Sodium-Glucose Cotransporter-2 Inhibitors at Discharge from Cardiology Hospitalization Department: Decoding A New Clinical Scenario
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design
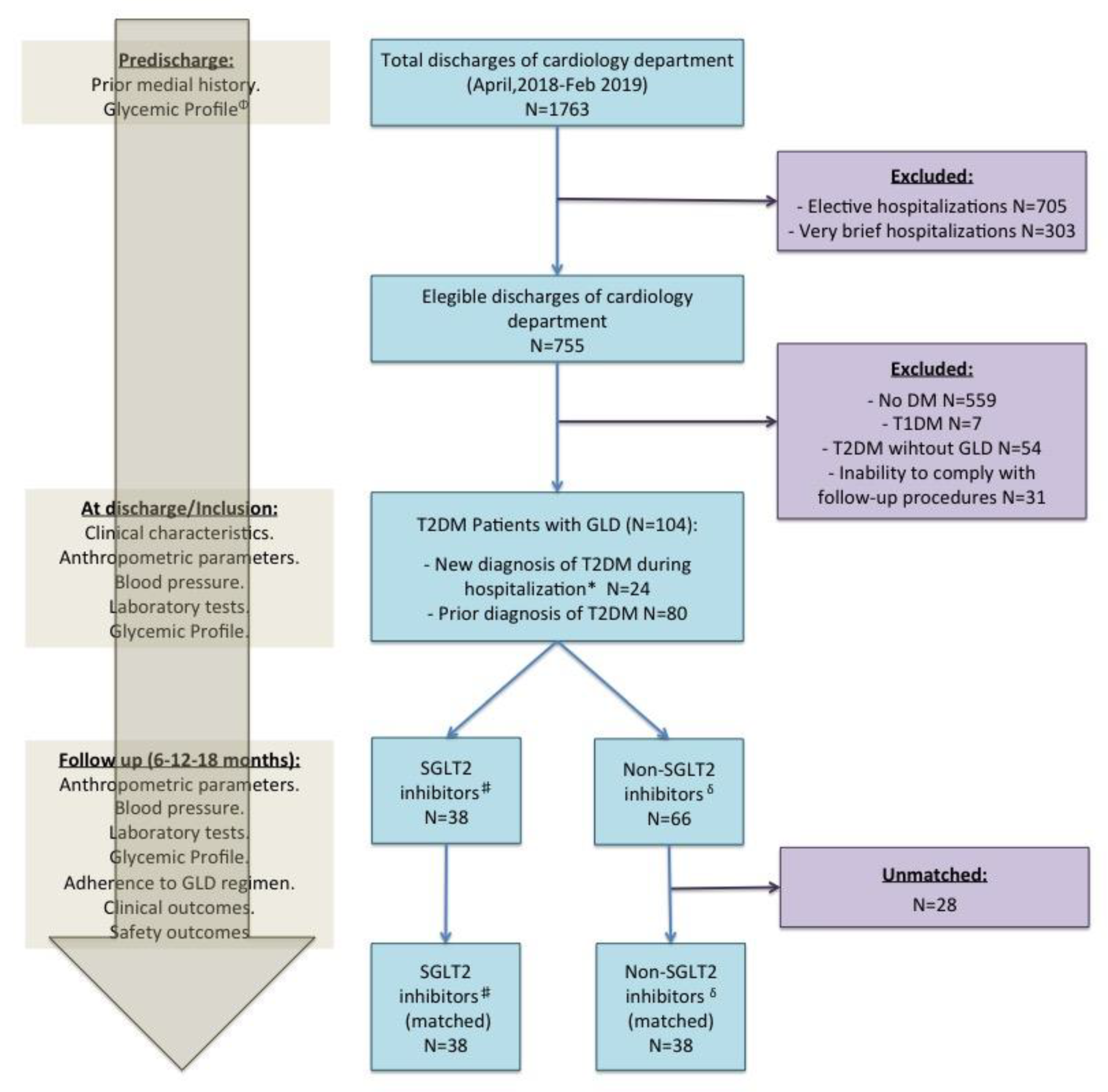
2.2. Patient Population
2.3. Study Procedures
- SGLT-2 inhibitor group: Patients who had initiated SGLT-2 inhibitors at discharge from the cardiology department (included empagliflozin, dapagliflozin or canagliflozin). These patients could be on other GLDs.
