Role of Type2 Inflammatory Biomarkers in Chronic Obstructive Pulmonary Disease
Abstract
:1. Introduction
2. Type2 Airway Inflammation in COPD
3. Relationship between COPD and Type2 Inflammation from the Perspective of Impaired Lung Development
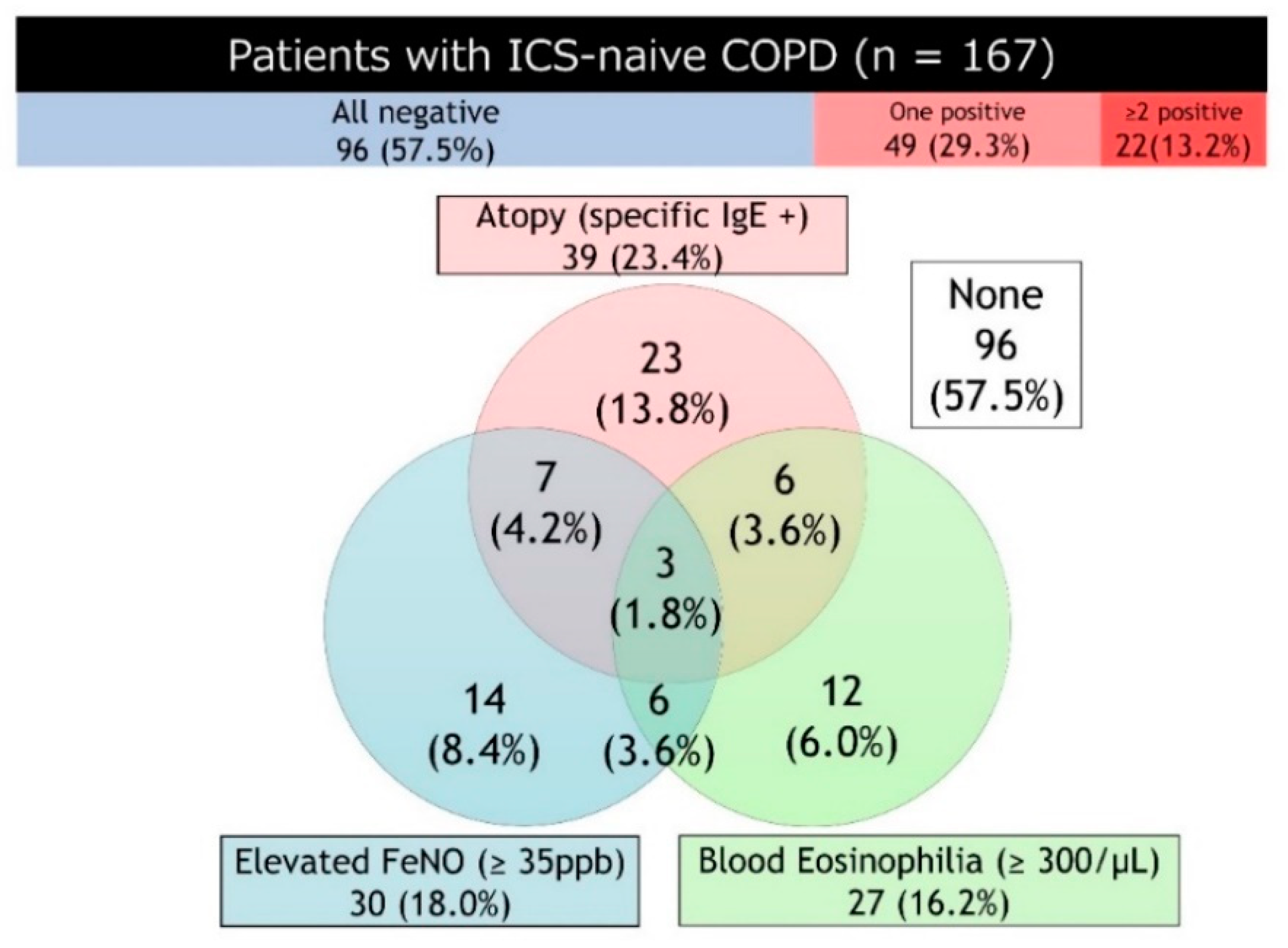
4. Existent Type2 Biomarkers in COPD
4.1. Sputum Eosinophils
4.2. Blood Eosinophils
4.2.1. Blood Eosinophil Levels in COPD and Modifying Factors
4.2.2. Blood Eosinophils as a Predictor for COPD Exacerbations
4.2.3. Relationship between Lung Function and Blood Eosinophils in COPD
4.2.4. Blood Eosinophils as a Biomarker of ICS Treatment Response in COPD
4.3. FeNO
4.3.1. FeNO Levels in COPD and Modifying Factors
4.3.2. Relationship to COPD Exacerbations in FeNO
4.3.3. Relationship between Lung Function and FeNO in COPD
4.3.4. FeNO as a Biomarker of the ICS Treatment Response in COPD
4.4. IgE, Atopy
4.4.1. Evaluation of Atopy in Patients with COPD
4.4.2. Atopy as a Biomarker of ICS Treatment Response in COPD
4.5. Composite Biomarkers
5. Emerging Type2 Biomarkers in COPD
5.1. Periostin
5.2. Chitinase-3-Like Protein 1 (YKL-40)
5.3. Eosinophil-Derived Neurotoxin (EDN)
5.4. Role of Biomarkers in Identifying Asthma-COPD Overlap (ACO)
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.; Han, M. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christenson, S.A.; Steiling, K.; van den Berge, M.; Hijazi, K.; Hiemstra, P.S.; Postma, D.S.; Lenburg, M.E.; Spira, A.; Woodruff, P.G. Asthma-COPD overlap. Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2015, 191, 758–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGeachie, M.J.; Yates, K.P.; Zhou, X.; Guo, F.; Sternberg, A.L.; Van Natta, M.L.; Wise, R.A.; Szefler, S.J.; Sharma, S.; Kho, A.T.; et al. Patterns of growth and decline in lung function in persistent childhood asthma. N. Engl. J. Med. 2016, 374, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- James, A.L.; Palmer, L.J.; Kicic, E.; Maxwell, P.S.; Lagan, S.E.; Ryan, G.F.; Musk, A.W. Decline in lung function in the Busselton Health Study: The effects of asthma and cigarette smoking. Am. J. Respir. Crit. Care Med. 2005, 171, 109–114. [Google Scholar] [CrossRef]
- Strunk, R.C.; Weiss, S.T.; Yates, K.P.; Tonascia, J.; Zeiger, R.S.; Szefler, S.J. Mild to moderate asthma affects lung growth in children and adolescents. J. Allergy Clin. Immunol. 2006, 118, 1040–1047. [Google Scholar] [CrossRef]
- Bisgaard, H.; Jensen, S.M.; Bønnelykke, K. Interaction between asthma and lung function growth in early life. Am. J. Respir. Crit. Care Med. 2012, 185, 1183–1189. [Google Scholar] [CrossRef]
- Morgan, W.J.; Stern, D.A.; Sherrill, D.L.; Guerra, S.; Holberg, C.J.; Guilbert, T.W.; Taussig, L.M.; Wright, A.L.; Martinez, F.D. Outcome of asthma and wheezing in the first 6 years of life: Follow-up through adolescence. Am. J. Respir. Crit. Care Med. 2005, 172, 1253–1258. [Google Scholar] [CrossRef] [Green Version]
- Håland, G.; Carlsen, K.C.; Sandvik, L.; Devulapalli, C.S.; Munthe-Kaas, M.C.; Pettersen, M.; Carlsen, K.H. Reduced lung function at birth and the risk of asthma at 10 years of age. N. Engl. J. Med. 2006, 355, 1682–1689. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.D.; Vercelli, D. Asthma. Lancet 2013, 382, 1360–1372. [Google Scholar] [CrossRef]
- Sears, M.R.; Greene, J.M.; Willan, A.R.; Wiecek, E.M.; Taylor, D.R.; Flannery, E.M.; Cowan, J.O.; Herbison, G.P.; Silva, P.A.; Poulton, R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N. Engl. J. Med. 2003, 349, 1414–1422. [Google Scholar] [CrossRef] [Green Version]
- Bui, D.S.; Lodge, C.J.; Burgess, J.A.; Lowe, A.J.; Perret, J.; Bui, M.Q.; Bowatte, G.; Gurrin, L.; Johns, D.P.; Thompson, B.R.; et al. Childhood predictors of lung function trajectories and future COPD risk: A prospective cohort study from the first to the sixth decade of life. Lancet Respir. Med. 2018, 6, 535–544. [Google Scholar] [CrossRef]
- Hikichi, M.; Mizumura, K.; Maruoka, S.; Gon, Y. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J. Thorac. Dis. 2019, 11, S2129–S2140. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis Management, and Prevention of Chronic Obstructive Pulmonary Disease (2020 REPORT). Available online: https://goldcopd.org/gold-reports/ (accessed on 1 May 2020).
