Early Intervention in Ulcerative Colitis: Ready for Prime Time?
Abstract
:1. Introduction
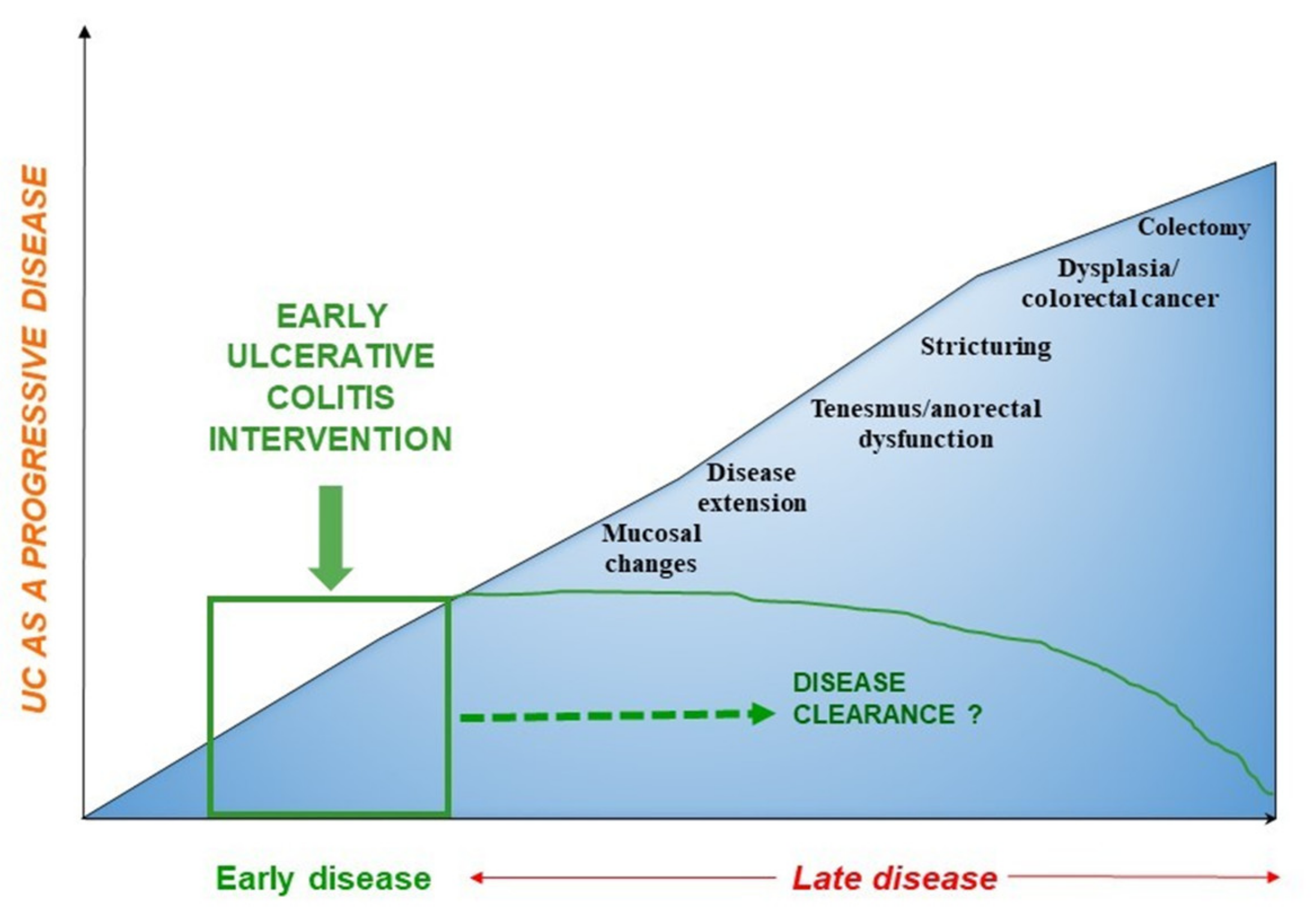
2. Ulcerative Colitis: A Progressive Disease
3. Early Disease: The Definition
4. Lessons from Early Intervention in CD
5. Evidence on Early Intervention in UC
6. Outlook
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar]
- Torres, J.; Billioud, V.; Sachar, D.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis as a progressive disease: The forgotten evidence. Inflamm. Bowel Dis. 2012, 18, 1356–1363. [Google Scholar]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar]
- Peyrin-Biroulet, L.; Bressenot, A.; Kampman, W. Histologic Remission: The Ultimate Therapeutic Goal in Ulcerative Colitis? Clin. Gastroenterol. Hepatol. 2014, 12, 929–934.e2. [Google Scholar]
- Danese, S.; Fiorino, G.; Peyrin-Biroulet, L. Early intervention in Crohn’s disease: Towards disease modification trials. Gut 2017, 66, 2179–2187. [Google Scholar]
- Danese, S.; Fiorino, G.; Fernandes, C.; Peyrin-Biroulet, L. Catching the Therapeutic Window of Opportunity in Early Crohn’s Disease. Curr. Drug Targets 2014, 15, 1056–1063. [Google Scholar]
- Bryant, R.V.; Costello, S.P.; Schoeman, S.; Sathananthan, D.; Knight, E.; Lau, S.Y.; Schoeman, M.N.; Mountifield, R.; Tee, D.; Travis, S.P.L.; et al. Limited uptake of ulcerative colitis “treat-to-target” recommendations in real-world practice. J. Gastroenterol. Hepatol. 2018, 33, 599–607. [Google Scholar]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Corrigendum: Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohn’s Colitis 2017, 11, 769–784. [Google Scholar]
- Le Berre, C.; Ananthakrishnan, A.N.; Danese, S.; Singh, S.; Peyrin-Biroulet, L. Ulcerative Colitis and Crohn’s Disease Have Similar Burden and Goals for Treatment. Clin. Gastroenterol. Hepatol. 2020, 18, 14–23. [Google Scholar]
- Williet, N.; Pillot, C.; Oussalah, A.; Billioud, V.; Chevaux, J.B.; Bresler, L.; Bigard, M.A.; Gueant, J.L.; Peyrin-Biroulet, L. Incidence of and impact of medications on colectomy in newly diagnosed ulcerative colitis in the era of biologics. Inflamm. Bowel Dis. 2012, 18, 1641–1646. [Google Scholar]
- Colombel, J.F.; Rutgeerts, P.; Reinisch, W.; Esser, D.; Wang, Y.; Lang, Y.; Marano, C.W.; Strauss, R.; Oddens, B.J.; Feagan, B.G.; et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011, 141, 1194–1201. [Google Scholar]
- Fumery, M.; Singh, S.; Dulai, P.S.; Gower-Rousseau, C.; Peyrin-Biroulet, L.; Sandborn, W.J. Natural History of Adult Ulcerative Colitis in Population-based Cohorts: A Systematic Review. Clin. Gastroenterol. Hepatol. 2018, 16, 343–356.e3. [Google Scholar]
- Ochsenkühn, T.; D’Haens, G. Current misunderstandings in the management of ulcerative colitis. Gut 2011, 60, 1294–1299. [Google Scholar]
- Meucci, G.; Vecchi, M.; Astegiano, M.; Beretta, L.; Cesari, P.; Dizioli, P.; Ferraris, L.; Panelli, M.R.; Prada, A.; Sostegni, R.; et al. The natural history of ulcerative proctitis: A multicenter, retrospective study. Am. J. Gastroenterol. 2000, 95, 469–473. [Google Scholar]
