Long-Term Outcomes of Switching from Fixed-Dose to As-Needed Regimen for Treating Submacular Hemorrhage Secondary to Polypoidal Choroidal Vasculopathy
Abstract
:1. Introduction
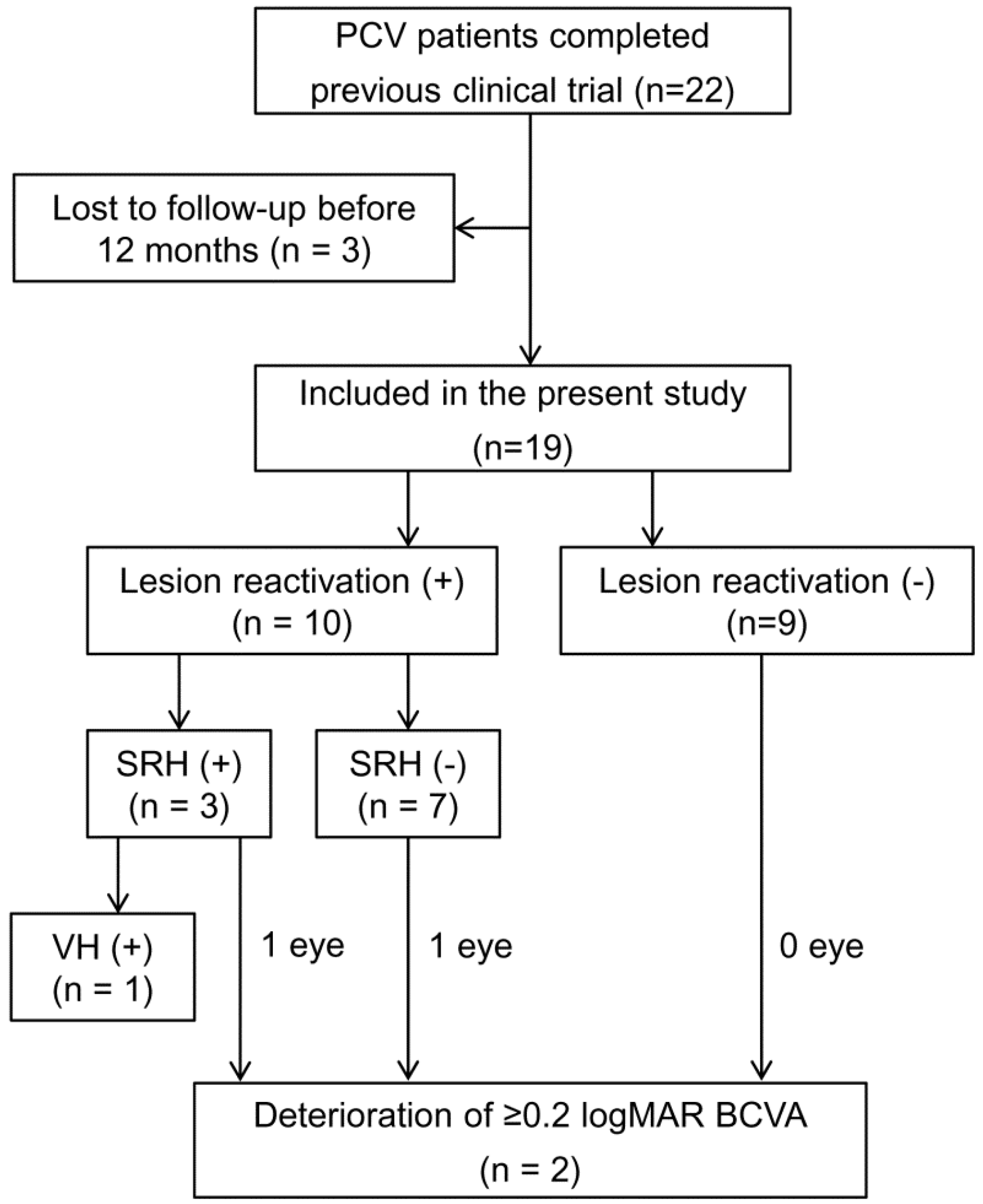
2. Materials and Methods
2.1. Patients
2.2. Examinations, Treatment, and Follow-Up
2.3. Outcome Measures
2.4. Statistical Analyses
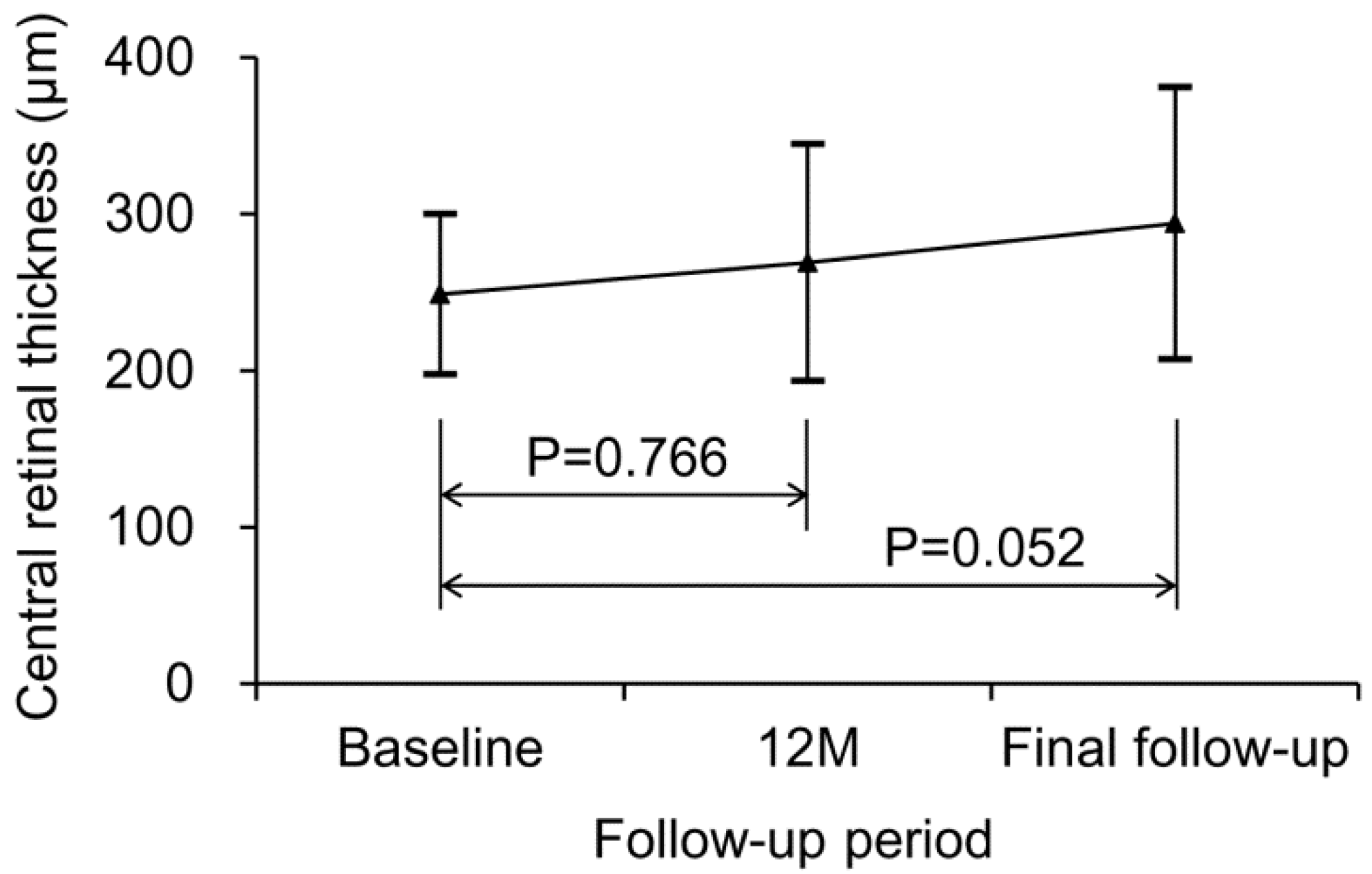
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Yannuzzi, L.A.; Sorenson, J.; Spaide, R.F.; Lipson, B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990, 10, 1–8. [Google Scholar] [CrossRef]
- Spaide, R.F.; Yannuzzi, L.A.; Slakter, J.S.; Sorenson, J.; Orlach, D.A. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 1995, 15, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dolz-Marco, R.; Messinger, J.D.; Sloan, K.R.; Ferrara, D.; Curcio, C.A.; Freund, K.B. Clinicopathologic Correlation of Aneurysmal Type 1 Neovascularization in Age-Related Macular Degeneration. Ophthalmol. Retina 2019, 3, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Wong, T.Y.; Cheung, C.M. Polypoidal Choroidal Vasculopathy in Asians. J. Clin. Med. 2015, 4, 782–821. [Google Scholar] [CrossRef]
- Chan, W.M.; Lam, D.S.; Lai, T.Y.; Liu, D.T.; Li, K.K.; Yao, Y.; Wong, T.H. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: One-year results of a prospective case series. Ophthalmology 2004, 111, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.H.; Chen, L.J.; Chen, S.J.; Chen, Y.; Giridhar, A.; Iida, T.; Kim, H.; Yuk Yau Lai, T.; Lee, W.K.; Li, X.; et al. Polypoidal choroidal vasculopathy: Evidence-based guidelines for clinical diagnosis and treatment. Retina 2013, 33, 686–716. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.G.; Lai, T.Y.Y.; Ruamviboonsuk, P.; Chen, S.J.; Chen, Y.; Freund, K.B.; Gomi, F.; Koh, A.H.; Lee, W.K.; Wong, T.Y. Polypoidal Choroidal Vasculopathy: Definition, Pathogenesis, Diagnosis, and Management. Ophthalmology 2018, 125, 708–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, T.H.; Lai, T.Y.Y.; Takahashi, K.; Wong, T.Y.; Chen, L.J.; Ruamviboonsuk, P.; Tan, C.S.; Lee, W.K.; Cheung, C.M.G.; Ngah, N.F.; et al. Comparison of Ranibizumab with or without Verteporfin Photodynamic Therapy for Polypoidal Choroidal Vasculopathy: The