The Effects of Longitudinal White Matter Hyperintensity Change on Cognitive Decline and Cortical Thinning over Three Years
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Acquisition of MR Images
2.3. Measurement of Longitudinal WMH Volume
2.4. Acquisition of [11C]PiB PET and Data Analysis
2.5. Cortical Thickness Analyses
2.6. Neuropsychological Tests
2.7. Statistical Analyses
2.8. Standard Protocol Approval, Registration, and Patient Consent
3. Results
3.1. Demographics
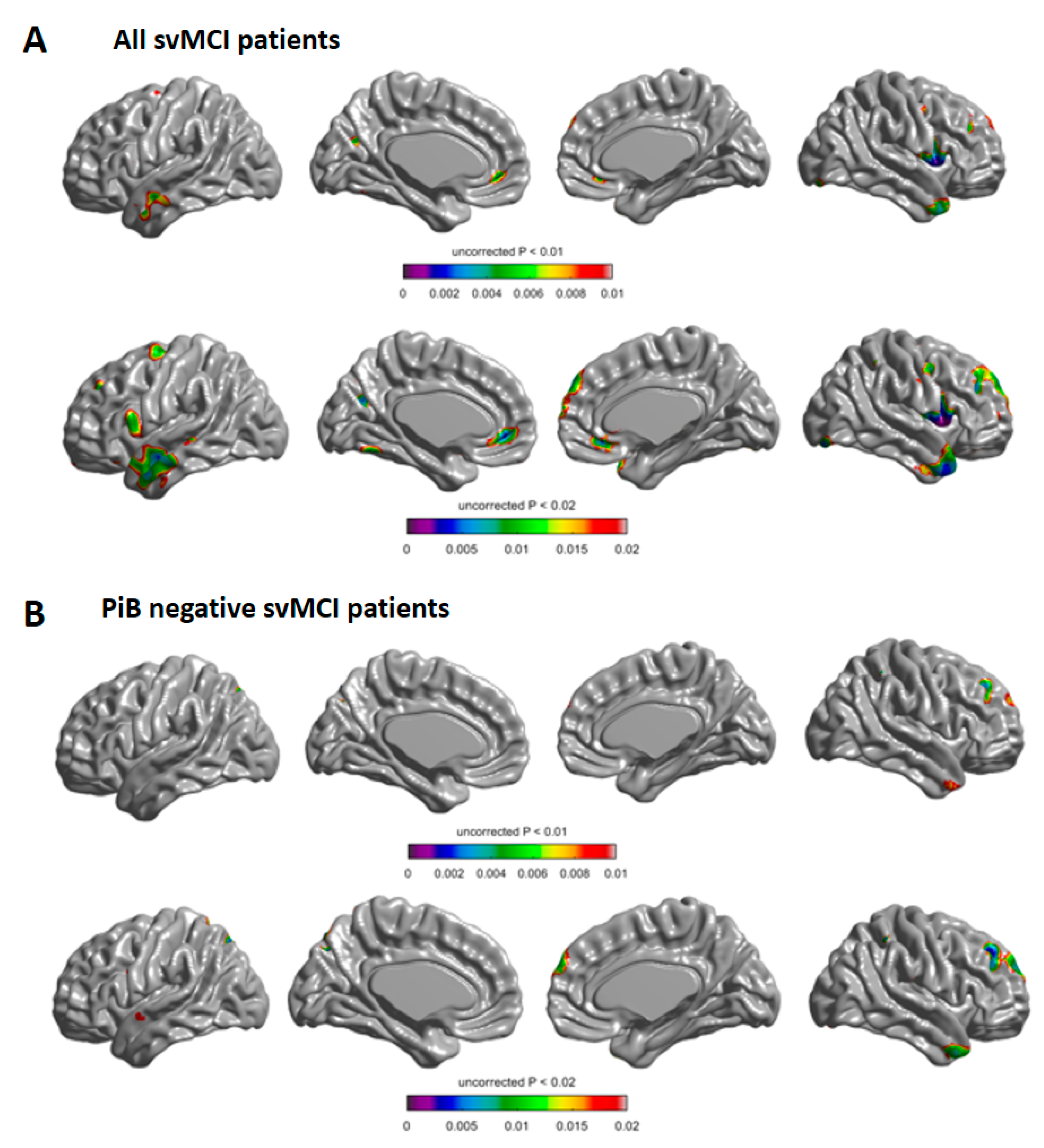
3.2. Longitudinal Change of Cortical Thickness
3.3. Longitudinal Change of Cognition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeCarli, C.; Murphy, D.; Tranh, M.; Grady, C.L.; Haxby, J.V.; Gillette, J.A.; Salerno, J.A.; Gonzales-Aviles, A.; Honvitz, B.; Rapoport, S.I.; et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology 1995, 45, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Ye, B.S.; Yoon, C.W.; Noh, Y.; Kim, G.H.; Cho, H.; Jeon, S.; Lee, J.M.; Kim, J.-H.; Seong, J.-K.; et al. Cortical thickness and hippocampal shape in pure vascular mild cognitive impairment and dementia of subcortical type. Eur. J. Neurol. 2014, 21, 744–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, S.W.; Ahn, J.; Yoon, U.; Im, K.; Lee, J.-M.; Kim, S.T.; Ahn, H.-J.; Chin, J.; Jeong, Y.; Na, D.L. Cortical Thinning in Vascular Mild Cognitive Impairment and Vascular Dementia of Subcortical Type. J. Neuroimaging 2010, 20, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Prins, N.D.; Scheltens, P. White matter hyperintensities, cognitive impairment and dementia: An update. Nat. Rev. Neurol. 2015, 11, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, E.J.; Prins, N.D.; Vrooman, H.A.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M.B. Progression of Cerebral Small Vessel Disease in Relation to Risk Factors and Cognitive Consequences. Stroke 2008, 39, 2712–2719. [Google Scholar] [CrossRef] [Green Version]
- Maillard, P.; Carmichael, O.; Fletcher, E.; Reed, B.; Mungas, D.; DeCarli, C. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology 2012, 79, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, L.; Sachdev, P.S.; Trollor, J.N.; Kochan, N.A.; Reppermund, S.; Brodaty, H.; Wen, W. Microstructural white matter changes in cognitively normal individuals at risk of amnestic MCI. Neurology 2012, 79, 748–754. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Doubal, F.N.; Valdes-Hernandez, M.; Wang, X.; Chappell, F.M.; Shuler, K.; Armitage, P.A.; Carpenter, T.C.; Dennis, M.S. Blood-brain barrier permeability and long-term clinical and imaging outcomes in cerebral small vessel disease. Stroke 2012, 44, 525–527. [Google Scholar] [CrossRef] [Green Version]
- Wardlaw, J.M.; Chappell, F.M.; Hernández, M.D.C.V.; Makin, S.D.; Staals, J.; Shuler, K.; Thrippleton, M.J.; Armitage, P.A.; Muñoz-Maniega, S.; Heye, A.K.; et al. White matter hyperintensity reduction and outcomes after minor stroke. Neurology 2017, 89, 1003–1010. [Google Scholar] [CrossRef] [Green Version]