- Gordon, I.O.; Agrawal, N.; Willis, E.; Goldblum, J.R.; Lopez, R.; Allende, D.; Liu, X.; Patil, D.Y.; Yerian, L.; El-Khider, F.; et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment. Pharmacol. Ther. 2018, 47, 922–939. [Google Scholar]
- Gore, R.M.; Stoker, J. Imaging of the Colon and Rectum: Inflammatory and Neoplastic Diseases. In Diseases of The Abdomen and Pelvis; Springer: Milano, Italy, 2006; pp. 74–83. [Google Scholar]
- Rutter, M.; Saunders, B.; Wilkinson, K.; Rumbles, S.; Schofield, G.; Kamm, M.; Williams, C.; Price, A.; Talbot, I.; Forbes, A. Severity of Inflammation Is a Risk Factor for Colorectal Neoplasia in Ulcerative Colitis. Gastroenterology 2004, 126, 451–459. [Google Scholar]
- Gupta, R.B.; Harpaz, N.; Itzkowitz, S.; Hossain, S.; Matula, S.; Kornbluth, A.; Bodian, C.; Ullman, T. Histologic Inflammation Is a Risk Factor for Progression to Colorectal Neoplasia in Ulcerative Colitis: A Cohort Study. Gastroenterology 2007, 133, 1099–1105. [Google Scholar]
- Rubin, D.T.; Huo, D.; Kinnucan, J.A.; Sedrak, M.S.; McCullom, N.E.; Bunnag, A.P.; Raun-Royer, E.P.; Cohen, R.D.; Hanauer, S.B.; Hart, J.; et al. Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: A case-control study. Clin. Gastroenterol. Hepatol. 2013, 11, 1601–1608.e4. [Google Scholar]
- Danese, S. Ulcerative colitis: A cinderella story. Curr. Drug Targets 2011, 12, 1372. [Google Scholar]
- Rocchi, A.; Benchimol, E.I.; Bernstein, C.N.; Bitton, A.; Feagan, B.; Panaccione, R.; Glasgow, K.W.; Fernandes, A.; Ghosh, S. Inflammatory bowel disease: A Canadian burden of illness review. Can. J. Gastroenterol. 2012, 26, 811–817. [Google Scholar]
- Peyrin-Biroulet, L.; Germain, A.; Patel, A.S.; Lindsay, J.O. Systematic review: Outcomes and post-operative complications following colectomy for ulcerative colitis. Aliment. Pharmacol. Ther. 2016, 44, 807–816. [Google Scholar]
- Ghosh, S.; Mitchell, R. Impact of inflammatory bowel disease on quality of life: Results of the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) patient survey. J. Crohn’s Colitis 2007, 1, 10–20. [Google Scholar]
- Gower-Rousseau, C.; Sarter, H.; Savoye, G.; Tavernier, N.; Fumery, M.; Sandborn, W.J.; Feagan, B.G.; Duhamel, A.; Guillon-Dellac, N.; Colombel, J.F.; et al. Validation of the inflammatory bowel disease disability index in a population-based cohort. Gut 2017, 66, 588–596. [Google Scholar]
- Peyrin-Biroulet, L.; Billioud, V.; D’Haens, G.; Panaccione, R.; Feagan, B.; Panés, J.; Danese, S.; Schreiber, S.; Ogata, H.; Hibi, T.; et al. Development of the Paris definition of early Crohn’s disease for disease-modification trials: Results of an international expert opinion process. Am. J. Gastroenterol. 2012, 107, 1770–1776. [Google Scholar]
- Van Der Kooij, S.M.; Goekoop-Ruiterman, Y.P.M.; De Vries-Bouwstra, J.K.; Güler-Yüksel, M.; Zwinderman, A.H.; Kerstens, P.J.S.M.; Van Der Lubbe, P.A.H.M.; De Beus, W.M.; Grillet, B.A.M.; Ronday, H.K.; et al. Drug-free remission, functioning and radiographic damage after 4 years of response-driven treatment in patients with recent-onset rheumatoid arthritis. Ann. Rheum. Dis. 2009, 68, 914–921. [Google Scholar]
