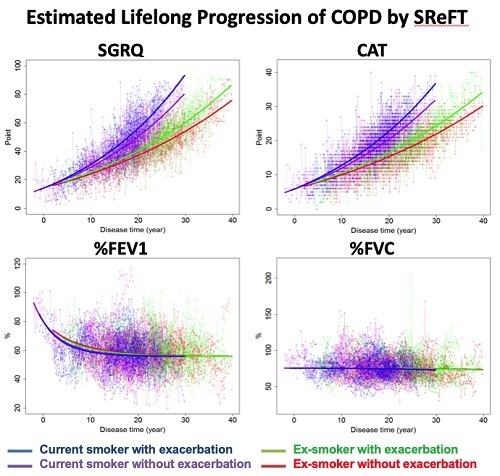
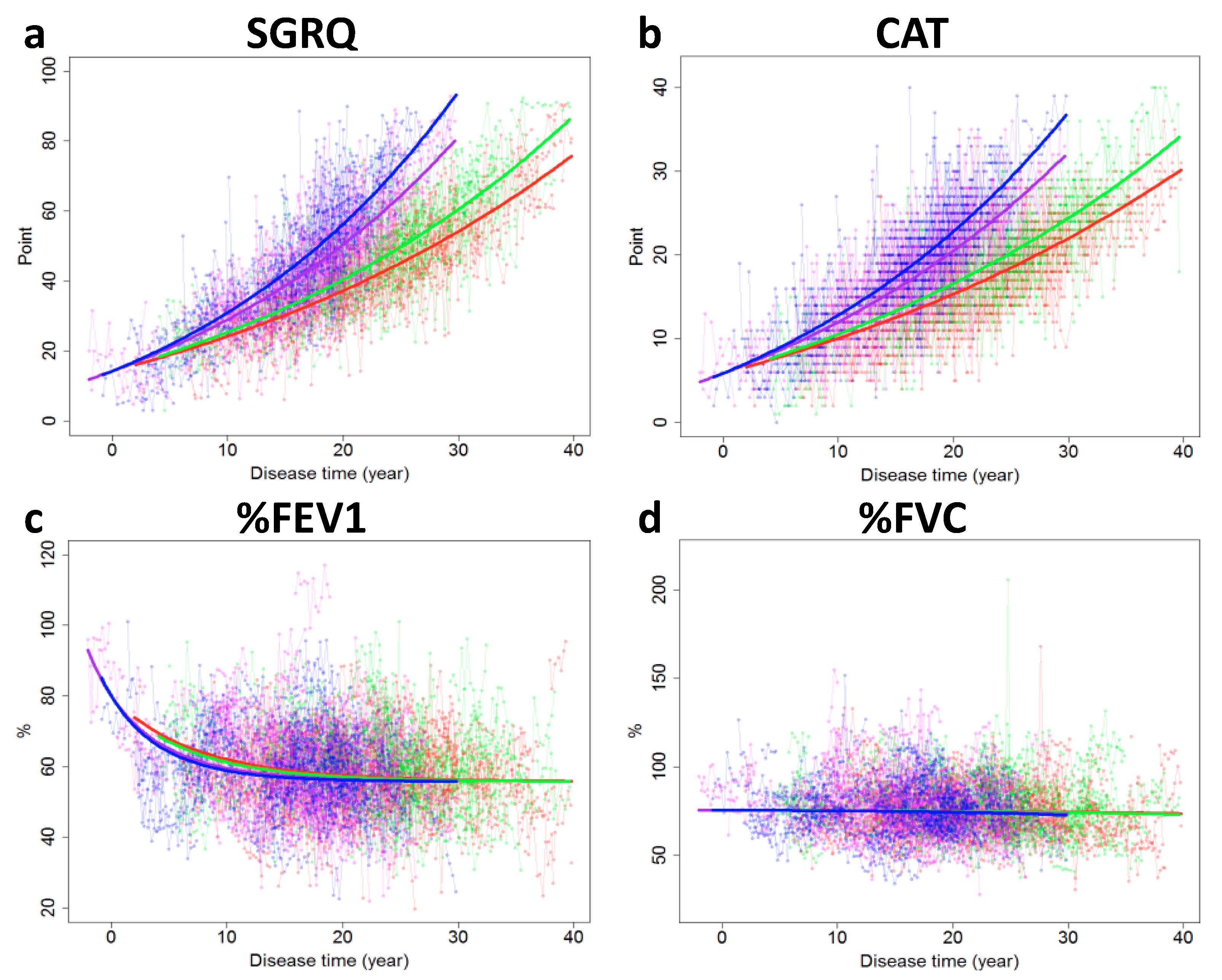
Scores of Health-Related Quality of Life Questionnaire Worsen Consistently in Patients of COPD: Estimating Disease Progression over 30 Years by SReFT with Individual Data Collected in SUMMIT Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
- COPD clinical study, available in Jan-2017;
- Double-blind, placebo-controlled, randomized clinical trials;
- Enrolled COPD patients;
- Clinical trials lasting more than or equal to 48 weeks;
- Clinical trials with more than 3000 patients;
- Clinical trials with continuous pulmonary function and HRQOL data;
- Clinical trials with available blood test data.