EVEREST II Randomized Clinical Trial. JAMA Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Chong Teo, K.Y.; Squirrell, D.M.; Nguyen, V.; Banerjee, G.; Cohn, A.; Barthelmes, D.; Gemmy Cheung, C.M.; Gillies, M. A Multicountry Comparison of Real-World Management and Outcomes of Polypoidal Choroidal Vasculopathy: Fight Retinal Blindness! Cohort. Ophthalmol. Retina 2019, 3, 220–229. [Google Scholar] [CrossRef]
- Kim, J.H.; Chang, Y.S.; Kim, J.W.; Kim, C.G. Characteristics of submacular hemorrhages in age-related macular degeneration. Optom. Vis. Sci. 2017, 94, 556–563. [Google Scholar] [CrossRef]
- Yiu, G.; Mahmoud, T.H. Subretinal hemorrhage. Dev. Ophthalmol. 2014, 54, 213–222. [Google Scholar] [PubMed]
- Kim, J.H.; Chang, Y.S.; Kim, C.G.; Lee, D.W.; Han, J.I. Hyperpigmented spots after treatment for submacular hemorrhage secondary to polypoidal choroidal vasculopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chang, Y.S.; Lee, D.W.; Kim, C.G.; Kim, J.W. Quantification of retinal changes after resolution of submacular hemorrhage secondary to polypoidal choroidal vasculopathy. Jpn. J. Ophthalmol. 2018, 62, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chang, Y.S.; Kim, J.W.; Kim, C.G.; Yoo, S.J.; Cho, H.J. Intravitreal anti-vascular endothelial growth factor for submacular hemorrhage from choroidal neovascularization. Ophthalmology 2014, 121, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, C.G.; Lee, D.W.; Yoo, S.J.; Lew, Y.J.; Cho, H.J.; Kim, J.Y.; Lee, S.H.; Kim, J.W. Intravitreal aflibercept for submacular hemorrhage secondary to neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Lee, J.M.; Byeon, S.H. Anti-vascular endothelial growth factor with or without pneumatic displacement for submacular hemorrhage. Am. J. Ophthalmol. 2015, 159, 904–914. [Google Scholar] [CrossRef]
- Kang, H.G.; Kang, H.; Byeon, S.H.; Kim, S.S.; Koh, H.J.; Lee, S.C.; Kim, M. Long-term visual outcomes for treatment of submacular haemorrhage secondary to polypoidal choroidal vasculopathy. Clin. Exp. Ophthalmol. 2018, 46, 916–925. [Google Scholar] [CrossRef]
- Miyamoto, N.; Mandai, M.; Oishi, A.; Nakai, S.; Honda, S.; Hirashima, T.; Oh, H.; Matsumoto, Y.; Uenishi, M.; Kurimoto, Y. Long-term results of photodynamic therapy or ranibizumab for polypoidal choroidal vasculopathy in LAPTOP study. Br. J. Ophthalmol. 2019, 103, 844–848. [Google Scholar] [CrossRef]