- Van Leijsen, E.M.; De Leeuw, F.-E.; Tuladhar, A.M. Disease progression and regression in sporadic small vessel disease–insights from neuroimaging. Clin. Sci. 2017, 131, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.-H.; Kim, H.-R.; Kim, W.; Yang, D.W. White Matter Hyperintensity in Ischemic Stroke Patients: It May Regress Over Time. J. Stroke 2015, 17, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Kloppenborg, R.P.; Nederkoorn, P.J.; Geerlings, M.I.; Berg, E.V.D. Presence and progression of white matter hyperintensities and cognition: A meta-analysis. Neurology 2014, 82, 2127–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, J.; McNeely, A.A.; Berezuk, C.; Gao, F.; Black, S.E. Dynamic Progression of White Matter Hyperintensities in Alzheimer’s Disease and Normal Aging: Results from the Sunnybrook Dementia Study. Front. Aging Neurosci. 2016, 8, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, P.; Zeestraten, E.; Lambert, C.; Chis-Ster, I.; Williams, O.; Lawrence, A.J.; Patel, B.; MacKinnon, A.D.; Barrick, T.R.; Markus, H.S. Progression of MRI markers in cerebral small vessel disease: Sample size considerations for clinical trials. Br. J. Pharmacol. 2015, 36, 228–240. [Google Scholar] [CrossRef]
- Schmidt, R.; Ropele, S.; Enzinger, C.; Petrovic, K.; Smith, S.M.; Schmidt, H.; Matthews, P.M.; Fazekas, F. White matter lesion progression, brain atrophy, and cognitive decline: The Austrian stroke prevention study. Ann. Neurol. 2005, 58, 610–616. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Hernández, M.C.V.; Muñoz-Maniega, S. What are White Matter Hyperintensities Made of? J. Am. Hear. Assoc. 2015, 4, 1140. [Google Scholar] [CrossRef] [Green Version]
- Al-Janabi, O.; Bauer, C.E.; Goldstein, L.B.; Murphy, R.R.; Bahrani, A.A.; Smith, C.D.; Wilcock, D.M.; Gold, B.T.; Jicha, G.A. White Matter Hyperintensity Regression: Comparison of Brain Atrophy and Cognitive Profiles with Progression and Stable Groups. Brain Sci. 2019, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Van Leijsen, E.M.; Bergkamp, M.; Van Uden, I.W.; Cooijmans, S.; Ghafoorian, M.; Van Der Holst, H.M.; Norris, D.G.; Kessels, R.P.; Platel, B.; Tuladhar, A.M.; et al. Cognitive consequences of regression of cerebral small vessel disease. Eur. Stroke J. 2018, 4, 85–89. [Google Scholar] [CrossRef]
- Lee, J.; Seo, S.W.; Yang, J.-J.; Jang, Y.K.; Lee, J.S.; Kim, Y.J.; Chin, J.; Lee, J.M.; Kim, S.T.; Lee, K.-H.; et al. Longitudinal cortical thinning and cognitive decline in patients with early- versus late-stage subcortical vascular mild cognitive impairment. Eur. J. Neurol. 2017, 25, 326–333. [Google Scholar] [CrossRef]
