Laparoscopic Outcomes after Normal Clinical and Ultrasound Findings in Young Women with Chronic Pelvic Pain: A Cross-Sectional Study
Abstract
:1. Introduction
2. Experimental Section
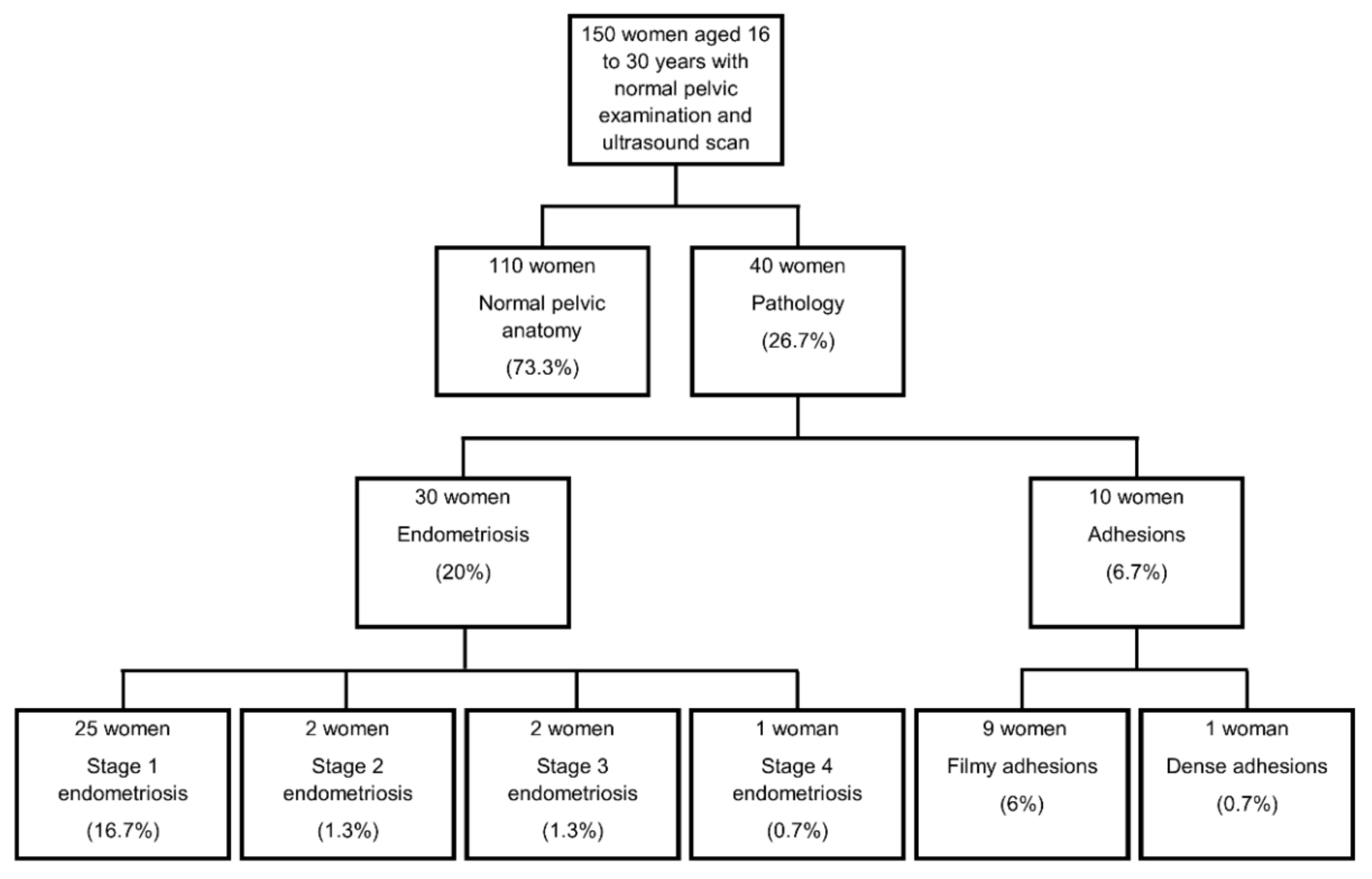
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- RCOG. The Initial management of chronic pelvic pain. In Green Top Guideline No. 41; Royal College of Obstetrics and Gynaecology: London, UK, 2012. [Google Scholar]
- Abercrombie, P.D.; Learman, L.A. Providing holistic care for women with chronic pelvic pain. J. Obstet. Gynecol. Neonatal. Nurs. 2012, 41, 668–679. [Google Scholar] [CrossRef]
- Chao, M.T.; Abercrombie, P.D.; Nakagawa, S.; Gregorich, S.E.; Learman, L.A.; Kuppermann, M. Prevalence and use of complementary health approaches among women with chronic pelvic pain in a prospective cohort study. Pain Med. 2015, 16, 328–340. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, T.Y.; Owens, A. The role of pelvic floor physical therapy in the treatment of pelvic and genital pain-related sexual dysfunction (CME). J. Sex. Med. 2008, 5, 513–523. [Google Scholar] [CrossRef]
- Till, S.R.; As-Sanie, S.; Schrepf, A. Psychology of chronic pelvic pain: Prevalence, neurobiological vulnerabilities, and treatment. Clin. Obstet. Gynecol. 2019, 62, 22–36. [Google Scholar] [CrossRef]
- Trutnovsky, G.; Plieseis, C.; Bjelic-Radisic, V.; BertholinyGalvez, M.C.; Tamussino, K.; Ulrich, D. Vulvodynia and chronic pelvic pain in a gynecologic outpatient clinic. J. Psychosom. Obstet. Gynaecol. 2019, 40, 243–247. [Google Scholar] [CrossRef]
- Latthe, P.; Latthe, M.; Say, L.; Gulmezoglu, M.; Khan, K.S. WHO systematic review of prevalence of chronic pelvic pain: A neglected reproductive health morbidity. BMC Public Health 2006, 6, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zondervan, K.T.; Yudkin, P.L.; Vessey, M.P.; Dawes, M.G.; Barlow, D.H.; Kennedy, S.H. Prevalence and incidence of chronic pelvic pain in primary care: Evidence from a national general practice database. Br. J. Obstet. Gynaecol. 1999, 106, 1149–1155. [Google Scholar] [CrossRef]
- Ballard, K.; Lowton, K.; Wright, J. What’s the delay? A qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil. Steril. 2006, 86, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, K.; Weissman-Fogel, I.; Lowenstein, L.; Abramov, L.; Granot, M. How does myofascial physical therapy attenuate pain in chronic pelvic pain syndrome? Pain Res. Manag. 