549
APA StyleAnnovazzi, A., Rea, S., Zoccali, C., Sciuto, R., Baldi, J., Anelli, V., Petrongari, M. G., Pescarmona, E., Biagini, R., & Ferraresi, V. (2020). Diagnostic and Clinical Impact of 18F-FDG PET/CT in Staging and Restaging Soft-Tissue Sarcomas of the Extremities and Trunk: Mono-Institutional Retrospective Study of a Sarcoma Referral Center. Journal of Clinical Medicine, 9(8), 2549. https://doi.org/10.3390/jcm9082549