- Non-SGLT-2 inhibitor group: Patients with GLDs but without SGLT-2 inhibitors at discharge from the cardiology department. These GLDs included any of (i) insulin, (ii) metformin, (iii) sulfonylurea, (iv) dipeptidyl peptidase 4 inhibitors, (v) glucagon-like peptide-1 receptor agonist (GLP1-ra), (vi) thiazolidinediones, or (vii) other GLDs (acarbose, bromocriptine, miglitol, nateglinide, and repaglinide).
2.4. Study Endpoints
2.5. Statistical Analysis
3. Results
3.1. Patients, GLD Regimens, and Follow-Up
3.2. Safety Outcomes
3.3. Efficacy Clinical Outcomes
3.4. Other Outcomes
4. Discussion
4.1. SGLT-2 Inhibitors and Cardiovascular Disease
4.2. Safety of Prescription of SGLT-2 Inhibitors at Hospital Discharge
4.3. Morbidity and Mortality Impact of SGLT-2 Inhibitors
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Monami, M.; Liistro, F.; Scatena, A.; Nreu, B.; Mannucci, E. Short and medium-term efficacy of sodium glucose co-transporter-2 (SGLT-2) inhibitors: A meta-analysis of randomized clinical trials. Diabetes Obes. Metab. 2018, 20, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Node, K. Exploration of the clinical benefits of sodium glucose co-transporter 2 inhibitors in diabetic patients with concomitant heart failure. Cardiovasc. Diabetol. 2018, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Lytvyn, Y.; Bjornstad, P.; Udell, J.A.; Lovshin, J.A.; Cherney, D.Z.I.; Yuliya, L.; Petter, B. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation 2017, 136, 1643–1658. [Google Scholar] [CrossRef]
- McMurray, J.J.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2019, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S98–S110. [Google Scholar] [CrossRef] [Green Version]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; De Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Baglioni, P.; Wiviott, S.D.; Raz, I.; Sabatine, M.S.; Akinci, B. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 1880–1881. [Google Scholar]
- Udell, J.A.; Yuan, Z.; Rush, T.; Sicignano, N.M.; Galitz, M.; Rosenthal, N. Cardiovascular Outcomes and Risks after Initiation of a Sodium Glucose Cotransporter 2 Inhibitor: Results from the EASEL population-based cohort study (Evidence for Cardiovascular Outcomes with Sodium Glucose Cotransporter 2 Inhibitors in the Real World). Circulation 2018, 137, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.S.; Siddiqi, T.J.; Memon, M.M.; Khan, M.S.; Rawasia, W.F.; Ayub, M.T.; Sreenivasan, J.; Golzar, Y. Sodium-glucose co-transporter 2 inhibitors and cardiovascular outcomes: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2018, 25, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Koufakis, T.; Mustafa, O.G.; Ajjan, R.A.; Garcia-Moll, X.; Zebekakis, P.; Dimitriadis, G.; Kotsa, K. The use of sodium-glucose co-transporter 2 inhibitors in the inpatient setting: Is the risk worth taking? J. Clin. Pharm. Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Sathiyakumar, V.; Singh, A.; McCarthy, C.P.; Qamar, A.; Januzzi, J.L.; Scirica, B.M.; Butler, J.; Cannon, C.P.; Bhatt, D.L. Prescriber Patterns of SGLT2i After Expansions of U.S. Food and Drug Administration Labeling. J. Am. Coll. Cardiol. 2018, 72, 3370–3372. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 41, S14–S31. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Jabbour, S.A.; Goldstein, B.J. Sodium glucose co-transporter 2 inhibitors: Blocking renal tubular reabsorption of glucose to improve glycaemic control in patients with diabetes. Int. J. Clin. Pract. 2008, 62, 1279–1284. [Google Scholar] [CrossRef]