- Pincus, T.; Gibofsky, A.; Weinblatt, M.E. Urgent care and tight control of rheumatoid arthritis as in diabetes and hypertension: Better treatments but a shortage of rheumatologists. Arthritis Rheum. 2002, 46, 851–854. [Google Scholar]
- Gremese, E.; Salaffi, F.; Bosello, S.L.; Ciapetti, A.; Bobbio-Pallavicini, F.; Caporali, R.; Ferraccioli, G. Very early rheumatoid arthritis as a predictor of remission: A multicentre real life prospective study. Ann. Rheum. Dis. 2013, 72, 858–862. [Google Scholar]
- Emery, P.; Breedveld, F.C.; Hall, S.; Durez, P.; Chang, D.J.; Robertson, D.; Singh, A.; Pedersen, R.D.; Koenig, A.S.; Freundlich, B. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): A randomised, double-blind, parallel treatment trial. Lancet 2008, 372, 375–382. [Google Scholar]
- Spencer, D.M.; Veldman, G.M.; Banerjee, S.; Willis, J.; Levine, A.D. Distinct inflammatory mechanisms mediate early versus late colitis in mice. Gastroenterology 2002, 122, 94–105. [Google Scholar]
- Zorzi, F.; Monteleone, I.; Sarra, M.; Calabrese, E.; Marafini, I.; Cretella, M.; Sedda, S.; Biancone, L.; Pallone, F.; Monteleone, G. Distinct Profiles of Effector Cytokines Mark the Different Phases of Crohn’s Disease. PLoS ONE 2013, 8, e54562. [Google Scholar]
- Veny, M.; Esteller, M.; Ricart, E.; Piqué, J.M.; Panés, J.; Salas, A. Late Crohn’s disease patients present an increase in peripheral Th17 cells and cytokine production compared with early patients. Aliment. Pharmacol. Ther. 2010, 31, 561–572. [Google Scholar]
- Schreiber, S.; Reinisch, W.; Colombel, J.F.; Sandborn, W.J.; Hommes, D.W.; Robinson, A.M.; Huang, B.; Lomax, K.G.; Pollack, P.F. Subgroup analysis of the placebo-controlled CHARM trial: Increased remission rates through 3years for adalimumab-treated patients with early Crohn’s disease. J. Crohn’s Colitis 2013, 7, 213–221. [Google Scholar]
- D’Haens, G.; Baert, F.; van Assche, G.; Caenepeel, P.; Vergauwe, P.; Tuynman, H.; De Vos, M.; van Deventer, S.; Stitt, L.; Donner, A.; et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: An open randomised trial. Lancet 2008, 371, 660–667. [Google Scholar]
- Colombel, J.F.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rutgeerts, P.; Tang, K.L.; Oortwijn, A.; Bevelander, G.S.; Cornillie, F.J.; Sandborn, W.J. Randomised clinical trial: Deep remission in biologic and immunomodulator naïve patients with Crohn’s disease—A SONIC post hoc analysis. Aliment. Pharmacol. Ther. 2015, 41, 734–746. [Google Scholar]
- Panaccione, R.; Löfberg, R.; Rutgeerts, P.; Sandborn, W.J.; Schreiber, S.; Berg, S.; Maa, J.F.; Petersson, J.; Robinson, A.M.; Colombel, J.F. Efficacy and safety of adalimumab by disease duration: Analysis of pooled data from crohn’s disease studies. J. Crohn’s Colitis 2019, 13, 725–734. [Google Scholar]