- Fung, A.E.; Lalwani, G.A.; Rosenfeld, P.J.; Dubovy, S.R.; Michels, S.; Feuer, W.J.; Puliafito, C.A.; Davis, J.L.; Flynn, H.W., Jr.; Esquiabro, M. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am. J. Ophthalmol. 2007, 143, 566–583. [Google Scholar] [CrossRef]
- Chin-Yee, D.; Eck, T.; Fowler, S.; Hardi, A.; Apte, R.S. A systematic review of as needed versus treat and extend ranibizumab or bevacizumab treatment regimens for neovascular age-related macular degeneration. Br. J. Ophthalmol. 2016, 100, 914–917. [Google Scholar] [CrossRef]
- Arendt, P.; Yu, S.; Munk, M.R.; Ebneter, A.; Wolf, S.; Zinkernagel, M.S. Exit strategy in a treat-and-extend regimen for exudative age-related macular degeneration. Retina 2019, 39, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Adrean, S.D.; Chaili, S.; Grant, S.; Pirouz, A. Recurrence Rate of Choroidal Neovascularization in Neovascular Age-Related Macular Degeneration Managed with a Treat-Extend-Stop Protocol. Ophthalmol. Retina 2018, 2, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Sloan, F.A.; Hanrahan, B.W. The effects of technological advances on outcomes for elderly persons with exudative age-related macular degeneration. JAMA Ophthalmol. 2014, 132, 456–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [Green Version]
- Veritti, D.; Sarao, V.; Missiroli, F.; Ricci, F.; Lanzetta, P. Twelve-month outcomes of intravitreal aflibercept for neovascular age-related macular degeneration: Fixed versus as-needed dosing. Retina 2019, 39, 2077–2083. [Google Scholar] [CrossRef]
- Augsburger, M.; Sarra, G.M.; Imesch, P. Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age-related macular degeneration: A comparative study. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1889–1895. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Park, S.J.; Byun, S.J.; Park, K.H.; Suh, H.S. Incremental economic burden associated with exudative age-related macular degeneration: A population-based study. BMC Health Serv. Res. 2019, 19, 828. [Google Scholar] [CrossRef]