2019, 2019, 6091257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grinberg, K.; Granot, M.; Lowenstein, L.; Abramov, L.; Weissman-Fogel, I. A common pronociceptive pain modulation profile typifying subgroups of chronic pelvic pain syndromes is interrelated with enhanced clinical pain. Pain 2017, 158, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Dydyk, A.M.; Gupta, N. Chronic Pelvic Pain; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Kuznetsov, L.; Dworzynski, K.; Davies, M.; Overton, C.; Guideline, C. Diagnosis and management of endometriosis: Summary of NICE guidance. BMJ 2017, 358, j3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NICE. Endometriosis: Diagnosis and management. In NICE Guideline 73; National Institute for Health and Care Excellence: London, UK, 2017. [Google Scholar]
- Dunselman, G.A.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Leyland, N.; Casper, R.; Laberge, P.; Singh, S.S.; Allen, L.; Arendas, K.; Leyland, N.; Allaire, C.; Awadalla, A.; Best, C.; et al. Endometriosis: Diagnosis and management. J. Obstet. Gynaecol. Can. 2010, 32, 107–134. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis: A committee opinion. Fertil. Steril. 2014, 101, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Vigano, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.W.; Daniels, J.; Hummelshoj, L.; Cox, E.; Cooper, K.G. Surgical removal of superficial peritoneal endometriosis for managing women with chronic pelvic pain: Time for a rethink? BJOG 2019, 126, 1414–1416. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Chapron, C.; Giudice, L.C.; Laufer, M.R.; Leyland, N.; Missmer, S.A.; Singh, S.S.; Taylor, H.S. Clinical diagnosis of endometriosis: A call to action. Am. J. Obstet. Gynecol. 2019, 220, 354.e1–354.e12. [Google Scholar] [CrossRef] [Green Version]
- Krantz, T.E.; Andrews, N.; Petersen, T.R.; Dunivan, G.C.; Montoya, M.; Swanson, N.; Wenzl, C.K.; Zambrano, J.R.; Komesu, Y.M. Adverse childhood experiences among gynecology patients with chronic pelvic pain. Obstet. Gynecol. 2019, 134, 1087–1095. [Google Scholar] [CrossRef]
- Singh, S.S.; Suen, M.W. Surgery for endometriosis: Beyond medical therapies. Fertil. Steril. 2017, 107, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Surrey, E.S.; Soliman, A.M.; Yang, H.; Du, E.X.; Su, B. Treatment patterns, complications, and health care utilization among endometriosis patients undergoing a laparoscopy or a hysterectomy: A retrospective claims analysis. Adv. Ther. 2017, 34, 2436–2451. [Google Scholar] [CrossRef] [Green Version]
- RCOG. Diagnostic laparoscopy. In Consent Advice No. 2; Royal College of Obstetrics and Gynaecology: London, UK, 2008. [Google Scholar]
- Chapron, C.; Fauconnier, A.; Goffinet, F.; Breart, G.; Dubuisson, J.B. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynaecologic pathology. Results of a meta-analysis. Hum. Reprod. 2002, 17, 1334–1342. [Google Scholar] [CrossRef]
- Hori, Y.; Committee, S.G. Diagnostic laparoscopy guidelines: This guideline was prepared by the SAGES Guidelines Committee and reviewed and approved by the Board of Governors of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), November 2007. Surg. Endosc. 2008, 22, 1353–1383. [Google Scholar] [CrossRef]