- Yun, J.H.; Lamb, A.; Chase, R.; Singh, D.; Parker, M.M.; Saferali, A.; Vestbo, J.; Tal-Singer, R.; Castaldi, P.J.; Silverman, E.K.; et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018, 141, 2037–2047.e2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Kolsum, U.; Brightling, C.E.; Locantore, N.; Agusti, A.; Tal-Singer, R.; ECLIPSE Investigators. Eosinophilic inflammation in COPD: Prevalence and clinical characteristics. Eur. Respir. J. 2014, 44, 1697–1700. [Google Scholar] [CrossRef] [Green Version]
- George, L.; Brightling, C.E. Eosinophilic airway inflammation: Role in asthma and chronic obstructive pulmonary disease. Ther. Adv. Chron. Dis. 2016, 7, 34–51. [Google Scholar] [CrossRef] [Green Version]
- Brusselle, G.G.; Maes, T.; Bracke, K.R. Eosinophils in the spotlight: Eosinophilic airway inflammation in nonallergic asthma. Nat. Med. 2013, 19, 977–979. [Google Scholar] [CrossRef]
- De Grove, K.C.; Provoost, S.; Verhamme, F.M.; Bracke, K.R.; Joos, G.F.; Maes, T.; Brusselle, G.G. Characterization and quantification of innate lymphoid cell subsets in human lung. PLoS ONE 2016, 11, e0145961. [Google Scholar] [CrossRef] [Green Version]
- Scanlon, S.T.; McKenzie, A.N. Type 2 innate lymphoid cells: New players in asthma and allergy. Curr. Opin. Immunol. 2012, 24, 707–712. [Google Scholar] [CrossRef]
- Byers, D.E.; Alexander-Brett, J.; Patel, A.C.; Agapov, E.; Dang-Vu, G.; Jin, X.; Wu, K.; You, Y.; Alevy, Y.; Girard, J.P.; et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J. Clin. Investig. 2013, 123, 3967–3982. [Google Scholar] [CrossRef]
- Wu, H.; Yang, S.; Wu, X.; Zhao, J.; Zhao, J.; Ning, Q.; Xu, Y.; Xie, J. Interleukin-33/ST2 signaling promotes production of interleukin-6 and interleukin-8 in systemic inflammation in cigarette smoke-induced chronic obstructive pulmonary disease mice. Biochem. Biophys. Res. Commun. 2014, 450, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Li, Y.; Li, M.; Li, M.; Liu, X.; McSharry, C.; Xu, D. Anti-interleukin-33 inhibits cigarette smoke-induced lung inflammation in mice. Immunology 2013, 138, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Miyata, M.; Ohba, T.; Ando, T.; Hatsushika, K.; Suenaga, F.; Shimokawa, N.; Ohnuma, Y.; Katoh, R.; Ogawa, H.; et al. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J. Allergy Clin. Immunol. 2008, 122, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; O’Connor, B.; Ratoff, J.; Meng, Q.; Fang, C.; Cousins, D.; Zhang, G.; Gu, S.; Gao, Z.; Shamji, B.; et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J. Immunol. 2008, 181, 2790–2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Shan, L.; Rahman, M.S.; Unruh, H.; Halayko, A.J.; Gounni, A.S. Constitutive and inducible thymic stromal lymphopoietin expression in human airway smooth muscle cells: Role in chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L375–L382. [Google Scholar] [CrossRef]
- Kim, S.W.; Rhee, C.K.; Kim, K.U.; Lee, S.H.; Hwang, H.G.; Kim, Y.I.; Kim, D.K.; Lee, S.D.; Oh, Y.M.; Yoon, H.K. Factors associated with plasma IL-33 levels in patients with chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.B.; Ma, L.J.; Qi, Y.; Zhang, G.J. Correlation of IL-33 gene polymorphism with chronic obstructive pulmonary disease. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6277–6282. [Google Scholar]
- Xia, J.; Zhao, J.; Shang, J.; Li, M.; Zeng, Z.; Zhao, J.; Wang, J.; Xu, Y.; Xie, J. Increased IL-33 expression in chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L619–L627. [Google Scholar] [CrossRef]
- Tworek, D.; Majewski, S.; Szewczyk, K.; Kiszalkiewicz, J.; Kurmanowska, Z.; Gorski, P.; Brzezianska-Lasota, E.; Kuna, P.; Antczak, A. The association between airway eosinophilic inflammation and IL-33 in stable non-atopic COPD. Respir. Res. 2018, 19, 108. [Google Scholar] [CrossRef]
- Papi, A.; Bellettato, C.M.; Braccioni, F.; Romagnoli, M.; Casolari, P.; Caramori, G.; Fabbri, L.M.; Johnston, S.L. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am. J. Respir. Crit. Care Med. 2006, 173, 1114–1121. [Google Scholar] [CrossRef]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A.; et al. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Yasuo, M.; Urushibata, K.; Hanaoka, M.; Koizumi, T.; Kubo, K. Airway inflammation during stable and acutely exacerbated chronic obstructive pulmonary disease. Eur. Respir. J. 2005, 25, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Saetta, M.; Di Stefano, A.; Maestrelli, P.; Turato, G.; Ruggieri, M.P.; Roggeri, A.; Calcagni, P.; Mapp, C.E.; Ciaccia, A.; Fabbri, L.M. Airway eosinophilia in chronic bronchitis during exacerbations. Am. J. Respir. Crit. Care Med. 1994, 150, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Schumann, D.M.; Tamm, M.; Kostikas, K.; Stolz, D. Stability of the blood eosinophilic phenotype in stable and exacerbated COPD. Chest 2019, 156, 456–465. [Google Scholar] [CrossRef]
- Kolsum, U.; Donaldson, G.C.; Singh, R.; Barker, B.L.; Gupta, V.; George, L.; Webb, A.J.; Thurston, S.; Brookes, A.J.; McHugh, T.D.; et al. Blood and sputum eosinophils in COPD; relationship with bacterial load. Respir. Res. 2017, 18, 88. [Google Scholar] [CrossRef]
- Orie, N. The Host Factor in Bronchitis; Charles C Thomas: Springfield, IL, USA, 1961. [Google Scholar]
- Eltboli, O.; Bafadhel, M.; Hollins, F.; Wright, A.; Hargadon, B.; Kulkarni, N.; Brightling, C. COPD exacerbation severity and frequency is associated with impaired macrophage efferocytosis of eosinophils. BMC Pulm. Med. 2014, 14, 112. [Google Scholar] [CrossRef] [Green Version]
- Leigh, R.; Pizzichini, M.M.; Morris, M.M.; Maltais, F.; Hargreave, F.E.; Pizzichini, E. Stable COPD: Predicting benefit from high-dose inhaled corticosteroid treatment. Eur. Respir. J. 2006, 27, 964–971. [Google Scholar] [CrossRef] [Green Version]