- Safroneeva, E.; Vavricka, S.R.; Fournier, N.; Pittet, V.; Peyrin-Biroulet, L.; Straumann, A.; Rogler, G.; Schoepfer, A.M. Impact of the early use of immunomodulators or TNF antagonists on bowel damage and surgery in Crohn’s disease. Aliment. Pharmacol. Ther. 2015, 42, 977–989. [Google Scholar]
- Frei, R.; Fournier, N.; Zeitz, J.; Scharl, M.; Morell, B.; Greuter, T.; Schreiner, P.; Misselwitz, B.; Safroneeva, E.; Schoepfer, A.M.; et al. Early Initiation of Anti-TNF is Associated with Favourable Long-term Outcome in Crohn’s Disease: 10-Year-Follow-up Data from the Swiss IBD Cohort Study. J. Crohn’s Colitis 2019, 13, 1292–1301. [Google Scholar]
- Ma, C.; Beilman, C.L.; Huang, V.W.; Fedorak, D.K.; Kroeker, K.I.; Dieleman, L.A.; Halloran, B.P.; Fedorak, R.N. Anti-TNF therapy within 2 years of Crohn’s disease diagnosis improves patient outcomes: A retrospective cohort study. Inflamm. Bowel Dis. 2016, 22, 870–879. [Google Scholar]
- Ungaro, R.C.; Aggarwal, S.; Topaloglu, O.; Lee, W.J.; Clark, R.; Colombel, J.F. Systematic review and meta-analysis: Efficacy and safety of early biologic treatment in adult and paediatric patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2020, 51, 831–842. [Google Scholar]
- Ungaro, R.C.; Yzet, C.; Bossuyt, P.; Baert, F.J.; Vanasek, T.; D’Haens, G.R.; Joustra, V.W.; Panaccione, R.; Novacek, G.; Reinisch, W.; et al. Deep Remission at 1 Year Prevents Progression of Early Crohn’s Disease. Gastroenterology 2020, 159, 139–147. [Google Scholar]
- Colombel, J.F.; Panaccione, R.; Bossuyt, P.; Lukas, M.; Baert, F.; Vaňásek, T.; Danalioglu, A.; Novacek, G.; Armuzzi, A.; Hébuterne, X.; et al. Effect of tight control management on Crohn’s disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet 2017, 390, 2779–2789. [Google Scholar]
- Berg, D.R.; Colombel, J.F.; Ungaro, R. The role of early biologic therapy in inflammatory bowel disease. Inflamm. Bowel Dis. 2019, 25, 1896–1905. [Google Scholar]
- Ma, C.; Beilman, C.L.; Huang, V.W.; Fedorak, D.K.; Wong, K.; Kroeker, K.I.; Dieleman, L.A.; Halloran, B.P.; Fedorak, R.N. Similar Clinical and Surgical Outcomes Achieved with Early Compared to Late Anti-TNF Induction in Mild-to-Moderate Ulcerative Colitis: A Retrospective Cohort Study. Can. J. Gastroenterol. Hepatol. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Oussalah, A.; Evesque, L.; Laharie, D.; Roblin, X.; Boschetti, G.; Nancey, S.; Filippi, J.; Flourié, B.; Hebuterne, X.; Bigard, M.A.; et al. A multicenter experience with infliximab for ulcerative colitis: Outcomes and predictors of response, optimization, colectomy, and hospitalization. Am. J. Gastroenterol. 2010, 105, 2617–2625. [Google Scholar]
- Murthy, S.K.; Greenberg, G.R.; Croitoru, K.; Nguyen, G.C.; Silverberg, M.S.; Steinhart, A.H. Extent of early clinical response to infliximab predicts long-term treatment success in active ulcerative colitis. Inflamm. Bowel Dis. 2015, 21, 2090–2096. [Google Scholar]
- Faleck, D.M.; Winters, A.; Chablaney, S.; Shashi, P.; Meserve, J.; Weiss, A.; Aniwan, S.; Koliani-Pace, J.L.; Kochhar, G.; Boland, B.S.; et al. Shorter Disease Duration Is Associated with Higher Rates of Response to Vedolizumab in Patients with Crohn’s Disease But Not Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2019, 17, 2497–2505.e1. [Google Scholar]