- McGowan, L.; Escott, D.; Luker, K.; Creed, F.; Chew-Graham, C. Is chronic pelvic pain a comfortable diagnosis for primary care practitioners: A qualitative study. BMC Fam. Pract. 2010, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Coxon, L.; Horne, A.W.; Vincent, K. Pathophysiology of endometriosis-associated pain: A review of pelvic and central nervous system mechanisms. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Laborda, E.; Clarke, A.; Carpenter, T. The threshold for laparoscopy for pelvic pain. Obstet. Gynaecol. 2010, 12, 7–12. [Google Scholar]
- Clemens, J.Q.; Kutch, J.J.; Mayer, E.A.; Naliboff, B.D.; Rodriguez, L.V.; Klumpp, D.J.; Schaeffer, A.J.; Kreder, K.J.; Clauw, D.J.; Harte, S.E.; et al. The multidisciplinary approach to the study of chronic pelvic pain (MAPP) research network*: Design and implementation of the Symptom Patterns Study (SPS). Neurourol. Urodyn. 2020. [Google Scholar] [CrossRef]
- Gambadauro, P.; Carli, V.; Hadlaczky, G. Depressive symptoms among women with endometriosis: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2019, 220, 230–241. [Google Scholar] [CrossRef]
- Facchin, F.; Barbara, G.; Saita, E.; Mosconi, P.; Roberto, A.; Fedele, L.; Vercellini, P. Impact of endometriosis on quality of life and mental health: Pelvic pain makes the difference. J. Psychosom. Obstet. Gynaecol. 2015, 36, 135–141. [Google Scholar] [CrossRef]
- Lagana, A.S.; La Rosa, V.L.; Rapisarda, A.M.C.; Valenti, G.; Sapia, F.; Chiofalo, B.; Rossetti, D.; Ban Frangez, H.; Bokal, E.V.; Vitale, S.G. Anxiety and depression in patients with endometriosis: Impact and management challenges. Int. J. Women’s Health 2017, 9, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Yosef, A.; Allaire, C.; Williams, C.; Ahmed, A.G.; Al-Hussaini, T.; Abdellah, M.S.; Wong, F.; Lisonkova, S.; Yong, P.J. Multifactorial contributors to the severity of chronic pelvic pain in women. Am. J. Obstet. Gynecol. 2016, 215, 760.e1–760.e14. [Google Scholar] [CrossRef]
- Grinberg, K.; Sela, Y.; Nissanholtz-Gannot, R. New insights about chronic pelvic pain syndrome (CPPS). Int. J. Environ. Res. Public Health 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Busacca, M.; Chiaffarino, F.; Candiani, M.; Vignali, M.; Bertulessi, C.; Oggioni, G.; Parazzini, F. Determinants of long-term clinically detected recurrence rates of deep, ovarian, and pelvic endometriosis. Am. J. Obstet. Gynecol. 2006, 195, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Tandoi, I.; Somigliana, E.; Riparini, J.; Ronzoni, S.; Vigano, P.; Candiani, M. High rate of endometriosis recurrence in young women. J. Pediatr. Adolesc. Gynecol. 