- Pizzichini, E.; Pizzichini, M.M.; Gibson, P.; Parameswaran, K.; Gleich, G.J.; Berman, L.; Dolovich, J.; Hargreave, F.E. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am. J. Respir. Crit.Care Med. 1998, 158, 1511–1517. [Google Scholar] [CrossRef]
- Brightling, C.E.; McKenna, S.; Hargadon, B.; Birring, S.; Green, R.; Siva, R.; Berry, M.; Parker, D.; Monteiro, W.; Pavord, I.D.; et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax 2005, 60, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Siva, R.; Green, R.H.; Brightling, C.E.; Shelley, M.; Hargadon, B.; McKenna, S.; Monteiro, W.; Berry, M.; Parker, D.; Wardlaw, A.J.; et al. Eosinophilic airway inflammation and exacerbations of COPD: A randomised controlled trial. Eur. Respir. J. 2007, 29, 906–913. [Google Scholar] [CrossRef]
- Brightling, C.E.; Monteiro, W.; Ward, R.; Parker, D.; Morgan, M.D.; Wardlaw, A.J.; Pavord, I.D. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: A randomised controlled trial. Lancet 2000, 356, 1480–1485. [Google Scholar] [CrossRef]
- Hastie, A.T.; Martinez, F.J.; Curtis, J.L.; Doerschuk, C.M.; Hansel, N.N.; Christenson, S.; Putcha, N.; Ortega, V.E.; Li, X.; Barr, R.G.; et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: An analysis of the SPIROMICS cohort. Lancet Respir. Med. 2017, 5, 956–967. [Google Scholar] [CrossRef]
- Bakakos, A.; Loukides, S.; Bakakos, P. Severe eosinophilic asthma. J. Clin. Med. 2019, 8, 1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, W.C., 3rd; Apter, A.J.; Dutmer, C.M.; Searing, D.A.; Szefler, S.J. Advances in asthma in 2016: Designing individualized approaches to management. J. Allergy Clin. Immunol. 2017, 140, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.F. Precision medicine in asthma: Linking phenotypes to targeted treatments. Curr. Opin. Pulm. Med. 2018, 24, 4–10. [Google Scholar] [CrossRef]
- Yancey, S.W.; Keene, O.N.; Albers, F.C.; Ortega, H.; Bates, S.; Bleecker, E.R.; Pavord, I. Biomarkers for severe eosinophilic asthma. J. Allergy Clin. Immunol. 2017, 140, 1509–1518. [Google Scholar] [CrossRef] [Green Version]
- Pignatti, P.; Visca, D.; Cherubino, F.; Zampogna, E.; Lucini, E.; Saderi, L.; Sotgiu, G.; Spanevello, A. Do blood eosinophils strictly reflect airway inflammation in COPD? Comparison with asthmatic patients. Respir. Res. 2019, 20, 145. [Google Scholar] [CrossRef] [Green Version]
- Malinovschi, A.; Janson, C.; Borres, M.; Alving, K. Simultaneously increased fraction of exhaled nitric oxide levels and blood eosinophil counts relate to increased asthma morbidity. J. Allergy Clin. Immunol. 2016, 138, 1301–1308.e1302. [Google Scholar] [CrossRef] [Green Version]
- Rabe, K.F.; Watz, H.; Baraldo, S.; Pedersen, F.; Biondini, D.; Bagul, N.; Hanauer, G.; Göhring, U.M.; Purkayastha, D.; Román, J.; et al. Anti-inflammatory effects of roflumilast in chronic obstructive pulmonary disease (ROBERT): A 16-week, randomised, placebo-controlled trial. Lancet Respir. Med. 2018, 6, 827–836. [Google Scholar] [CrossRef]
- Eltboli, O.; Mistry, V.; Barker, B.; Brightling, C.E. Relationship between blood and bronchial submucosal eosinophilia and reticular basement membrane thickening in chronic obstructive pulmonary disease. Respirology 2015, 20, 667–670. [Google Scholar] [CrossRef]
- Kolsum, U.; Damera, G.; Pham, T.H.; Southworth, T.; Mason, S.; Karur, P.; Newbold, P.; Singh, D. Pulmonary inflammation in patients with chronic obstructive pulmonary disease with higher blood eosinophil counts. J. Allergy Clin. Immunol. 2017, 140, 1181–1184.e1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turato, G.; Semenzato, U.; Bazzan, E.; Biondini, D.; Tinè, M.; Torrecilla, N.; Forner, M.; Marin, J.M.; Cosio, M.G.; Saetta, M. Blood eosinophilia neither reflects tissue eosinophils nor worsens clinical outcomes in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018, 197, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Vedel-Krogh, S.; Nielsen, S.F.; Lange, P.; Vestbo, J.; Nordestgaard, B.G. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen general population study. Am. J. Respir. Crit. Care Med. 2016, 193, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Watz, H.; Tetzlaff, K.; Wouters, E.F.; Kirsten, A.; Magnussen, H.; Rodriguez-Roisin, R.; Vogelmeier, C.; Fabbri, L.M.; Chanez, P.; Dahl, R.; et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: A post-hoc analysis of the WISDOM trial. Lancet Respir. Med. 2016, 4, 390–398. [Google Scholar] [CrossRef]
- Suzuki, M.; Makita, H.; Konno, S.; Shimizu, K.; Kimura, H.; Kimura, H.; Nishimura, M. Asthma-like features and clinical course of chronic obstructive pulmonary disease. An analysis from the Hokkaido COPD cohort study. Am. J. Respir. Crit. Care Med. 2016, 194, 1358–1365. [Google Scholar] [CrossRef]
- Oshagbemi, O.A.; Burden, A.M.; Braeken, D.C.W.; Henskens, Y.; Wouters, E.F.M.; Driessen, J.H.M.; Maitland-van der Zee, A.H.; de Vries, F.; Franssen, F.M.E. Stability of blood eosinophils in patients with chronic obstructive pulmonary disease and in control subjects, and the impact of sex, age, smoking, and baseline counts. Am. J. Respir. Crit. Care Med. 2017, 195, 1402–1404. [Google Scholar] [CrossRef] [Green Version]
- Casanova, C.; Celli, B.R.; de-Torres, J.P.; Martínez-Gonzalez, C.; Cosio, B.G.; Pinto-Plata, V.; de Lucas-Ramos, P.; Divo, M.; Fuster, A.; Peces-Barba, G.; et al. Prevalence of persistent blood eosinophilia: Relation to outcomes in patients with COPD. Eur. Respir. J. 2017, 50, 1701162. [Google Scholar] [CrossRef]
- Kovalszki, A.; Weller, P.F. Eosinophilia. Prim. Care. 2016, 43, 607–617. [Google Scholar] [CrossRef] [Green Version]
- Schleich, F.; Corhay, J.L.; Louis, R. Blood eosinophil count to predict bronchial eosinophilic inflammation in COPD. Eur. Respir. J. 2016, 47, 1562–1564. [Google Scholar] [CrossRef]
