Association of Regional Bone Synthetic Activities of Vertebral Corners and Vertebral Bodies Quantified Using 18F-Fluoride Positron Emission Tomography with Bone Mineral Density on Dual Energy X-ray Absorptiometry in Patients with Ankylosing Spondylitis
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design and Subjects
2.2. Imaging Protocol
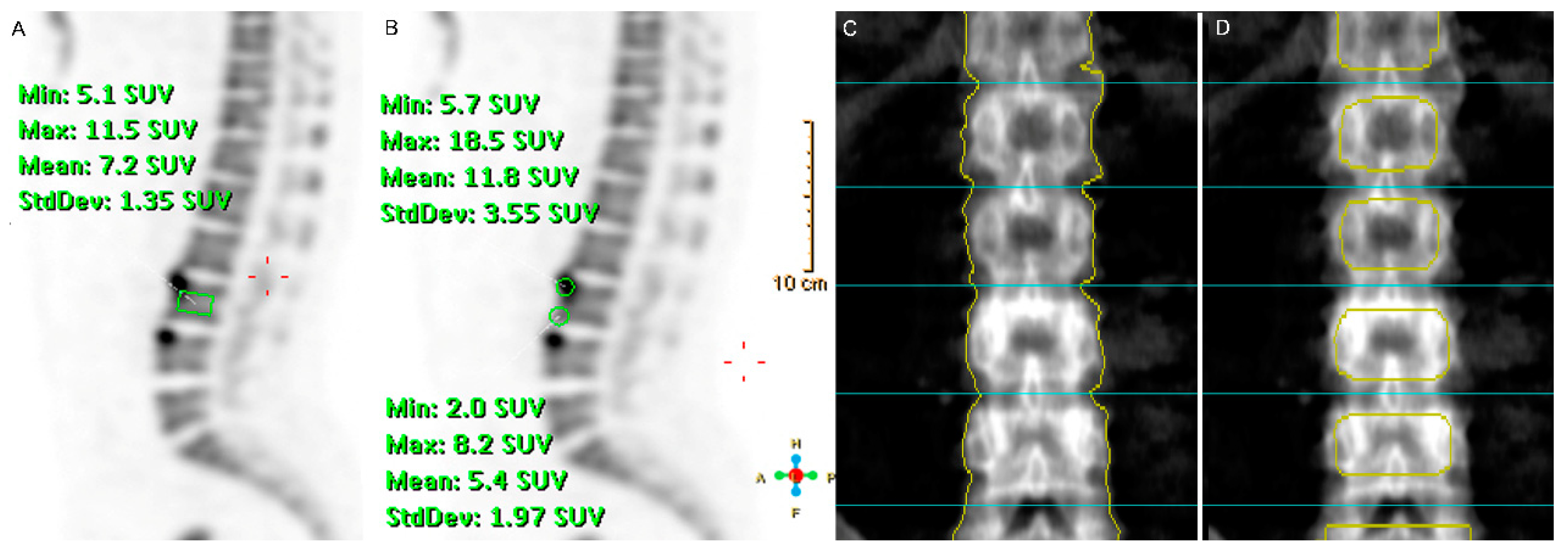
2.3. Imaging Analyses and Interpretation
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schett, G.; Rudwaleit, M. Can we stop progression of ankylosing spondylitis? Best Pract. Res. Clin. Rheumatol. 2010, 24, 363–371. [Google Scholar] [CrossRef]
- Carter, S.; Lories, R.J. Osteoporosis: A paradox in ankylosing spondylitis. Curr. Osteoporos. Rep. 2011, 9, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Arends, S.; Spoorenberg, A.; Efde, M.; Bos, R.; Leijsma, M.K.; Bootsma, H.; Veeger, N.J.; Brouwer, E.; van der Veer, E. Higher bone turnover is related to spinal radiographic damage and low bone mineral density in ankylosing spondylitis patients with active disease: A cross-sectional analysis. PLoS ONE 2014, 9, e99685. [Google Scholar] [CrossRef] [PubMed]
- Pray, C.; Feroz, N.I.; Nigil Haroon, N. Bone Mineral Density and Fracture Risk in Ankylosing Spondylitis: A Meta-Analysis. Calcif. Tissue Int. 2017, 101, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, E.; Lorentzon, M.; Gothlin, J.; Mellstrom, D.; Geijer, M.; Ohlsson, C.; Atkinson, E.J.; Khosla, S.; Carlsten, H.; Forsblad-d’Elia, H. Bone microarchitecture in ankylosing spondylitis and the association with bone mineral density, fractures, and syndesmophytes. Arthritis Res. Ther. 2013, 15, R179. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Li, X.-M.; Wang, G.-S.; Tao, J.-H.; Chen, Z.; Ma, Y.; Li, X.-P. The association between ankylosing spondylitis and the risk of any, hip, or vertebral fracture. Medicine 2017, 96, e8458. [Google Scholar] [CrossRef]
- Sohn, D.H.; Jeong, H.; Roh, J.S.; Lee, H.N.; Kim, E.; Koh, J.H.; Lee, S.G. Serum CCL11 level is associated with radiographic spinal damage in patients with ankylosing spondylitis. Rheumatol. Int. 2018, 38, 1455–1464. [Google Scholar] [CrossRef]
- Blake, G.M.; Siddique, M.; Frost, M.L.; Moore, A.E.; Fogelman, I. Imaging of site specific bone turnover in osteoporosis using positron emission tomography. Curr. Osteoporos. Rep. 2014, 12, 475–485. [Google Scholar] [CrossRef]
- Puri, T.; Frost, M.L.; Curran, K.M.; Siddique, M.; Moore, A.E.; Cook, G.J.; Marsden, P.K.; Fogelman, I.; Blake, G.M. Differences in regional bone metabolism at the spine and hip: A quantitative study using (18)F-fluoride positron emission tomography. Osteoporos. Int. 2013, 24, 633–639. [Google Scholar] [CrossRef]