- Fazekas, F.; Kleinert, R.; Offenbacher, H.; Schmidt, R.; Kleinert, G.; Payer, F.; Radner, H.; Lechner, H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993, 43, 1683. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Yoon, U.; Park, J.-S.; Seo, S.W.; Kim, J.-H.; Kim, S.T.; Kim, S.I.; Na, D.L.; Lee, J.-M. Fully automated pipeline for quantification and localization of white matter hyperintensity in brain magnetic resonance image. Int. J. Imaging Syst. Technol. 2011, 21, 193–200. [Google Scholar] [CrossRef]
- Carmichael, O.; Schwarz, C.G.; Drucker, D.; Fletcher, E.; Harvey, D.; Beckett, L.A.; Jack, C.R.; Weiner, M.; DeCarli, C.; Initiative, A.D.N. Longitudinal Changes in White Matter Disease and Cognition in the First Year of the Alzheimer Disease Neuroimaging Initiative. Arch. Neurol. 2010, 67, 1370–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, C.C.; Ellis, K.; Rimajova, M.; Bourgeat, P.; Pike, K.E.; Jones, G.R.; Fripp, J.; Tochon-Danguy, H.; Morandeau, L.; O’Keefe, G.; et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol. Aging 2010, 31, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, S.H.; Kim, G.H.; Seo, S.W.; Park, H.K.; Oh, S.J.; Kim, J.S.; Cheong, H.-K.; Na, D.L. Identification of pure subcortical vascular dementia using 11C-Pittsburgh compound B. Neurology 2011, 77, 18–25. [Google Scholar] [CrossRef]
- Kim, H.J.; Yang, J.J.; Kwon, H.; Kim, C.; Lee, J.M.; Chun, P.; Kim, Y.J.; Jung, N.-Y.; Chin, J.; Kim, S.; et al. Relative impact of amyloid-β, lacunes, and downstream imaging markers on cognitive trajectories. Brain 2016, 139, 2516–2527. [Google Scholar] [CrossRef] [Green Version]
- Collins, D.L.; Neelin, P.; Peters, T.M.; Evans, A.C. Automatic 3D Intersubject Registration of MR Volumetric Data in Standardized Talairach Space. J. Comput. Assist. Tomogr. 1994, 18, 192–205. [Google Scholar] [CrossRef]
- Sled, J.G.; Zijdenbos, A.; Evans, A. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med Imaging 1998, 17, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Zijdenbos, A.; Forghani, R.; Evans, A. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: Application to multiple sclerosis. IEEE Trans. Med Imaging 2002, 21, 1280–1291. [Google Scholar] [CrossRef]
- Kim, J.S.; Singh, V.; Lee, J.K.; Lerch, J.; Ad-Dab’Bagh, Y.; Macdonald, D.; Lee, J.M.; Kim, S.I.; Evans, A.C. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. NeuroImage 2005, 27, 210–221. [Google Scholar] [CrossRef]
- Im, K.; Lee, J.-M.; Lee, J.; Shin, Y.W.; Kim, I.Y.; Kwon, J.S.; Kim, S.I. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. NeuroImage 2006, 31, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Lyttelton, O.; Boucher, M.; Robbins, S.; Evans, A.C. An unbiased iterative group registration template for cortical surface analysis. NeuroImage 2007, 34, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.