- Zeiger, R.S.; Schatz, M.; Li, Q.; Chen, W.; Khatry, D.B.; Gossage, D.; Tran, T.N. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J. Allergy Clin. Immunol. Pract. 2014, 2, 741–750. [Google Scholar] [CrossRef]
- Zeiger, R.S.; Tran, T.N.; Butler, R.K.; Schatz, M.; Li, Q.; Khatry, D.B.; Martin, U.; Kawatkar, A.A.; Chen, W. Relationship of blood eosinophil count to exacerbations in chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. Pract. 2018, 6, 944–954.e945. [Google Scholar] [CrossRef] [PubMed]
- Adir, Y.; Hakrush, O.; Shteinberg, M.; Schneer, S.; Agusti, A. Circulating eosinophil levels do not predict severe exacerbations in COPD: A retrospective study. ERJ Open Res. 2018, 4, 00022–2018. [Google Scholar] [CrossRef] [PubMed]
- Zysman, M.; Deslee, G.; Caillaud, D.; Chanez, P.; Escamilla, R.; Court-Fortune, I.; Nesme-Meyer, P.; Perez, T.; Paillasseur, J.L.; Pinet, C.; et al. Relationship between blood eosinophils, clinical characteristics, and mortality in patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1819–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerkhof, M.; Sonnappa, S.; Postma, D.S.; Brusselle, G.; Agustí, A.; Anzueto, A.; Jones, R.; Papi, A.; Pavord, I.; Pizzichini, E.; et al. Blood eosinophil count and exacerbation risk in patients with COPD. Eur. Respir. J. 2017, 50, 1700761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.H.; Park, H.Y.; Kang, D.; Cho, J.; Kwon, S.O.; Park, J.H.; Lee, J.S.; Oh, Y.M.; Sin, D.D.; Kim, W.J.; et al. Serial blood eosinophils and clinical outcome in patients with chronic obstructive pulmonary disease. Respir. Res. 2018, 19, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hancox, R.J.; Pavord, I.D.; Sears, M.R. Associations between blood eosinophils and decline in lung function among adults with and without asthma. Eur. Respir. J. 2018, 51, 1702536. [Google Scholar] [CrossRef]
- Nishimura, M.; Makita, H.; Nagai, K.; Konno, S.; Nasuhara, Y.; Hasegawa, M.; Shimizu, K.; Betsuyaku, T.; Ito, Y.M.; Fuke, S.; et al. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 185, 44–52. [Google Scholar] [CrossRef]
- Suzuki, M.; Makita, H.; Konno, S.; Shimizu, K.; Nasuhara, Y.; Nagai, K.; Akiyama, Y.; Fuke, S.; Saito, H.; Igarashi, T.; et al. Annual change in FEV(1) in elderly 10-year survivors with established chronic obstructive pulmonary disease. Sci. Rep. 2019, 9, 2073. [Google Scholar] [CrossRef] [Green Version]
- Price, D.B.; Voorham, J.; Brusselle, G.; Clemens, A.; Kostikas, K.; Stephens, J.W.; Park, H.Y.; Roche, N.; Fogel, R. Inhaled corticosteroids in COPD and onset of type 2 diabetes and osteoporosis: Matched cohort study. NPJ Prim. Care Respir. Med. 2019, 29, 38. [Google Scholar] [CrossRef] [Green Version]
- Ernst, P.; Saad, N.; Suissa, S. Inhaled corticosteroids in COPD: The clinical evidence. Eur.Respir. J. 2015, 45, 525–537. [Google Scholar] [CrossRef] [Green Version]
- Pascoe, S.; Barnes, N.; Brusselle, G.; Compton, C.; Criner, G.J.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Hartley, B.; Lange, P.; et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: Analysis of the IMPACT trial. Lancet Respir. Med. 2019, 7, 745–756. [Google Scholar] [CrossRef]
- Ferguson, G.T.; Rabe, K.F.; Martinez, F.J.; Fabbri, L.M.; Wang, C.; Ichinose, M.; Bourne, E.; Ballal, S.; Darken, P.; DeAngelis, K.; et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): A double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir. Med. 2018, 6, 747–758. [Google Scholar] [PubMed]
- Barnes, N.C.; Sharma, R.; Lettis, S.; Calverley, P.M. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur. Respir. J. 2016, 47, 1374–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vestbo, J.; Papi, A.; Corradi, M.; Blazhko, V.; Montagna, I.; Francisco, C.; Cohuet, G.; Vezzoli, S.; Scuri, M.; Singh, D. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): A double-blind, parallel group, randomised controlled trial. Lancet. 2017, 389, 1919–1929. [Google Scholar] [CrossRef] [Green Version]
- Pascoe, S.; Locantore, N.; Dransfield, M.T.; Barnes, N.C.; Pavord, I.D. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: A secondary analysis of data from two parallel randomised controlled trials. Lancet Respir. Med. 2015, 3, 435–442. [Google Scholar] [CrossRef]
- Papi, A.; Vestbo, J.; Fabbri, L.; Corradi, M.; Prunier, H.; Cohuet, G.; Guasconi, A.; Montagna, I.; Vezzoli, S.; Petruzzelli, S.; et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): A double-blind, parallel group, randomised controlled trial. Lancet 2018, 391, 1076–1084. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Guasconi, A.; Vestbo, J.; Jones, P.; Agusti, A.; Paggiaro, P.; Wedzicha, J.A.; Singh, D. Blood eosinophils: A biomarker of response to extrafine beclomethasone/Formoterol in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2015, 192, 523–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bafadhel, M.; Peterson, S.; De Blas, M.A.; Calverley, P.M.; Rennard, S.I.; Richter, K.; Fagerås, M. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: A post-hoc analysis of three randomised trials. Lancet Respir. Med. 2018, 6, 117–126. [Google Scholar] [CrossRef]
- Lipson, D.A.; Barnhart, F.; Brealey, N.; Brooks, J.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Jones, C.E.; et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N. Engl. J. Med. 2018, 378, 1671–1680. [Google Scholar] [CrossRef]