- Mandel, M.D.; Balint, A.; Golovics, P.A.; Vegh, Z.; Mohas, A.; Szilagyi, B.; Szabo, A.; Kurti, Z.; Kiss, L.S.; Lovasz, B.D.; et al. Decreasing trends in hospitalizations during anti-TNF therapy are associated with time to anti-TNF therapy: Results from two referral centres. Dig. Liver Dis. 2014, 46, 985–990. [Google Scholar]
- Im, J.P.; Ye, B.D.; Kim, Y.S.; Kim, J.S. Changing treatment paradigms for the management of inflammatory bowel disease. Korean J. Intern. Med. 2018, 33, 28–35. [Google Scholar]
- Danese, S.; Roda, G.; Peyrin-Biroulet, L. Evolving therapeutic goals in ulcerative colitis: Towards disease clearance. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 1–2. [Google Scholar]
- Dal Buono, A.; Roda, G.; Argollo, M.; Peyrin-Biroulet, L.; Danese, S. Histological healing: Should it be considered as a new outcome for ulcerative colitis? Expert Opin. Biol. Ther. 2020, 20, 407–412. [Google Scholar]
- Olén, O.; Askling, J.; Sachs, M.C.; Neovius, M.; Smedby, K.E.; Ekbom, A.; Ludvigsson, J.F. Mortality in adult-onset and elderly-onset IBD: A nationwide register-based cohort study 1964–2014. Gut 2020, 69, 453–461. [Google Scholar]
- Allen, P.B.; Peyrin-Biroulet, L. Moving towards disease modification in inflammatory bowel disease therapy. Curr. Opin. Gastroenterol. 2013, 29, 397–404. [Google Scholar]
- Beaugerie, L.; Kirchgesner, J. Balancing Benefit vs Risk of Immunosuppressive Therapy for Individual Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2019, 17, 370–379. [Google Scholar]
- Smolen, J.S.; Van Der Heijde, D.M.F.M.; St. Clair, E.W.; Emery, P.; Bathon, J.M.; Keystone, E.; Maini, R.N.; Kalden, J.R.; Schiff, M.; Baker, D.; et al. Predictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate with or without concomitant infliximab: Results from the ASPIRE trial. Arthritis Rheum. 2006, 54, 702–710. [Google Scholar]
- Zallot, C.; Peyrin-Biroulet, L. Clinical risk factors for complicated disease: How reliable are they? Dig. Dis. 2013, 30 (Suppl. S3), 67–72. [Google Scholar]
- Zhao, M.; Burisch, J. The Sooner the Better? Cost-effectiveness of Early Biological Therapy in Patients with Crohn’s Disease. J. Crohn’s Colitis 2020, 14, 573–574. [Google Scholar]
- Torres, J.; Caprioli, F.; Katsanos, K.H.; Lobatón, T.; Micic, D.; Zerôncio, M.; Van Assche, G.; Lee, J.C.; Lindsay, J.O.; Rubin, D.T.; et al. Predicting outcomes to optimize disease management in inflammatory bowel diseases. J. Crohn’s Colitis 2016, 10, 1385–1394. [Google Scholar]
- Palmela, C.; Torres, J.; Cravo, M. New Trends in Inflammatory Bowel Disease. GE Port. J. Gastroenterol. 2015, 22, 103–111. [Google Scholar]
- Billiet, T.; Ferrante, M.; Van Assche, G. The Use of Prognostic Factors in Inflammatory Bowel Diseases. Curr. Gastroenterol. Rep. 2014, 16, 416. [Google Scholar]