-J.; Chin, J.; Park, A.; Lee, B.H.; Suh, M.K.; Seo, S.W.; Na, D.L. Seoul Neuropsychological Screening Battery-Dementia Version (SNSB-D): A Useful Tool for Assessing and Monitoring Cognitive Impairments in Dementia Patients. J. Korean Med Sci. 2010, 25, 1071–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.H.; Park, Y.H.; Lee, D.; Kim, J.P.; Chin, J.; Ahn, Y.; Park, S.B.; Kim, H.J.; Jang, H.; Jung, Y.H.; et al. The Cortical Neuroanatomy Related to Specific Neuropsychological Deficits in Alzheimer’s Continuum. Dement. Neurocognitive Disord. 2019, 18, 77–95. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.; Cho, H.; Jang, Y.K.; Lee, J.S.; Jang, H.; Kim, Y.; Kim, K.W.; Ryu, Y.H.; Choi, J.Y.; et al. Assessment of Extent and Role of Tau in Subcortical Vascular Cognitive Impairment Using 18F-AV1451 Positron Emission Tomography Imaging. JAMA Neurol. 2018, 75, 999–1007. [Google Scholar] [CrossRef] [Green Version]
- Scheltens, P.; Barkhof, F.; Leys, D.; Pruvo, J.; Nauta, J.; Vermersch, P.; Steinling, M.; Valk, J. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J. Neurol. Sci. 1993, 114, 7–12. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Sandercock, P.; Dennis, M.; Starr, J.; Kalimo, H. Is Breakdown of the Blood-Brain Barrier Responsible for Lacunar Stroke, Leukoaraiosis, and Dementia? Stroke 2003, 34, 806–812. [Google Scholar] [CrossRef]
- Tomimoto, H.; Akiguchi, I.; Suenaga, T.; Nishimura, M.; Wakita, H.; Nakamura, S.; Kimura, J. Alterations of the Blood-Brain Barrier and Glial Cells in White-Matter Lesions in Cerebrovascular and Alzheimer’s Disease Patients. Stroke 1996, 27, 2069–2074. [Google Scholar] [CrossRef]
- Jang, H.; Kwon, H.; Yang, J.-J.; Hong, J.; Kim, Y.; Kim, K.W.; Lee, J.S.; Jang, Y.K.; Kim, S.T.; Lee, K.H.; et al. Correlations between Gray Matter and White Matter Degeneration in Pure Alzheimer’s Disease, Pure Subcortical Vascular Dementia, and Mixed Dementia. Sci. Rep. 2017, 7, 9541. [Google Scholar] [CrossRef]
| WMH Progression (n = 70) | WMH Regression (n = 17) | p-Value | |
|---|---|---|---|
| Age (mean ± SD), years | 72.1 ± 6.5 | 71.8 ± 8.7 | 0.158 |
| Female, n (%) | 39 (55.7) | 12 (70.6) | 0.264 |
| Education (mean ± SD), years | 9.9 ± 5.5 | 8.1 ± 5.0 | 0.229 |
| Cardiovascular risk factors, n (%) | |||
| BMI (kg/m2) | 25.05 ± 4.39 | 22.97 ± 3.47 | 0.073 |
| Hypertension | 54 (77.1) | 13 (76.5) | 1.000 |
| Diabetes mellitus | 15 (21.4) | 5 (29.4) | 0.526 |
| Hyperlipidaemia | 21 (30) | 3 (17.6) | 0.378 |
| APOE4, n (%) | 17 (24.3) | 6 (35.3) | 0.370 |
| Anti-platelet agent, n (%) | 62 (88.6) | 14 (82.4) | 0.443 |
| Anti-coagulant agent, n (%) | 1 (1.4) | 0 (0.0) | 0.014 |
| Baseline SVD markers | |||
| WMH (mean ± SD), mL | 40.98 ± 29.13 | 38.97 ± 24.72 | 0.794 |
| Microbleed (mean ± SD), n | 5.2 ± 12.2 | 3.6 ± 6.3 | 0.648 |
| Lacunae (mean ± SD), n | 6.3 ± 7.3 | 4.3 ± 4.1 | 0.254 |
| PiB PET | |||
| PiB SUVR | 1.48 ± 0.40 | 1.53 ± 0.38 | 0.711 |
| PiB positive (SUVR > 1.5), n (%) | 18/63 (28.6) | 4/10 (40) | 0.476 |