- Chapman, K.R.; Hurst, J.R.; Frent, S.M.; Larbig, M.; Fogel, R.; Guerin, T.; Banerji, D.; Patalano, F.; Goyal, P.; Pfister, P.; et al. Long-term triple therapy DE-escalation to indacaterol/Glycopyrronium in patients with chronic obstructive pulmonary disease (Sunset): A randomized, double-blind, triple-dummy clinical trial. Am. J. Respir. Crit. Care Med. 2018, 198, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Song, J.H.; Lee, C.H.; Kim, J.W.; Lee, W.Y.; Jung, J.Y.; Park, J.H.; Jung, K.S.; Yoo, K.H.; Park, Y.B.; Kim, D.K. Clinical implications of blood eosinophil count in patients with non-asthma-COPD overlap syndrome COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 2455–2464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshagbemi, O.A.; Franssen, F.M.E.; Braeken, D.C.W.; Henskens, Y.; Wouters, E.F.M.; Maitland-van der Zee, A.H.; Burden, A.M.; de Vries, F. Blood eosinophilia, use of inhaled corticosteroids, and risk of COPD exacerbations and mortality. Pharmacoepidemiol. Drug Saf. 2018, 27, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Herland, K.; Akselsen, J.P.; Skjønsberg, O.H.; Bjermer, L. How representative are clinical study patients with asthma or COPD for a larger “real life” population of patients with obstructive lung disease? Respir. Med. 2005, 99, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oishi, K.; Hirano, T.; Hamada, K.; Uehara, S.; Suetake, R.; Yamaji, Y.; Ito, K.; Asami-Noyama, M.; Edakuni, N.; Matsunaga, K. Characteristics of 2017 GOLD COPD group A: A multicenter cross-sectional CAP study in Japan. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3901–3907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera López, C.; Casanova Macario, C.; Marín Trigo, J.M.; de-Torres, J.P.; Sicilia Torres, R.; González, J.M.; Polverino, F.; Divo, M.; Pinto Plata, V.; Zulueta, J.J.; et al. Comparison of the 2017 and 2015 global initiative for chronic obstructive lung disease reports. Impact on grouping and outcomes. Am. J. Respir. Crit. Care Med. 2018, 197, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, Y.; Oishi, K.; Hamada, K.; Ohteru, Y.; Chikumoto, A.; Murakawa, K.; Matsuda, K.; Suetake, R.; Murata, Y.; Ito, K.; et al. Detection of type2 biomarkers for response in COPD. J. Breath Res. 2020, 14, 026007. [Google Scholar] [CrossRef]
- Smith, A.D.; Cowan, J.O.; Brassett, K.P.; Herbison, G.P.; Taylor, D.R. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N. Engl. J. Med. 2005, 352, 2163–2173. [Google Scholar] [CrossRef] [Green Version]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.C.; Plummer, A.L.; Taylor, D.R.; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels for Clinical. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef] [Green Version]
- Tee, K.H.; Hui, K.P. Clips, spirometry, and submaximal inhalation for exhaled nitric oxide. Am. J. Respir. Crit. Care Med. 2005, 172, 931–932. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. 2020 GINA Report, Global Strategy for Asthma Management and Prevention, Updated 2020. Available online: http://ginasthma.org (accessed on 20 April 2020).
- Alving, K.; Malinovschi, A. Basic aspects of exhaled nitric oxide. Eur. Respir. Soc. Monograph. 2010, 49, 1–31. [Google Scholar]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Maestrelli, P.; Páska, C.; Saetta, M.; Turato, G.; Nowicki, Y.; Monti, S.; Formichi, B.; Miniati, M.; Fabbri, L.M. Decreased haem oxygenase-1 and increased inducible nitric oxide synthase in the lung of severe COPD patients. Eur. Respir. J. 2003, 21, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.T.; Liu, X.S.; Xu, Y.J.; Ni, W.; Chen, S.X. Expression of nitric oxide synthase isoenzyme in lung tissue of smokers with and without chronic obstructive pulmonary disease. Chin. Med. J. (Engl.) 2015, 128, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Corradi, M.; Mutti, A. Nitric oxide synthase isoforms in lung parenchyma of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 181, 3–4. [Google Scholar] [CrossRef]
- Lu, Z.; Huang, W.; Wang, L.; Xu, N.; Ding, Q.; Cao, C. Exhaled nitric oxide in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2695–2705. [Google Scholar] [CrossRef] [Green Version]
- Michils, A.; Louis, R.; Peché, R.; Baldassarre, S.; Van Muylem, A. Exhaled nitric oxide as a marker of asthma control in smoking patients. Eur. Respir. J. 2009, 33, 1295–1301. [Google Scholar] [CrossRef]
- Matsunaga, K.; Hirano, T.; Akamatsu, K.; Koarai, A.; Sugiura, H.; Minakata, Y.; Ichinose, M. Exhaled nitric oxide cutoff values for asthma diagnosis according to rhinitis and smoking status in Japanese subjects. Allergol. Int. 2011, 60, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Olin, A.C.; Rosengren, A.; Thelle, D.S.; Lissner, L.; Bake, B.; Torén, K. Height, age, and atopy are associated with fraction of exhaled nitric oxide in a large adult general population sample. Chest 2006, 130, 1319–1325. [Google Scholar] [CrossRef]
- Kharitonov, S.A.; Yates, D.H.; Barnes, P.J. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am. J. Respir. Crit. Care Med. 1996, 153, 454–457. [Google Scholar] [CrossRef]
- Kunisaki, K.M.; Rice, K.L.; Janoff, E.N.; Rector, T.S.; Niewoehner, D.E. Exhaled nitric oxide, systemic inflammation, and the spirometric response to inhaled fluticasone propionate in severe chronic obstructive pulmonary disease: A prospective study. Ther. Adv. Respir. Dis. 2008, 2, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.X.; Lin, Y.; Lin, J.; He, S.S.; Chen, M.F.; Wu, X.M.; Xu, Y.Z. Relationship between Fractional Exhaled Nitric Oxide Level and Efficacy of Inhaled Corticosteroid in Asthma-COPD Overlap Syndrome Patients with Different Disease Severity. J. Korean Med. Sci. 2017, 32, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Camargo, C.A., Jr.; Hasegawa, K. Fractional exhaled nitric oxide levels in asthma-COPD overlap syndrome: Analysis of the National Health and Nutrition Examination Survey, 2007–2012. Int. J. Chron Obstruct. Pulmon. Dis. 2016, 11, 2149–2155. [Google Scholar] [CrossRef] [Green Version]