- Kuriyama, M.; Kato, J.; Fujimoto, T.; Nasu, J.; Miyaike, J.; Morita, T.; Okada, H.; Suzuki, S.; Shiode, J.; Yamamoto, H.; et al. Risk factors and indications for colectomy in ulcerative colitis patients are different according to patient’s clinical background. Dis. Colon Rectum 2006, 49, 1307–1315. [Google Scholar]
- Colombel, J.F.; Narula, N.; Peyrin-Biroulet, L. Management Strategies to Improve Outcomes of Patients with Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 351–361. [Google Scholar]
- Schoepfer, A.M.; Vavricka, S.; Zahnd-Straumann, N.; Straumann, A.; Beglinger, C. Monitoring inflammatory bowel disease activity: Clinical activity is judged to be more relevant than endoscopic severity or biomarkers. J. Crohn’s Colitis 2012, 6, 412–418. [Google Scholar]

| Author (Year) | Ref | Study Design | Study Population (Number) | Definition of Early UC | Outcomes | Significant Results |
|---|---|---|---|---|---|---|
| Ma C., et al. (2016) | [44] | Retrospective Observational | UC (115) | Within 3 years of diagnosis | Colectomy UC-related hospitalization Secondary loss of response | Early initiators vs. late initiators 6 for 100 PY vs. 2.7 per 100 PY, p = 0.13 43.9% vs. 27.6%, p = 0.7 49.1 vs. 58.6%, p = 0.31 |
| Oussalah A., et al. (2010) | [45] | Retrospective Observational | UC (191) | ≤50 months | Predictors of short- and long-term outcomes of IFX | Short duration at IFX initiation predicts hospitalization: HR = 2.14, 95% CI = 1.25–3.66, p = 0.006 |
| Murthy S.K., et al. (2015) | [46] | Retrospective Observational | UC (213) | Not available | Annual SFR IFX failure with discontinuation colectomy | Longer disease duration aOR = 2.1; 95% CI, 1.2–3.5, p = 0.061 aHR = 0.59; 95% CI, 0.40–0.87, p = 0.0198 aHR = 0.49; 95% CI, 0.28–0.85, p = 0.0048 |
| Faleck D.M., et al. (2019) | [47] | Retrospective Observational | IBD (1087) [CD (650)/UC (417)] | ≤2 years | Clinical remission rates CSFR Endoscopic remission within 6 months with VDZ | Early-stage vs. late-stage CD aHR = 1.59; 95% CI, 1.02–2.49, p < 0.2 aHR = 3.39; 95% CI, 1.66–6.9, p < 0.2 aHR = 1.90; 95% CI, 1.06–3.39, p < 0.2 |
| Mandel M.D., et al. (2014) | [48] | Retrospective Observational | IBD (194) [CD (152)/UC (42)] | Within 3 years from diagnosis | Hospitalization rates and predictors during anti-TNF therapy | Early anti-TNF exposure CD OR = 0.60, 95% CI 0.48–0.75, p < 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solitano, V.; D’Amico, F.; Zacharopoulou, E.; Peyrin-Biroulet, L.; Danese, S. Early Intervention in Ulcerative Colitis: Ready for Prime Time? J. Clin. Med. 2020, 9, 2646. https://doi.org/10.3390/jcm9082646
Solitano V, D’Amico F, Zacharopoulou E, Peyrin-Biroulet L, Danese S. Early Intervention in Ulcerative Colitis: Ready for Prime Time? Journal of Clinical Medicine. 2020; 9(8):2646. https://doi.org/10.3390/jcm9082646
Chicago/Turabian StyleSolitano, Virginia, Ferdinando D’Amico, Eirini Zacharopoulou, Laurent Peyrin-Biroulet, and Silvio Danese. 2020. "Early Intervention in Ulcerative Colitis: Ready for Prime Time?" Journal of Clinical Medicine 9, no. 8: 2646. https://doi.org/10.3390/jcm9082646