2011, 24, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Chao, X.P.; Leng, J.H.; Zhang, W.; Zhang, J.J.; Dai, Y.; Shi, J.H.; Jia, S.Z.; Xu, X.X.; Chen, S.K.; et al. Risk factors for postoperative recurrence of ovarian endometriosis: Long-term follow-up of 358 women. J. Ovarian Res. 2019, 12, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, J.; Ziebland, S.; Kennedy, S. “People sometimes react funny if they’re not told enough”: Women’s views about the risks of diagnostic laparoscopy. Health Expect. 2002, 5, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Guerriero, S.; Condous, G.; van den Bosch, T.; Valentin, L.; Leone, F.P.; Van Schoubroeck, D.; Exacoustos, C.; Installe, A.J.; Martins, W.P.; Abrao, M.S.; et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: A consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet. Gynecol. 2016, 48, 318–332. [Google Scholar] [CrossRef]
- Armour, M.; Ferfolja, T.; Curry, C.; Hyman, M.S.; Parry, K.; Chalmers, K.J.; Smith, C.A.; MacMillan, F.; Holmes, K. The prevalence and educational impact of pelvic and menstrual pain in Australia: A national online survey of 4202 young women aged 13–25. J. Pediatr. Adolesc. Gynecol. 2020. [Google Scholar] [CrossRef]
- Peters, A.A.; van Dorst, E.; Jellis, B.; van Zuuren, E.; Hermans, J.; Trimbos, J.B. A randomized clinical trial to compare two different approaches in women with chronic pelvic pain. Obstet. Gynecol. 1991, 77, 740–744. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Aged 16–30 years | Aged < 16 years or > 30 years |
| No previous gynaecological surgery | Previous gynaecological surgery |
| Chronic pelvic pain | No chronic pelvic pain |
| Normal pelvic clinical examination | Pathology at pelvic clinical examination |
| Normal pelvic USS | Pathology on pelvic USS |
| Non-pregnant | Pregnant |
| Demographics | All Women (150) | Endometriosis at Laparoscopy (30) | No Endometriosis at Laparoscopy (120) |
|---|---|---|---|
| Age, mean (SD) | 24.5 (3.8) | 23.5 (4.4) | 23.4 (3.6) |
| BMI, mean (SD) | 24.5 (4.1) | 25 (4.0) | 24.4 (4.1) |
| Age at menarche, mean (SD) | 12.3 (1.5) | 12.5 (1) | 12.3 (1.5) |
| Nulliparous, n (%) | 102 (68%) | 25 (83.3%) | 77 (64.2%) |
| Irritable bowel syndrome, n (%) | 19 (12.7%) | 5 (16.7%) | 14 (11.7%) |
| Anxiety, n (%) | 16 (10.7%) | 4 (13.3%) | 12 (10%) |
| Depression, n (%) | 16 (10.7%) | 6 (20%) | 10 (8.3%) |
| Asthma, n (%) | 12 (8%) | 4 (13.3%) | 8 (6.7%) |
| Heartburn, n (%) | 11 (7.3%) | 3 (10%) | 8 (6.7%) |
| Migraine, n (%) | 6 (4%) | 1 (3.3%) | 5 (4.2%) |
| PCOS, n (%) | 6 (4%) | 0 (0%) | 6 (5%) |
| Ruptured ovarian cyst, n (%) | 3 (2%) | 1 (3.3%) | 2 (1.7%) |
| IDDM, n (%) | 2 (1.3%) | 0 (0%) | 2 (1.7%) |
| Anaemia, n (%) | 2 (1.3%) | 0 (0%) | 2 (1.7%) |
| Eczema, n (%) | 2 (1.3%) | 1 (3.3%) | 1 (0.8%) |
| Vit B12 deficiency, n (%) | 1 (0.7%) | 0 (0%) | 1 (0.8%) |
| Haemochromatosis, n (%) | 1 (0.7%) | 0 (0%) | 1 (0.8%) |