- Targher, G.; Dauriz, M.; Laroche, C.; Temporelli, P.L.; Hssanein, M.; Seferovic, P.M.; Drozdz, J.; Ferrari, R.; Anker, S.; Coats, A.; et al. In-hospital and 1-year mortality associated with diabetes in patients with acute heart failure: Results from the ESC-HFA Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Komaru, Y.; Takeuchi, T.; Suzuki, L.; Asano, T.; Urayama, K.Y. Recurrent cardiovascular events in patients with newly diagnosed acute coronary syndrome: Influence of diabetes and its management with medication. J. Diabetes Its Complicat. 2020, 34, 107511. [Google Scholar] [CrossRef]
- Ferrannini, G.; De Bacquer, D.; De Backer, G.; Kotseva, K.; Mellbin, L.; Wood, D.; Ryden, L.; Collaborators, E.V. Screening for Glucose Perturbations and Risk Factor Management in Dysglycemic Patients with Coronary Artery Disease—A Persistent Challenge in Need of Substantial Improvement: A Report From ESC EORP EUROASPIRE V. Diabetes Care 2020, 43, 726–733. [Google Scholar] [CrossRef] [Green Version]
- De la Hera, J.M.; Delgado, E.; Hernández, E.; García-Ruiz, J.M.; Vegas, J.M.; Avanzas, P.; Lozano, I.; Barriales-Villa, R.; Hevia, S.; Martín, J.S.; et al. Prevalence and outcome of newly detected diabetes in patients who undergo percutaneous coronary intervention. Eur. Heart J. 2009, 30, 2614–2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, S.V.; De Lemos, J.A.; Rosenson, R.S.; Ballantyne, C.M.; Liu, Y.; Mues, K.E.; Alam, S.; Elliott-Davey, M.; Bhatt, D.L.; Cannon, C.P.; et al. Use of Guideline-Recommended Risk Reduction Strategies Among Patients With Diabetes and Atherosclerotic Cardiovascular Disease. Circulation 2019, 140, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Fonarow, G.C.; Lippmann, S.J.; Mi, X.; Heidenreich, P.A.; Yancy, C.W.; Greiner, M.; Hammill, B.G.; Hardy, N.C.; Turner, S.J.; et al. Early adoption of sacubitril/valsartan for patients with heart failure with reduced ejection fraction: Insights from Get with the Guidelines–Heart Failure (GWTG-HF). JACC Heart Fail. 2017, 5, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Wachter, R.; Senni, M.; Belohlavek, J.; Straburzynska-Migaj, E.; Witte, K.K.; Kobalava, Z.; Fonseca, C.; Goncalvesova, E.; Cavusoglu, Y.; Fernandez, A.; et al. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: Primary results of the randomised TRANSITION study. Eur. J. Heart Fail. 2019, 21, 998–1007. [Google Scholar] [CrossRef] [Green Version]
- Damman, K.; Beusekamp, J.C.; Boorsma, E.M.; Swart, H.P.; Smilde, T.D.; Elvan, A.; Van Eck, J.M.; Heerspink, H.J.; Voors, A.A. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur. J. Heart Fail. 2020, 22, 713–722. [Google Scholar] [CrossRef] [Green Version]



| Glucose-Lowering Drugs | Unmatched | Propensity-Score Matched | ||||