- Frost, M.L.; Compston, J.E.; Goldsmith, D.; Moore, A.E.; Blake, G.M.; Siddique, M.; Skingle, L.; Fogelman, I. (18)F-fluoride positron emission tomography measurements of regional bone formation in hemodialysis patients with suspected adynamic bone disease. Calcif. Tissue Int. 2013, 93, 436–447. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Schiepers, C.; Lake, R.; Dadparvar, S.; Berenji, G.R. Clinical utility of (18)F-fluoride PET/CT in benign and malignant bone diseases. Bone 2012, 50, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Irmler, I.M.; Gebhardt, P.; Hoffmann, B.; Opfermann, T.; Figge, M.T.; Saluz, H.P.; Kamradt, T. 18 F-Fluoride positron emission tomography/computed tomography for noninvasive in vivo quantification of pathophysiological bone metabolism in experimental murine arthritis. Arthritis Res Ther. 2014, 16, R155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, M.L.; Moore, A.E.; Siddique, M.; Blake, G.M.; Laurent, D.; Borah, B.; Schramm, U.; Valentin, M.A.; Pellas, T.C.; Marsden, P.K.; et al. (1)(8)F-fluoride PET as a noninvasive imaging biomarker for determining treatment efficacy of bone active agents at the hip: A prospective, randomized, controlled clinical study. J. Bone Miner. Res. 2013, 28, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.K.; Virk, M.S.; Feeley, B.T.; Stout, D.B.; Chatziioannou, A.F.; Lieberman, J.R. Characterization of osteolytic, osteoblastic, and mixed lesions in a prostate cancer mouse model using 18F-FDG and 18F-fluoride PET/CT. J. Nucl. Med. 2008, 49, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, A.L.; Tanner, S.F.; Waller, M.L.; Hensor, E.M.; Burns, A.; Jeavons, A.P.; Bury, R.F.; Emery, P.; McGonagle, D. High-resolution [18F]fluoride positron emission tomography of the distal interphalangeal joint in psoriatic arthritis—A bone-enthesis-nail complex. Rheumatology 2013, 52, 898–904. [Google Scholar] [CrossRef] [Green Version]
- Raynor, W.; Houshmand, S.; Gholami, S.; Emamzadehfard, S.; Rajapakse, C.S.; Blomberg, B.A.; Werner, T.J.; Hoilund-Carlsen, P.F.; Baker, J.F.; Alavi, A. Evolving Role of Molecular Imaging with (18)F-Sodium Fluoride PET as a Biomarker for Calcium Metabolism. Curr. Osteoporos. Rep. 2016, 14, 115–125. [Google Scholar] [CrossRef]