| Juvenille arthritis, n (%) | 1 (0.7%) | 0 (0%) | 1 (0.8%) |
| Deaf, n (%) | 1 (0.7%) | 0 (0%) | 1 (0.8%) |
| Hypermobility, n (%) | 1 (0.7%) | 0 (0%) | 1 (0.8%) |
| Fibromyalgia, n (%) | 1 (0.7%) | 0 (0%) | 1 (0.8%) |
| Diverticulitis, n (%) | 1 (0.7%) | 0 (0%) | 1 (0.8%) |
| Psoriasis, n (%) | 1 (0.7%) | 0 (0%) | 1 (0.8%) |
| Vaginismus, n (%) | 1 (0.7%) | 1 (3.3%) | 0 (0%) |
| Lactose intolerance, n (%) | 1 (0.7%) | 0 (0%) | 1 (0.8%) |
| Hypothyroid, n (%) | 1 (0.7%) | 0 (0%) | 1 (0.8%) |
| Appendiectomy, n (%) | 7 (4.7%) | 0 (0%) | 7 (5.8%) |
| Tonsilectomy, n (%) | 5 (3.3%) | 0 (0%) | 5 (4.2%) |
| Gromit and adenoids, n (%) | 2 (1.3%) | 1 (3.3%) | 1 (0.8%) |
| Breast augmentation, n (%) | 2 (1.3%) | 0 (0%) | 2 (1.7%) |
| Breast reduction, n (%) | 2 (1.3%) | 1 (3.3%) | 1 (0.8%) |
| Rhinoplasty, n (%) | 1 (0.7%) | 1 (3.3%) | 0 (0%) |
| Arthroscopy, n (%) | 1 (0.7%) | 0 (0%) | 1 (0.8%) |
| Kidney stone removal, n (%) | 1 (0.7%) | 0 (0%) | 1 (0.8%) |
| Current Medication/Contraceptive Use | All Women (150) | Endometriosis at Laparoscopy (30) | No Endometriosis at Laparoscopy (120) |
|---|---|---|---|
| Analgesia | 62 (41.3%) | 11 (36.7%) | 51 (42.5%) |
| Antidepressants | 14 (9.3%) | 6 (20%) | 8 (6.7%) |
| Anti-anxiety | 9 (6%) | 2 (6.7%) | 7 (5.8%) |
| Inhalers | 7 (4.7%) | 4 (13.3%) | 3 (2.5%) |
| Antacids | 7 (4.7%) | 3 (10%) | 4 (3.3%) |
| IBS treatment | 7 (4.7%) | 1 (3.3%) | 6 (5%) |
| Laxatives | 3 (2%) | 1 (3.3%) | 2 (1.7%) |
| Insulin | 2 (1.3%) | 0 (0%) | 2 (1.7%) |
| Folic acid | 1 (0.7%) | 0 (0%) | 1 (0.8%) |
| Vitamin B12 | 1 (0.7%) | 0 (0%) | 1 (0.8%) |
| Levothyroxine | 1 (0.7%) | 0 (0%) | 1 (0.8%) |
| Iron | 1 (0.7%) | 0 (0%) | 1 (0.8%) |
| COCP | 52 (34.7%) | 14 (46.7%) | 38 (31.7%) |
| POP | 20 (13.3%) | 4 (13.3%) | 16 (13.3%) |
| Depo | 10 (6.7%) | 2 (6.7%) | 8 (6.7%) |
| Implant | 10 (6.7%) | 2 (6.7%) | 8 (6.7%) |
| Mirena | 6 (4%) | 1 (3%) | 5 (4.2%) |
| Cu coil | 2 (1.3%) | 0 (0%) | 2 (1.7%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tempest, N.; Efstathiou, E.; Petros, Z.; Hapangama, D.K. Laparoscopic Outcomes after Normal Clinical and Ultrasound Findings in Young Women with Chronic Pelvic Pain: A Cross-Sectional Study. J. Clin. Med. 2020, 9, 2593. https://doi.org/10.3390/jcm9082593
Tempest N, Efstathiou E, Petros Z, Hapangama DK. Laparoscopic Outcomes after Normal Clinical and Ultrasound Findings in Young Women with Chronic Pelvic Pain: A Cross-Sectional Study. Journal of Clinical Medicine. 2020; 9(8):2593. https://doi.org/10.3390/jcm9082593
Chicago/Turabian StyleTempest, Nicola, Ekaterina Efstathiou, Zena Petros, and Dharani K. Hapangama. 2020. "Laparoscopic Outcomes after Normal Clinical and Ultrasound Findings in Young Women with Chronic Pelvic Pain: A Cross-Sectional Study" Journal of Clinical Medicine 9, no. 8: 2593. https://doi.org/10.3390/jcm9082593