- Cosío, B.G.; Pérez de Llano, L.; Lopez Viña, A.; Torrego, A.; Lopez-Campos, J.L.; Soriano, J.B.; Martinez Moragon, E.; Izquierdo, J.L.; Bobolea, I.; Callejas, J.; et al. Th-2 signature in chronic airway diseases: Towards the extinction of asthma-COPD overlap syndrome? Eur. Respir. J. 2017, 49, 1602397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, D.D.; Zhou, A.Y.; Shuang, Q.C.; Chen, P. The value of fractionated exhaled nitric oxide in the diagnosis of asthma-chronic obstructive pulmonary disease overlap syndrome. Zhonghua Jie He He Hu Xi Za Zhi 2017, 40, 98–101. [Google Scholar] [PubMed]
- Kobayashi, S.; Hanagama, M.; Yamanda, S.; Ishida, M.; Yanai, M. Inflammatory biomarkers in asthma-COPD overlap syndrome. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 2117–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.J.; Huang, X.Y.; Liu, Y.L.; Lin, G.P.; Xie, C.M. Importance of fractional exhaled nitric oxide in the differentiation of asthma-COPD overlap syndrome, asthma, and COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 2385–2390. [Google Scholar]
- Alcázar-Navarrete, B.; Romero-Palacios, P.J.; Ruiz-Sancho, A.; Ruiz-Rodriguez, O. Diagnostic performance of the measurement of nitric oxide in exhaled air in the diagnosis of COPD phenotypes. Nitric Oxide 2016, 54, 67–72. [Google Scholar] [CrossRef]
- Tamada, T.; Sugiura, H.; Takahashi, T.; Matsunaga, K.; Kimura, K.; Katsumata, U.; Takekoshi, D.; Kikuchi, T.; Ohta, K.; Ichinose, M. Biomarker-based detection of asthma-COPD overlap syndrome in COPD populations. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 2169–2176. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.J.; Lin, G.P.; Huang, X.Y.; Liu, Y.L.; Zeng, Z.M.; Guo, Y.B. Evaluation of the characteristics of asthma in severe and extremely severe COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 2663–2671. [Google Scholar] [CrossRef] [Green Version]
- Bhowmik, A.; Seemungal, T.A.; Donaldson, G.C.; Wedzicha, J.A. Effects of exacerbations and seasonality on exhaled nitric oxide in COPD. Eur. Respir. J. 2005, 26, 1009–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antus, B.; Barta, I.; Horvath, I.; Csiszer, E. Relationship between exhaled nitric oxide and treatment response in COPD patients with exacerbations. Respirology 2010, 15, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.G.; Villaverde, J.M.; Togores, B.; Bosch, M. Serial measurements of exhaled nitric oxide during exacerbations of chronic obstructive pulmonary disease. Eur. Respir. J. 1999, 14, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Lázár, Z.; Kelemen, Á.; Gálffy, G.; Losonczy, G.; Horváth, I.; Bikov, A. Central and peripheral airway nitric oxide in patients with stable and exacerbated chronic obstructive pulmonary disease. J. Breath Res. 2018, 12, 036017. [Google Scholar] [CrossRef]
- Xia, Q.; Pan, P.; Wang, Z.; Lu, R.; Hu, C. Fractional exhaled nitric oxide in bronchial inflammatory lung diseases. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014, 39, 365–370. [Google Scholar]
- Sanders, S.P.; Proud, D.; Permutt, S.; Siekierski, E.S.; Yachechko, R.; Liu, M.C. Role of nasal nitric oxide in the resolution of experimental rhinovirus infection. J. Allergy Clin. Immunol. 2004, 113, 697–702. [Google Scholar] [CrossRef]
- Viniol, C.; Vogelmeier, C.F. Exacerbations of COPD. Eur. Respir. Rev. 2018, 27, 170103. [Google Scholar] [CrossRef] [Green Version]
- Alcázar-Navarrete, B.; Ruiz Rodríguez, O.; Conde Baena, P.; Romero Palacios, P.J.; Agusti, A. Persistently elevated exhaled nitric oxide fraction is associated with increased risk of exacerbation in COPD. Eur. Respir. J. 2018, 51, 1701457. [Google Scholar] [CrossRef] [Green Version]
- Dweik, R.A.; Sorkness, R.L.; Wenzel, S.; Hammel, J.; Curran-Everett, D.; Comhair, S.A.; Bleecker, E.; Busse, W.; Calhoun, W.J.; Castro, M.; et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am. J. Respir. Crit. Care Med. 2010, 181, 1033–1041. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, K.; Hirano, T.; Oka, A.; Ito, K.; Edakuni, N. Persistently high exhaled nitric oxide and loss of lung function in controlled asthma. Allergol. Int. 2016, 65, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, K.; Katoh, N.; Fujieda, S.; Izuhara, K.; Oishi, K. Dupilumab: Basic aspects and applications to allergic diseases. Allergol. Int. 2020, 69, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Dummer, J.F.; Epton, M.J.; Cowan, J.O.; Cook, J.M.; Condliffe, R.; Landhuis, C.E.; Smith, A.D.; Taylor, D.R. Predicting corticosteroid response in chronic obstructive pulmonary disease using exhaled nitric oxide. Am. J. Respir. Crit. Care Med. 2009, 180, 846–852. [Google Scholar] [CrossRef] [PubMed]
- De Laurentiis, G.; Maniscalco, M.; Cianciulli, F.; Stanziola, A.; Marsico, S.; Lundberg, J.O.; Weitzberg, E.; Sofia, M. Exhaled nitric oxide monitoring in COPD using a portable analyzer. Pulm. Pharmacol. Ther. 2008, 21, 689–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehouck, A.; Carremans, C.; De Bent, K.; Decramer, M.; Janssens, W. Alveolar and bronchial exhaled nitric oxide in chronic obstructive pulmonary disease. Respir. Med. 2010, 104, 1020–1026. [Google Scholar] [CrossRef] [Green Version]
- Beg, M.F.; Alzoghaibi, M.A.; Abba, A.A.; Habib, S.S. Exhaled nitric oxide in stable chronic obstructive pulmonary disease. Ann. Thorac Med. 2009, 4, 65–70. [Google Scholar]
- Ansarin, K.; Chatkin, J.M.; Ferreira, I.M.; Gutierrez, C.A.; Zamel, N.; Chapman, K.R. Exhaled nitric oxide in chronic obstructive pulmonary disease: Relationship to pulmonary function. Eur. Respir. J. 2001, 17, 934–938. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, H.; Wang, Y.; Lu, Y.; Gao, Y.; Lu, Y.; Zheng, C.; Yin, D.; Wang, S.; Huang, K. Fractional exhaled nitric oxide is associated with the severity of stable COPD. COPD 2020, 17, 121–127. [Google Scholar] [CrossRef]
- Lim, C.S.; Rani, F.A.; Tan, L.E. Response of exhaled nitric oxide to inhaled corticosteroids in patients with stable COPD: A systematic review and meta-analysis. Clin. Respir. J. 2018, 12, 218–226. [Google Scholar] [CrossRef]