- Bruijnen, S.T.G.; Verweij, N.J.F.; van Duivenvoorde, L.M.; Bravenboer, N.; Baeten, D.L.P.; van Denderen, C.J.; van der Horst-Bruinsma, I.E.; Voskuyl, A.E.; Custers, M.; van de Ven, P.M.; et al. Bone formation in ankylosing spondylitis during anti-tumour necrosis factor therapy imaged by 18F-fluoride positron emission tomography. Rheumatology 2018, 57, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Frost, M.L.; Cook, G.J.; Blake, G.M.; Marsden, P.K.; Fogelman, I. The relationship between regional bone turnover measured using 18F-fluoride positron emission tomography and changes in BMD is equivalent to that seen for biochemical markers of bone turnover. J. Clin. Densitom. 2007, 10, 46–54. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, I.J.; Kim, K.Y.; Kim, H.Y.; Park, K.J.; Kim, S.J.; Park, E.K.; Jeon, Y.K.; Yang, B.Y.; Kim, G.T. Assessment of bone synthetic activity in inflammatory lesions and syndesmophytes in patients with ankylosing spondylitis: The potential role of 18F-fluoride positron emission tomography-magnetic resonance imaging. Clin. Exp. Rheumatol. 2015, 33, 90–97. [Google Scholar]
- Park, E.K.; Pak, K.; Park, J.H.; Kim, K.; Kim, S.J.; Kim, I.J.; Kim, G.T.; Lee, S.G. Baseline increased 18F-fluoride uptake lesions at vertebral corners on positron emission tomography predict new syndesmophyte development in ankylosing spondylitis: A 2-year longitudinal study. Rheumatol. Int. 2017, 37, 765–773. [Google Scholar] [CrossRef]
- van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Deminger, A.; Klingberg, E.; Lorentzon, M.; Geijer, M.; Gothlin, J.; Hedberg, M.; Rehnberg, E.; Carlsten, H.; Jacobsson, L.T.; Forsblad-d’Elia, H. Which measuring site in ankylosing spondylitis is best to detect bone loss and what predicts the decline: Results from a 5-year prospective study. Arthritis Res. Ther. 2017, 19, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maksymowych, W.P.; Chiowchanwisawakit, P.; Clare, T.; Pedersen, S.J.; Ostergaard, M.; Lambert, R.G. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: Evidence of a relationship between inflammation and new bone formation. Arthritis Rheum. 2009, 60, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Nakajima, H.; Miyazaki, T.; Yayama, T.; Kawahara, H.; Kobayashi, S.; Tsuchida, T.; Okazawa, H.; Fujibayashi, Y.; Baba, H. Effects of alendronate on bone metabolism in glucocorticoid-induced osteoporosis measured by 18F-fluoride PET: A prospective study. J. Nucl. Med. 2009, 50, 1808–1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, R.A.; Choi, Y.; Huang, S.C.; Hoh, C.K.; Dahlbom, M.; Schiepers, C.; Satyamurthy, N.; Barrio, J.R.; Phelps, M.E. Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J. Nucl. Med. 1992, 33, 633–642. [Google Scholar] [PubMed]
- Piert, M.; Zittel, T.T.; Becker, G.A.; Jahn, M.; Stahlschmidt, A.; Maier, G.; Machulla, H.J.; Bares, R. Assessment of porcine bone metabolism by dynamic. J. Nucl. Med. 2001, 42, 1091–1100. [Google Scholar] [PubMed]