- Prenner, J.L.; Halperin, L.S.; Rycroft, C.; Hogue, S.; Williams Liu, Z.; Seibert, R. Disease Burden in the Treatment of Age-Related Macular Degeneration: Findings From a Time-and-Motion Study. Am. J. Ophthalmol. 2015, 160, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, V.; Gusenbauer, K.; Mak, M.; Barbosa, J.; Mohammad Mohaghegh, P.S.; Popovic, M. Waiting room educational media effect on preinjection anxiety for initial intravitreal injections. Can. J. Ophthalmol. 2016, 51, 71–75. [Google Scholar] [CrossRef]
- Segal, O.; Segal-Trivitz, Y.; Nemet, A.Y.; Cohen, P.; Geffen, N.; Mimouni, M. Anxiety levels and perceived pain intensity during intravitreal injections. Acta Ophthalmol. 2016, 94, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Munk, M.R.; Arendt, P.; Yu, S.; Ceklic, L.; Huf, W.; Ebneter, A.; Wolf, S.; Zinkernagel, M.S. The Impact of the Vitreomacular Interface in Neovascular Age-Related Macular Degeneration in a Treat-and-Extend Regimen with Exit Strategy. Ophthalmol. Retina 2018, 2, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chang, Y.S.; Lee, D.W.; Kim, C.G.; Kim, J.W. Incidence and Timing of the First Recurrence in Neovascular Age-Related Macular Degeneration: Comparison Between Ranibizumab and Aflibercept. J. Ocul. Pharmacol. Ther. 2017, 33, 445–451. [Google Scholar] [CrossRef]
- Tsujikawa, A.; Ojima, Y.; Yamashiro, K.; Nakata, I.; Ooto, S.; Tamura, H.; Nakanishi, H.; Hayashi, H.; Otani, A.; Yoshimura, N. Association of lesion size and visual prognosis to polypoidal choroidal vasculopathy. Am. J. Ophthalmol. 2011, 151, 961–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.M.; Koh, H.J. Long-term visual outcome and prognostic factors after intravitreal ranibizumab injections for polypoidal choroidal vasculopathy. Am. J. Ophthalmol. 2013, 156, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Hikichi, T. Six-year outcomes of antivascular endothelial growth factor monotherapy for polypoidal choroidal vasculopathy. Br. J. Ophthalmol. 2018, 102, 97–101. [Google Scholar] [CrossRef]
- Wong, T.Y.; Ogura, Y.; Lee, W.K.; Iida, T.; Chen, S.J.; Mitchell, P.; Gemmy Cheung, C.M.; Zhang, Z.; Leal, S.; Ishibashi, T. PLANET Investigators. Efficacy and Safety of Intravitreal Aflibercept for Polypoidal Choroidal Vasculopathy: Two-Year Results of the Aflibercept in Polypoidal Choroidal Vasculopathy Study. Am. J. Ophthalmol. 2019, 204, 80–89. [Google Scholar] [CrossRef]