2.2. Analysis by Linear Mixed-Effect Model
2.3. Analysis by SReFT
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Health Statistics 2008; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Barnes, P.J.; Burney, P.G.J.; Silverman, E.K.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F.M. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Prim. 2015, 1, 15076. [Google Scholar] [CrossRef]
- Laborin, R.L. Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21st century. Int. J. Environ. Res. Public Health 2009, 6, 209–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, C.M.; Sciurba, F.C. Diagnosis and outpatient management of chronic obstructive pulmonary disease. JAMA 2019, 321, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.-P.; Niti, M.; Tan, W.-C.; Cao, Z.; Ong, K.-C.; Eng, P. Depressive symptoms and chronic obstructive pulmonary disease. Arch. Intern. Med. 2007, 167, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Quirk, F.H.; Baveystock, C. The St George’s respiratory questionnaire. Respir. Med. 1991, 85, 25–31. [Google Scholar] [CrossRef]
- Jones, P.W. COPD assessment test—Rationale, development, validation and performance. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10, 269–271. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease; Global Initiative for Chronic Obstructive Lung Disease, Inc.: Fontana, WI, USA, 2020. [Google Scholar]
- Bhavani, S.; Tsai, C.-L.; Perusich, S.; Hesselbacher, S.; Coxson, H.; Pandit, L.; Corry, D.B.; Kheradmand, F. Clinical and immunological factors in emphysema progression. Five-year prospective longitudinal exacerbation study of chronic obstructive pulmonary disease (LES-COPD). Am. J. Respir. Crit. Care Med. 2015, 192, 1171–1178. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Salcedo, P.; Divo, M.; Casanova, C.; Pinto-Plata, V.; De Torres, J.P.; Côté, C.; Cabrera, C.; Zagaceta, J.; Rodriguez-Roisin, R.; Zulueta, J.J.; et al. Disease progression in young patients with COPD: Rethinking the Fletcher and Peto model. Eur. Respir. J. 2014, 44, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Zafari, Z.; Sin, D.D.; Postma, D.S.; Löfdahl, C.-G.; Vonk, J.; Bryan, S.; Lam, S.; Tammemagi, C.M.; Khakban, R.; Man, S.P.; et al. Individualized prediction of lung-function decline in chronic obstructive pulmonary disease. Can. Med. Assoc. J. 2016, 188, 1004–1011. [Google Scholar] [CrossRef] [Green Version]
- Ross, J.C.; Castaldi, P.J.; Cho, M.H.; Hersh, C.P.; Rahaghi, F.N.; Sanchez-Ferrero, G.V.; Parker, M.M.; Litonjua, A.A.; Sparrow, D.; Dy, J.G.; et al. Longitudinal modeling of lung function trajectories in smokers with and without chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018, 198, 1033–1042. [Google Scholar] [CrossRef]
- Hankinson, J.L.; Odencrantz, J.R.; Fedan, K.B. Spirometric reference values from a sample of the general U.S. Population. Am. J. Respir. Crit. Care Med. 1999, 159, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Ståhl, E.; Lindberg, A.; Jansson, S.-A.; Rönmark, E.; Svensson, K.; Andersson, F.; Löfdahl, C.-G.; Lundbäck, B. Health-related quality of life is related to COPD disease severity. Health Qual. Life Outcomes 2005, 3, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, T.; Tokuda, K.; Hisaka, A.; Honma, M.; Kijima, S.; Takatoku, H.; Iwatsubo, T.; Moritoyo, T.; Suzuki, H.; Initiative, A.D.N. A novel method to estimate long-term chronological changes from fragmented observations in disease progression. Clin. Pharmacol. Ther. 2018, 105, 436–447. [Google Scholar] [CrossRef] [Green Version]