- Aznar, M.C.; Sersar, R.; Saabye, J.; Ladefoged, C.N.; Andersen, F.L.; Rasmussen, J.H.; Lofgren, J.; Beyer, T. Whole-body PET/MRI: The effect of bone attenuation during MR-based attenuation correction in oncology imaging. Eur. J. Radiol. 2014, 83, 1177–1183. [Google Scholar] [CrossRef]
- Schramm, G.; Maus, J.; Hofheinz, F.; Petr, J.; Lougovski, A.; Beuthien-Baumann, B.; Oehme, L.; Platzek, I.; van den Hoff, J. Correction of quantification errors in pelvic and spinal lesions caused by ignoring higher photon attenuation of bone in [18F]NaF PET/MR. Med. Phys. 2015, 42, 6468–6476. [Google Scholar] [CrossRef]
- Shevroja, E.; Lamy, O.; Kohlmeier, L.; Koromani, F.; Rivadeneira, F.; Hans, D. Use of Trabecular Bone Score (TBS) as a Complementary Approach to Dual-energy X-ray Absorptiometry (DXA) for Fracture Risk Assessment in Clinical Practice. J. Nucl. Med. 2017, 20, 334–345. [Google Scholar] [CrossRef]

| Characteristics | Patients with AS (n = 12) |
|---|---|
| Males, n (%) | 12 (100) |
| Age (years), median (IQR) | 42 (32–42.8) |
| Disease duration (years), median (IQR) | 7.5 (2.4–10.1) |
| HLA-B27 positive, n (%) | 12 (100) |
| mSASSS, median (IQR) | 9 (5.3–28) |
| BMI (kg/m2), median (IQR), | 26.3 (22.9–27.6) |
| ESR (mm/hr), median (IQR) | 13 (5.8–20) |
| CRP (mg/dL), median (IQR) | 0.33 (0.06–0.6) |
| BASDAI, median (IQR) | 3.5 (1.2–6) |
| BASFI, median (IQR) | 2.4 (0.7–3.8) |
| BASMI, median (IQR) | 2.8 (1.3–4.4) |
| NSAID use, n (%) | 12 (100) |
| SSZ use, n (%) | 12 (100) |
| TNF-α inhibitor use, n (%) | 0 (0) |
| Lumbar Vertebrae (n = 48) | |
|---|---|
| 18F-Fluoride SUVmean of vertebral corner a, mean ± SD | 5.24 ± 0.9 |
| 18F-fluoride SUVmean of vertebral body, mean ± SD | 5.03 ± 0.9 |
| Conventional BMD of vertebra (g/cm2), mean ± SD | 1.19 ± 0.21 |
| Alternative BMD of vertebra (g/cm2), mean ± SD | 1.03 ± 0.22 |
| Dependent Variable | Independent Variable | Univariable Model | |
|---|---|---|---|
| Unstandardized β (SE) | p Value | ||
| Conventional BMD of vertebra (g/cm2) | 18F-fluoride SUVmean of vertebral corner a | 0.112 (0.028) | <0.001 |
| Conventional BMD of vertebra (g/cm2) | 18F-fluoride SUVmean of vertebral body | 0.103 (0.042) | 0.014 |
| Alternative BMD of vertebra (g/cm2) | 18F-fluoride SUVmean of vertebral corner a | 0.123 (0.031) | <0.001 |
| Alternative BMD of vertebra (g/cm2) | 18F-fluoride SUVmean of vertebral body | 0.158 (0.041) | <0.001 |
| 18F-fluoride SUVmean of vertebral corner a | 18F-fluoride SUVmean of vertebral body | 0.711 (0.102) | <0.001 |
| Dependent Variable | Independent Variable | Univariable Model | Multivariable Model b | ||
|---|---|---|---|---|---|
| Unstandardized β (SE) | p Value | Unstandardized β (SE) | p Value | ||