- Clini, E.; Cremona, G.; Campana, M.; Scotti, C.; Pagani, M.; Bianchi, L.; Giordano, A.; Ambrosino, N. Production of endogenous nitric oxide in chronic obstructive pulmonary disease and patients with cor pulmonale. Correlates with echo-Doppler assessment. Am. J. Respir. Crit. Care Med. 2000, 162, 446–450. [Google Scholar] [CrossRef]
- Zietkowski, Z.; Kucharewicz, I.; Bodzenta-Lukaszyk, A. The influence of inhaled corticosteroids on exhaled nitric oxide in stable chronic obstructive pulmonary disease. Respir. Med. 2005, 99, 816–824. [Google Scholar] [CrossRef] [Green Version]
- Johansson, S.G.O.; Haahtela, T.; World Allergy Organization. Prevention of Allergy and Allergic Asthma: World Allergy Organization Project Report and Guidelines; Karger Medical and Scientific Publishers: Basel, Switzerland, 2004. [Google Scholar]
- O’Hehir, R.E.; Holgate, S.T.; Sheikh, A. Middleton’s Allergy Essentials E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Sluiter, H.J.; Koëter, G.H.; de Monchy, J.G.; Postma, D.S.; de Vries, K.; Orie, N.G. The Dutch hypothesis (chronic non-specific lung disease) revisited. Eur. Respir. J. 1991, 4, 479–489. [Google Scholar] [PubMed]
- Sherrill, D.L.; Lebowitz, M.D.; Halonen, M.; Barbee, R.A.; Burrows, B. Longitudinal evaluation of the association between pulmonary function and total serum IgE. Am. J. Respir. Crit. Care Med. 1995, 152, 98–102. [Google Scholar] [CrossRef]
- Everaerts, S.; Lagrou, K.; Dubbeldam, A.; Lorent, N.; Vermeersch, K.; Van Hoeyveld, E.; Bossuyt, X.; Dupont, L.J.; Vanaudenaerde, B.M.; Janssens, W. Sensitization to Aspergillus fumigatus as a risk factor for bronchiectasis in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 2629–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.; Liu, X.; Sun, Y. The prevalence of increased serum IgE and Aspergillus sensitization in patients with COPD and their association with symptoms and lung function. Respir. Res. 2014, 15, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bafadhel, M.; McKenna, S.; Agbetile, J.; Fairs, A.; Desai, D.; Mistry, V.; Morley, J.P.; Pancholi, M.; Pavord, I.D.; Wardlaw, A.J.; et al. Aspergillus fumigatus during stable state and exacerbations of COPD. Eur. Respir. J. 2014, 43, 64–71. [Google Scholar] [CrossRef]
- Fattahi, F.; ten Hacken, N.H.; Löfdahl, C.G.; Hylkema, M.N.; Timens, W.; Postma, D.S.; Vonk, J.M. Atopy is a risk factor for Respir.atory symptoms in COPD patients: Results from the EUROSCOP study. Respir. Res. 2013, 14, 10. [Google Scholar] [CrossRef] [Green Version]
- Jamieson, D.B.; Matsui, E.C.; Belli, A.; McCormack, M.C.; Peng, E.; Pierre-Louis, S.; Curtin-Brosnan, J.; Breysse, P.N.; Diette, G.B.; Hansel, N.N. Effects of allergic phenotype on Respir.atory symptoms and exacerbations in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 188, 187–192. [Google Scholar] [CrossRef]
- Bożek, A.; Rogala, B. IgE-dependent sensitization in patients with COPD. Ann. Agric. Environ. Med. 2018, 25, 417–420. [Google Scholar] [CrossRef]
- Hu, H.; Huang, H.; Zheng, P.; Li, L.; Cai, C.; Li, N.; Sun, B. The sensitization characteristics of adult Chinese patients diagnosed with chronic respiratory diseases. Asian Pac. J. Allergy Immunol. 2019. [Google Scholar]
- Neves, M.C.; Neves, Y.C.; Mendes, C.M.; Bastos, M.N.; Camelier, A.A.; Queiroz, C.F.; Mendoza, B.F.; Lemos, A.C.; D’Oliveira, A., Jr. Evaluation of atopy in patients with COPD. J. Bras. Pneumol. 2013, 39, 296–305. [Google Scholar] [CrossRef] [Green Version]
- De Soyza, A.; Aliberti, S. Bronchiectasis and Aspergillus: How are they linked? Med. Mycol. 2017, 55, 69–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akamatsu, K.; Matsunaga, K.; Sugiura, H.; Koarai, A.; Hirano, T.; Minakata, Y.; Ichinose, M. Improvement of airflow limitation by fluticasone propionate/Salmeterol in chronic obstructive pulmonary disease: What is the specific marker? Front. Pharmacol. 2011, 2, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oishi, K.; Hirano, T.; Suetake, R.; Ohata, S.; Yamaji, Y.; Ito, K.; Edakuni, N.; Matsunaga, K. A trial of oral corticosteroids for persistent systemic and airway inflammation in severe asthma. Immun. Inflamm. Dis. 2017, 5, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S.; Ichinose, M. Definition and diagnosis of asthma-COPD overlap (ACO). Allergol. Int. 2018, 67, 172–178. [Google Scholar] [CrossRef]
- Sin, D.D.; Miravitlles, M.; Mannino, D.M.; Soriano, J.B.; Price, D.; Celli, B.R.; Leung, J.M.; Nakano, Y.; Park, H.Y.; Wark, P.A.; et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur. Respir. J. 2016, 48, 664–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takayama, Y.; Ohnishi, H.; Ogasawara, F.; Oyama, K.; Kubota, T.; Yokoyama, A. Clinical utility of fractional exhaled nitric oxide and blood eosinophils counts in the diagnosis of asthma-COPD overlap. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2525–2532. [Google Scholar] [CrossRef] [Green Version]
- Çolak, Y.; Afzal, S.; Nordestgaard, B.G.; Marott, J.L.; Lange, P. Combined value of exhaled nitric oxide and blood eosinophils in chronic airway disease: The Copenhagen general population study. Eur. Respir. J. 2018, 52, 1800616. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, K.; Hirano, T.; Kawayama, T.; Tsuburai, T.; Nagase, H.; Aizawa, H.; Akiyama, K.; Ohta, K.; Ichinose, M. Reference ranges for exhaled nitric oxide fraction in healthy Japanese adult population. Allergol. Int. 2010, 59, 363–367. [Google Scholar] [CrossRef]
- Matsunaga, K.; Kuwahira, I.; Hanaoka, M.; Saito, J.; Tsuburai, T.; Fukunaga, K.; Matsumoto, H.; Sugiura, H.; Ichinose, M. An official JRS statement: The principles of fractional exhaled nitric oxide (FeNO) measurement and interpretation of the results in clinical practice. Respir. Investig. 2020. [Google Scholar] [CrossRef]