- Vestbo, J.; Anderson, J.A.; Brook, R.D.; ACalverley, P.M.; Celli, B.R.; Crim, C.; Martínez, F.; Yates, J.; Newby, D.E.; SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): A double-blind randomised controlled trial. Lancet 2016, 387, 1817–1826. [Google Scholar] [CrossRef]
- Hankinson, J.L.; Kawut, S.M.; Shahar, E.; Smith, L.J.; Stukovsky, K.H.; Barr, R.G. Performance of american thoracic society-recommended spirometry reference values in a multiethnic sample of adults: The multi-ethnic study of atherosclerosis (MESA) lung study. Chest 2009, 137, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Dartois, C.; Brendel, K.; Comets, E.; Laffont, C.M.; Laveille, C.; Tranchand, B.; Mentré, F.; Lemenuel-Diot, A.; Girard, P. Overview of model-building strategies in population PK/PD analyses: 2002–2004 literature survey. Br. J. Clin. Pharmacol. 2007, 64, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.S.; Brashier, B.B.; Londhe, J.; Pyasi, K.; Vincent, V.; Kajale, S.S.; Tambe, S.; Mandani, K.; Nair, A.; Mak, S.M.; et al. Phenotypic comparison between smoking and non-smoking chronic obstructive pulmonary disease. Respir. Res. 2020, 21, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Soler-Cataluña, J.J.; Martinez-Garcia, M.; Roman, S.; Salcedo, E.; Navarro, M.; Ochando, R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005, 60, 925–931. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Yang, N.; Ge, Z.; Yan, M.; Wu, N.; Liu, Y. Body mass index of patients with chronic obstructive pulmonary disease is associated with pulmonary function and exacerbations: A retrospective real world research. J. Thorac. Dis. 2018, 10, 5086–5099. [Google Scholar] [CrossRef]
- Han, M.K.; Postma, D.; Mannino, D.M.; Giardino, N.D.; Buist, S.; Curtis, J.L.; Martinez, F.J. Gender and Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2007, 176, 1179–1184. [Google Scholar] [CrossRef] [Green Version]
- Seemungal, T.A.R.; Donaldson, G.C.; Paul, E.A.; Bestall, J.; Jeffries, D.J.; Wedzicha, J.A. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 157, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Pinto, L.M.; Morogan, A.; Bourbeau, J. The COPD assessment test: A systematic review. Eur. Respir. J. 2014, 44, 873–884. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Pinto, L.; Aaron, S.D.; Marciniuk, D.D.; O’Donnell, D.E.; Walker, B.L.; Fitzgerald, J.M.; Sin, D.; Marciniuk, D.D.; O’Donnell, D.E.; et al. The COPD Assessment Test. Chest 2016, 150, 1069–1079. [Google Scholar] [CrossRef]
- Fletcher, C.; Peto, R. The natural history of chronic airflow obstruction. BMJ 1977, 1, 1645–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaher, C.; Halbert, R.; Dubois, R.; George, D.; Nonikov, D. Smoking-related diseases: The importance of COPD. Int. J. Tuberc. Lung Dis. 2004, 8, 1423–1428. [Google Scholar]
- Ramírez-Venegas, A.; Sansores, R.H.; Quintana-Carrillo, R.H.; Velázquez-Uncal, M.; Hernández-Zenteno, R.J.; Sánchez-Romero, C.; Velázquez-Montero, A.; Flores-Trujillo, F. FEV1Decline in patients with chronic obstructive pulmonary disease associated with biomass exposure. Am. J. Respir. Crit. Care Med. 2014, 190, 996–1002. [Google Scholar] [CrossRef]
- Karloh, M.; Rocha, S.A.V.; Pizzichini, M.M.M.; Cavalli, F.; Matte, D.L.; Pizzichini, E. Is the COPD assessment test sensitive for differentiating COPD patients from active smokers and nonsmokers without lung function impairment? A population-based study. J. Bras. Pneumol. 2018, 44, 213–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St George’s University of London Health Status Research. Available online: http://www.healthstatus.sgul.ac.uk (accessed on 26 July 2020).