| Conventional BMD (g/cm2) | 18F-fluoride SUVmean of vertebral corner a | 0.112 (0.028) | <0.001 | 0.092 (0.029) | 0.001 |
| 18F-fluoride SUVmean of vertebral body | 0.103(0.042) | 0.014 | 0.069 (0.039) | 0.075 | |
| Disease duration (months) | 0.002 (0.001) | 0.025 | 0.002 (0.001) | 0.038 | |
| CRP (mg/dL) | −0.153 (0.106) | 0.146 | −0.164 (0.091) | 0.071 | |
| Age (years) | −0.001 (0.007) | 0.854 | |||
| BMI (kg/m2) | 0.013 (0.013) | 0.338 | |||
| BASDAI | −0.017 (0.023) | 0.467 | |||
| Alternative BMD (g/cm2) | 18F-fluoride SUVmean of vertebral corner a | 0.123 (0.031) | <0.001 | 0.085 (0.032) | 0.009 |
| 18F-fluoride SUVmean of vertebral body | 0.158 (0.041) | <0.001 | 0.114 (0.044) | 0.01 | |
| BMI (kg/m2) | 0.017 (0.013) | 0.187 | 0.005 (0.013) | 0.702 | |
| Disease duration (months) | 0.001 (0.001) | 0.585 | |||
| CRP (mg/dL) | −0.067 (0.104) | 0.5558 | |||
| Age (years) | −0.006 (0.007) | 0.35 | |||
| BASDAI | −0.004 (0.024) | 0.877 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Pak, K.; Kim, I.-J.; Kim, S.-J.; Sohn, D.H.; Kim, A.; Lee, S.-G. Association of Regional Bone Synthetic Activities of Vertebral Corners and Vertebral Bodies Quantified Using 18F-Fluoride Positron Emission Tomography with Bone Mineral Density on Dual Energy X-ray Absorptiometry in Patients with Ankylosing Spondylitis. J. Clin. Med. 2020, 9, 2656. https://doi.org/10.3390/jcm9082656
Kim K, Pak K, Kim I-J, Kim S-J, Sohn DH, Kim A, Lee S-G. Association of Regional Bone Synthetic Activities of Vertebral Corners and Vertebral Bodies Quantified Using 18F-Fluoride Positron Emission Tomography with Bone Mineral Density on Dual Energy X-ray Absorptiometry in Patients with Ankylosing Spondylitis. Journal of Clinical Medicine. 2020; 9(8):2656. https://doi.org/10.3390/jcm9082656
Chicago/Turabian StyleKim, Keunyoung, Kyoungjune Pak, In-Joo Kim, Seong-Jang Kim, Dong Hyun Sohn, Aran Kim, and Seung-Geun Lee. 2020. "Association of Regional Bone Synthetic Activities of Vertebral Corners and Vertebral Bodies Quantified Using 18F-Fluoride Positron Emission Tomography with Bone Mineral Density on Dual Energy X-ray Absorptiometry in Patients with Ankylosing Spondylitis" Journal of Clinical Medicine 9, no. 8: 2656. https://doi.org/10.3390/jcm9082656
APA StyleKim, K., Pak, K., Kim, I. -J., Kim, S. -J., Sohn, D. H., Kim, A., & Lee, S. -G. (2020). Association of Regional Bone Synthetic Activities of Vertebral Corners and Vertebral Bodies Quantified Using 18F-Fluoride Positron Emission Tomography with Bone Mineral Density on Dual Energy X-ray Absorptiometry in Patients with Ankylosing Spondylitis. Journal of Clinical Medicine, 9(8), 2656. https://doi.org/10.3390/jcm9082656