| Cortical thickness (mean ± SD), mm | 2.84 ± 0.16 | 2.77 ± 0.18 | 0.130 |
| Cognition | |||
| Language | 40.20 ± 9.08 | 36.24 ± 12.22 | 0.223 |
| Visuospatial function | 27.07 ± 7.97 | 27.56 ± 7.79 | 0.820 |
| Memory | 75.76 ± 17.54 | 76.88 ± 21.60 | 0.845 |
| Executive function | 97.40 ± 35.74 | 87.88 ± 26.80 | 0.230 |
| MMSE | 25.91 ± 3.13 | 24.82 ± 3.84 | 0.290 |
| CDR-SB | 1.29 ± 1.07 | 1.74 ± 1.64 | 0.295 |
| Mean follow-up duration | 34.01 ± 10.13 | 29.06 ± 13.48 | 0.095 |
| All svMCI (n = 87) | PiB Negative svMCI (n = 51) | |||
|---|---|---|---|---|
| B (SE) | p-Value | B (SE) | p-Value | |
| Global thickness | −0.034 (0.012) | 0.006 | −0.027 (0.012) | 0.030 |
| Frontal lobe | −0.041 (0.013) | 0.002 | −0.028 (0.013) | 0.033 |
| Temporal lobe | −0.040 (0.016) | 0.014 | −0.035 (0.015) | 0.024 |
| Parietal lobe | −0.034 (0.012) | 0.005 | −0.032 (0.012) | 0.009 |
| Occipital lobe | −0.025 (0.011) | 0.029 | −0.019 (0.014) | 0.173 |
| All svMCI (n = 87) | PiB negative svMCI (n = 51) | |||
|---|---|---|---|---|
| B (SE) | p-Value | B (SE) | p-Value | |
| Cognition | ||||
| Language | 1.076 (0.699) | 0.129 | 0.869 (0.693) | 0.217 |
| Visuospatial function | −0.277 (0.712) | 0.699 | 0.949 (0.831) | 0.260 |
| Memory | 1.873 (2.189) | 0.395 | 2.792 (2.442) | 0.259 |
| Executive function | 2.348 (3.396) | 0.492 | 0.564 (3.912) | 0.886 |
| MMSE | 0.419 (0.386) | 0.281 | 0.301 (0.402) | 0.457 |
| CDR-SB | −0.034 (0.226) | 0.880 | −0.0665 (0.239) | 0.782 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.J.; Lee, D.K.; Jang, Y.K.; Jang, H.; Kim, S.E.; Cho, S.H.; Kim, J.P.; Jung, Y.H.; Kim, E.-J.; Na, D.L.; et al. The Effects of Longitudinal White Matter Hyperintensity Change on Cognitive Decline and Cortical Thinning over Three Years. J. Clin. Med. 2020, 9, 2663. https://doi.org/10.3390/jcm9082663
Kim SJ, Lee DK, Jang YK, Jang H, Kim SE, Cho SH, Kim JP, Jung YH, Kim E-J, Na DL, et al. The Effects of Longitudinal White Matter Hyperintensity Change on Cognitive Decline and Cortical Thinning over Three Years. Journal of Clinical Medicine. 2020; 9(8):2663. https://doi.org/10.3390/jcm9082663
Chicago/Turabian StyleKim, Seung Joo, Dong Kyun Lee, Young Kyoung Jang, Hyemin Jang, Si Eun Kim, Soo Hyun Cho, Jun Pyo Kim, Young Hee Jung, Eun-Joo Kim, Duk L. Na, and et al. 2020. "The Effects of Longitudinal White Matter Hyperintensity Change on Cognitive Decline and Cortical Thinning over Three Years" Journal of Clinical Medicine 9, no. 8: 2663. https://doi.org/10.3390/jcm9082663
APA StyleKim, S. J., Lee, D. K., Jang, Y. K., Jang, H., Kim, S. E., Cho, S. H., Kim, J. P., Jung, Y. H., Kim, E.-J., Na, D. L., Lee, J.-M., Seo, S. W., & Kim, H. J. (2020). The Effects of Longitudinal White Matter Hyperintensity Change on Cognitive Decline and Cortical Thinning over Three Years. Journal of Clinical Medicine, 9(8), 2663. https://doi.org/10.3390/jcm9082663