|---|---|---|---|---|---|---|
| SGLT-2 Inhibitors (n = 38) | Non-SGLT-2 Inhibitors (n = 66) | p-Value | SGLT-2 Inhibitors (n = 38) | Non-SGLT-2 Inhibitors (n = 38) | p-Value | |
| Metformin | 35 (92%) | 34 (52%) | <0.001 | 35 (92%) | 31 (82%) | 0.309 |
| Dipeptidyl peptidase 4 inhibitors | 8 (21%) | 18 (27%) | 0.638 | 8 (21%) | 8 (21%) | 1 |
| Sulfonylurea | 2 (5%) | 3 (5%) | 1 | 2 (5%) | 2 (5%) | 1 |
| Insulin | 9 (24%) | 21 (32%) | 0.511 | 9 (24%) | 7 (18%) | 0.778 |
| Sodium-glucose cotransporter 2 inhibitors | 38 (100%) | 0 | – | 38 (100%) | 0 | – |
| Glucagon-like peptide 1 receptor agonist | 3 (8%) | 1 (2%) | 0.271 | 3 (8%) | 1 (3%) | 0.615 |
| Characteristics | Unmatched | Propensity-Score Matched | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SGLT-2 Inhibitors (n = 38) | Non-SGLT-2 Inhibitors (n = 66) | SGLT-2 Inhibitors (n = 38) | Non-SGLT-2 Inhibitors (n = 38) | |||||||
| n/Mean | %/SD | n/Mean | %/SD | p-Value | n/Mean | %/SD | n/Mean | %/SD | p-Value | |
| Prior history | ||||||||||
| Age (years) | 65.7 | 10.6 | 74 | 11.3 | <0.001 | 65.7 | 10.6 | 69.4 | 11.4 | 0.152 |
| Male sex n (%) | 25 | 65.8 | 43 | 63.2 | 0.947 | 25 | 65.8 | 25 | 65.8 | 1 |
| Smoke n (%) | 6 | 15.8 | 6 | 9.1 | 0.303 | 6 | 15.8 | 4 | 10.5 | 0.197 |
| Hypertension n (%) | 21 | 55.3 | 36 | 63.2 | 0.944 | 21 | 55.3 | 16 | 42.1 | 0.359 |
| History of chronic kidney disease Ψ n (%) | 2 | 5.3 | 22 | 33.3 | 0.001 | 2 | 5.3 | 3 | 7.89 | 1 |
| History of cerebrovascular disease n (%) | 0 | 0 | 8 | 12.1 | 0.025 | 0 | 0 | 0 | 0 | 1 |
| History of heart failure n (%) | 5 | 13.1 | 15 | 22.7 | 0.233 | 5 | 13.1 | 7 | 18.4 | 0.753 |
| History of coronary artery disease n (%) | 11 | 28.9 | 18 | 27.3 | 0.858 | 11 | 28.9 | 10 | 26.32 | 1 |
| Discharge diagnosis | ||||||||||
| STEMI n (%) | 6 | 15.8 | 4 | 6.1 | 0.105 | 6 | 15.8 | 2 | 5.3 | 0.135 |
| NSTEMI n (%) | 8 | 21.1 | 21 | 31.8 | 0.238 | 8 | 21.1 | 13 | 34.2 | 0.200 |
| Unstable Angina n (%) | 9 | 23.7 | 10 | 15.2 | 0.278 | 9 | 23.7 | 9 | 23.7 | 1 |
| Stable heart disease n (%) | 3 | 7.9 | 4 | 6.1 | 0.719 | 3 | 7.9 | 2 | 5.3 | 0.644 |
| Heart failure n (%) | 7 | 18.4 | 15 | 22.7 | 0.605 | 7 | 18.4 | 15 | 22.7 | 0.605 |
| Arrhythmia n (%) | 4 | 10.5 | 8 | 12.1 | 0.806 | 4 | 10.5 | 8 | 12.1 | 0.806 |
| Others n (%) | 1 | 2.6 | 3 | 4.5 | 0.625 | 1 | 2.6 | 3 | 4.5 | 0.625 |
| Anthropometrical parameters, laboratory tests, and glycemic profile (at inclusion) | ||||||||||
| Systolic blood pressure (mmHg) | 124.8 | 16.3 | 127.3 | 16.5 | 0.453 | 124.789 | 16.28 | 125.189 | 13.737 | 0.909 |
| Diastolic blood pressure (mmHg) | 69.9 | 8.9 | 65.8 | 12.8 | 0.057 | 69.947 | 8.88 | 68.622 | 13.149 | 0.612 |
| Body mass index Φ (%) | 29.091 | 4.485 | 28.321 | 5.327 | 0.453 | 29.091 | 4.485 | 28.72 | 6.081 | 0.776 |
| Fasting plasma glucose (mg/dL) | 137.447 | 49.465 | 145.97 | 64.839 | 0.453 | 137.447 | 49.465 | 147.632 | 66.039 | 0.449 |
| Glycated hemoglobin (%) | 7.787 | 1.596 | 7.285 | 1.554 | 0.124 | 7.787 | 1.596 | 7.286 | 1.619 | 0.182 |
| eGFR φ (mL/min/1.73 m2) | 77.343 | 18.148 | 58.19 | 27.425 | <0.001 | 77.343 | 18.148 | 71.886 | 21.351 | 0.253 |