- Doble, B.; Finkelstein, E.A.; Tian, Y.; Saxena, N.; Patil, S.; Wong, T.Y.; Cheung, C.M.G. Cost-effectiveness of Intravitreal Ranibizumab with Verteporfin Photodynamic Therapy Compared With Ranibizumab Monotherapy for Patients with Polypoidal Choroidal Vasculopathy. JAMA Ophthalmol. 2020, 138, 251–259. [Google Scholar] [CrossRef]

| Characteristic | |
|---|---|
| Age | 67.7 ± 7.0 |
| Sex | |
| Men | 16 (84.2%) |
| Women | 3 (15.8%) |
| Hypertension | 10 (52.6%) |
| Diabetes mellitus | 4 (21.1%) |
| Best-corrected visual acuity, logMAR | 0.26 ± 0.34 |
| Central retinal thickness, μm | 248.8 ± 51.3 |
| Serous pigment epithelial detachment | 3 (15.8%) |
| Presence of intraretinal/subretinal fluid or hemorrhage | 0 |
| Characteristics | P * | β | 95% CI |
|---|---|---|---|
| Age | 0.647 | ||
| Sex | 0.134 | ||
| Best-corrected visual acuity | 0.388 | ||
| Central retinal thickness | 0.572 | ||
| Number of polypoidal lesions | 0.657 | ||
| Size of the lesion | 0.009 | 32.000 | 2.394–427.744 |
| Serous pigment epithelial detachment | 0.455 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Kim, J.W.; Kim, C.G.; Lee, D.W. Long-Term Outcomes of Switching from Fixed-Dose to As-Needed Regimen for Treating Submacular Hemorrhage Secondary to Polypoidal Choroidal Vasculopathy. J. Clin. Med. 2020, 9, 2637. https://doi.org/10.3390/jcm9082637
Kim JH, Kim JW, Kim CG, Lee DW. Long-Term Outcomes of Switching from Fixed-Dose to As-Needed Regimen for Treating Submacular Hemorrhage Secondary to Polypoidal Choroidal Vasculopathy. Journal of Clinical Medicine. 2020; 9(8):2637. https://doi.org/10.3390/jcm9082637
Chicago/Turabian StyleKim, Jae Hui, Jong Woo Kim, Chul Gu Kim, and Dong Won Lee. 2020. "Long-Term Outcomes of Switching from Fixed-Dose to As-Needed Regimen for Treating Submacular Hemorrhage Secondary to Polypoidal Choroidal Vasculopathy" Journal of Clinical Medicine 9, no. 8: 2637. https://doi.org/10.3390/jcm9082637
APA StyleKim, J. H., Kim, J. W., Kim, C. G., & Lee, D. W. (2020). Long-Term Outcomes of Switching from Fixed-Dose to As-Needed Regimen for Treating Submacular Hemorrhage Secondary to Polypoidal Choroidal Vasculopathy. Journal of Clinical Medicine, 9(8), 2637. https://doi.org/10.3390/jcm9082637