- Matsumoto, H. Role of serum periostin in the management of asthma and its comorbidities. Respir. Investig. 2020, 58, 144–154. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Matsumoto, H.; Izuhara, K.; Tohda, Y.; Kita, H.; Horiguchi, T.; Kuwabara, K.; Tomii, K.; Otsuka, K.; Fujimura, M.; et al. Increased periostin associates with greater airflow limitation in patients receiving inhaled corticosteroids. J. Allergy Clin. Immunol. 2013, 132, 305–312.e303. [Google Scholar] [CrossRef] [Green Version]
- Park, H.Y.; Lee, H.; Koh, W.J.; Kim, S.; Jeong, I.; Koo, H.K.; Kim, T.H.; Kim, J.W.; Kim, W.J.; Oh, Y.M.; et al. Association of blood eosinophils and plasma periostin with FEV1 response after 3-month inhaled corticosteroid and long-acting beta2-agonist treatment in stable COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantelou, E.; Papaioannou, A.I.; Loukides, S.; Bartziokas, K.; Papaporfyriou, A.; Papatheodorou, G.; Bakakos, P.; Papiris, S.; Koulouris, N.; Kostikas, K. Serum periostin in patients hospitalized for COPD exacerbations. Cytokine 2017, 93, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Carpaij, O.A.; Muntinghe, F.O.W.; Wagenaar, M.B.; Habing, J.W.; Timens, W.; Kerstjens, H.A.M.; Nawijn, M.C.; Kunz, L.I.Z.; Hiemstra, P.S.; Tew, G.W.; et al. Serum periostin does not reflect type 2-driven inflammation in COPD. Respir. Res. 2018, 19, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, X.; Wang, D.; Liu, S.; Ma, Y.; Li, Z.; Tian, P.; Fan, H. The YKL-40 protein is a potential biomarker for COPD: A meta-analysis and systematic review. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 409–418. [Google Scholar] [CrossRef]
- James, A.J.; Reinius, L.E.; Verhoek, M.; Gomes, A.; Kupczyk, M.; Hammar, U.; Ono, J.; Ohta, S.; Izuhara, K.; Bel, E.; et al. Increased ykl-40 and chitotriosidase in asthma and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2016, 193, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Hinks, T.S.C.; Brown, T.; Lau, L.C.K.; Rupani, H.; Barber, C.; Elliott, S.; Ward, J.A.; Ono, J.; Ohta, S.; Izuhara, K.; et al. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J. Allergy Clin. Immunol. 2016, 138, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Majewski, S.; Tworek, D.; Szewczyk, K.; Kiszałkiewicz, J.; Kurmanowska, Z.; Brzeziańska-Lasota, E.; Jerczyńska, H.; Antczak, A.; Piotrowski, W.J.; Górski, P. Overexpression of chitotriosidase and YKL-40 in peripheral blood and sputum of healthy smokers and patients with chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 1611–1631. [Google Scholar] [CrossRef] [Green Version]
- Gon, Y.; Ito, R.; Hattori, T.; Hiranuma, H.; Kumasawa, F.; Kozu, Y.; Endo, D.; Koyama, D.; Shintani, Y.; Eriko, T.; et al. Serum eosinophil-derived neurotoxin: Correlation with persistent airflow limitation in adults with house-dust mite allergic asthma. Allergy Asthma Proc. 2015, 36, e113–e120. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.H.; Yang, E.M.; Kwon, E.; Jung, C.G.; Kim, S.C.; Choi, Y.; Cho, Y.S.; Kim, C.K.; Park, H.S. Serum levels of eosinophil-derived neurotoxin: A biomarker for asthma severity in adult asthmatics. Allergy Asthma Immunol. Res. 2019, 11, 394–405. [Google Scholar] [CrossRef]
- An, J.; Lee, J.H.; Sim, J.H.; Song, W.J.; Kwon, H.S.; Cho, Y.S.; Moon, H.B.; Kim, C.K.; Kim, T.B. Serum Eosinophil-derived neurotoxin better reflect asthma control status than blood eosinophil counts. J. Allergy Clin. Immunol. Pract. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, H.; Luo, Z.; Mou, S.; Liu, J.; Liu, C.; Deng, S.; Jiang, Y.; Lin, J.; Wu, C.; et al. Plasma YKL-40 and NGAL are useful in distinguishing ACO from asthma and COPD. Respir. Res. 2018, 19, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Llano, L.P.; Cosío, B.G.; Iglesias, A.; de Las Cuevas, N.; Soler-Cataluña, J.J.; Izquierdo, J.L.; López-Campos, J.L.; Calero, C.; Plaza, V.; Miravitlles, M.; et al. Mixed Th2 and non-Th2 inflammatory pattern in the asthma-COPD overlap: A network approach. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 591–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gon, Y.; Maruoka, S.; Ito, R.; Mizumura, K.; Kozu, Y.; Hiranuma, H.; Hattori, T.; Takahashi, M.; Hikichi, M.; Hashimoto, S. Utility of serum YKL-40 levels for identification of patients with asthma and COPD. Allergol. Int. 2017, 66, 624–626. [Google Scholar] [CrossRef]
- Shirai, T.; Hirai, K.; Gon, Y.; Maruoka, S.; Mizumura, K.; Hikichi, M.; Holweg, C.; Itoh, K.; Inoue, H.; Hashimoto, S. Combined assessment of serum periostin and ykl-40 may identify asthma-COPD overlap. J. Allergy Clin. Immunol. Pract. 2019, 7, 134–145.e131. [Google Scholar] [CrossRef]
- Shirai, T.; Hirai, K.; Gon, Y.; Maruoka, S.; Mizumura, K.; Hikichi, M.; Itoh, K.; Hashimoto, S. Combined assessment of serum eosinophil-derived neurotoxin and ykl-40 may identify asthma-COPD overlap. Allergol. Int. 2020, S1323–S8930, 30070. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oishi, K.; Matsunaga, K.; Shirai, T.; Hirai, K.; Gon, Y. Role of Type2 Inflammatory Biomarkers in Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2020, 9, 2670. https://doi.org/10.3390/jcm9082670
Oishi K, Matsunaga K, Shirai T, Hirai K, Gon Y. Role of Type2 Inflammatory Biomarkers in Chronic Obstructive Pulmonary Disease. Journal of Clinical Medicine. 2020; 9(8):2670. https://doi.org/10.3390/jcm9082670
Chicago/Turabian StyleOishi, Keiji, Kazuto Matsunaga, Toshihiro Shirai, Keita Hirai, and Yasuhiro Gon. 2020. "Role of Type2 Inflammatory Biomarkers in Chronic Obstructive Pulmonary Disease" Journal of Clinical Medicine 9, no. 8: 2670. https://doi.org/10.3390/jcm9082670
APA StyleOishi, K., Matsunaga, K., Shirai, T., Hirai, K., & Gon, Y. (2020). Role of Type2 Inflammatory Biomarkers in Chronic Obstructive Pulmonary Disease. Journal of Clinical Medicine, 9(8), 2670. https://doi.org/10.3390/jcm9082670