| LDL-cholesterol (mg/dL) | 84.211 | 36.1 | 84.523 | 38.861 | 0.967 | 84.211 | 36.1 | 89.622 | 44.013 | 0.563 |
| LVEF (%) | 52.71 | 11.536 | 53.861 | 12.856 | 0.667 | 52.71 | 11.536 | 51.848 | 12.592 | 0.776 |
| Duration of T2DM (months) | 66.9 | 0.2 | 66.9 | 0.3 | 0.977 | 66.9 | 0.2 | 67.0 | 0.2 | 0.261 |
| Cardiovascular therapies | ||||||||||
| Antiplatelet agents n (%) | 26 | 68.4 | 46 | 69.7 | 0.892 | 26 | 68.42 | 27 | 71.05 | 1 |
| Anticoagulants n (%) | 6 | 15.8 | 12 | 18.2 | 0.756 | 6 | 15.79 | 5 | 13.16 | 1 |
| ACE inhibitor or ARB n (%) | 26 | 68.4 | 25 | 37.9 | 0.003 | 26 | 68.42 | 15 | 39.47 | 0.021 |
| MRA n (%) | 1 | 2.6 | 4 | 6.1 | 0.431 | 1 | 2.63 | 4 | 10.53 | 0.358 |
| Beta-blocker n (%) | 25 | 65.8 | 44 | 66.7 | 0.927 | 25 | 65.79 | 26 | 68.42 | 1 |
| Statins n (%) | 29 | 76.3 | 48 | 72.7 | 0.688 | 29 | 76.32 | 27 | 71.05 | 0.794 |
| Ezetimibe n (%) | 3 | 7.9 | 12 | 18.2 | 0.15 | 3 | 7.89 | 8 | 21.05 | 0.191 |
| Loop diuretics n (%) | 6 | 15.8 | 25 | 37.9 | 0.018 | 6 | 15.79 | 11 | 28.95 | 0.271 |
| Safety Outcome | SGLT-2 Inhibitors (n = 38) | Non-SGLT-2 Inhibitors (n = 38) | p-Value |
|---|---|---|---|
| Discontinuation of SGLT-2 inhibitors | 1 (3%) | – | – |
| Discontinuation of other GLDs | 10 (26%) | 10 (26%) | 1 |
| Worsening renal function | 1 (2.6%) | 2 (5.3%) | 0.94 |
| Death from renal cause | 0 | 0 | 1 |
| Hospitalization for renal cause | 0 | 2 (5.3%) | 0.16 |
| Hospitalization for hepatic injury, metabolic acidosis, ketoacidosis, or diabetic ketoacidosis. | 0 | 0 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozado, J.; García Iglesias, D.; Soroa, M.; Junco-Vicente, A.; Barja, N.; Adeba, A.; Vigil-Escalera, M.; Alvarez, R.; Torres Saura, F.; Capín, E.; et al. Sodium-Glucose Cotransporter-2 Inhibitors at Discharge from Cardiology Hospitalization Department: Decoding A New Clinical Scenario. J. Clin. Med. 2020, 9, 2600. https://doi.org/10.3390/jcm9082600
Rozado J, García Iglesias D, Soroa M, Junco-Vicente A, Barja N, Adeba A, Vigil-Escalera M, Alvarez R, Torres Saura F, Capín E, et al. Sodium-Glucose Cotransporter-2 Inhibitors at Discharge from Cardiology Hospitalization Department: Decoding A New Clinical Scenario. Journal of Clinical Medicine. 2020; 9(8):2600. https://doi.org/10.3390/jcm9082600
Chicago/Turabian StyleRozado, José, Daniel García Iglesias, Miguel Soroa, Alejandro Junco-Vicente, Noemí Barja, Antonio Adeba, María Vigil-Escalera, Rut Alvarez, Francisco Torres Saura, Esmeralda Capín, and et al. 2020. "Sodium-Glucose Cotransporter-2 Inhibitors at Discharge from Cardiology Hospitalization Department: Decoding A New Clinical Scenario" Journal of Clinical Medicine 9, no. 8: 2600. https://doi.org/10.3390/jcm9082600
APA StyleRozado, J., García Iglesias, D., Soroa, M., Junco-Vicente, A., Barja, N., Adeba, A., Vigil-Escalera, M., Alvarez, R., Torres Saura, F., Capín, E., García, L., Rodriguez, M. L., Calvo, D., Moris, C., Delgado, E., & de la Hera, J. M. (2020). Sodium-Glucose Cotransporter-2 Inhibitors at Discharge from Cardiology Hospitalization Department: Decoding A New Clinical Scenario. Journal of Clinical Medicine, 9(8), 2600. https://doi.org/10.3390/jcm9082600