- COPD Assessment Test. Available online: https://www.catestonline.org (accessed on 26 July 2020).

| Biomarker | Smoking Status | Previous Exacerbation * | Intercept | Slope |
|---|---|---|---|---|
| SGRQ | Ex-smk | Yes | 42.1 (0.935) | 0.355 (0.369) |
| Ex-smk | No | 47.5 (1.06) | −0.549 (0.468) | |
| Smk | Yes | 42.1 (0.893) | 0.213 (0.445) | |
| Smk | No | 45.7 (1.24) | −0.127 (0.525) | |
| CAT | Ex-smk | Yes | 16.8 (0.374) | 0.176 (0.150) |
| Ex-smk | No | 18.6 (0.483) | −0.0373 (0.216) | |
| Smk | Yes | 17.8 (0.353) | 0.0966 (0.176) | |
| Smk | No | 18.8 (0.505) | −0.0995 (0.243) | |
| %FEV1 | Ex-smk | Yes | 58.7 (0.612) | −0.710 (0.246) |
| Ex-smk | No | 60.6 (0.671) | −0.735 (0.331) | |
| Smk | Yes | 60.5 (0.625) | −1.28 (0.272) | |
| Smk | No | 60.0 (0.729) | −1.17 (0.353) | |
| %FVC | Ex-smk | Yes | 76.1 (0.827) | −0.710 (0.329) |
| Ex-smk | No | 77.0 (0.922) | −0.0832 (0.434) | |
| Smk | Yes | 78.2 (0.849) | −1.27 (0.428) | |
| Smk | No | 77.0 (0.923) | −0.651 (0.429) |
| Model | Smoking Status | Exacerbation History | BMI at Baseline | Gender | Interaction | Object Function |
|---|---|---|---|---|---|---|
| Base model | N | N | N | N | NA | 554.7 |
| Y | N | N | N | NA | 413.2 | |
| N | Y | N | N | NA | 549.1 | |
| N | N | Y | N | NA | 544.9 | |
| N | N | N | Y | NA | 547.1 | |
| Y | Y | N | N | N | 403.8 | |
| Final model | Y | Y | N | N | Y | 397.1 |
| Y | N | Y | N | N | 419.2 | |
| Y | N | Y | N | Y | 399.8 | |
| Y | N | N | Y | N | 438.3 | |
| Y | N | N | Y | Y | 453.5 | |
| Y | Y | Y | N | N | 434.1 | |
| Y | Y | Y | N | Y | 414.3 |
| Parameter | Biomarker | ||||
|---|---|---|---|---|---|
| SGRQ | CAT | %FEV1 | %FVC | ||
| α | −1.83 | −1.80 | 1.62 * | 0.0159 | |
| β | 0.0835 | 0.0864 | −0.347 | −0.00131 | |
| γ | 0.0411 | 0.0361 | −0.19 | 0.0889 | |
| Inter-subject variability (ω) | α | 0.142 | 0.151 | 0.00 ** | 0.829 |
| β | 0.00 ** | 0.00 ** | 0.182 | 0.00 ** | |
| γ | 0.00464 | 0.00359 | 0.00 ** | 0.128 | |
| Intra-subject variability (σ) | 0.529 | 0.584 | 0.515 | 0.524 | |
| Ex-smk | desm | 0.711 | |||
| Non-exa | dnex | 0.906 | |||
| Interaction (Smk, Non-exa) | dinter | 0.995 | |||
| Item | Current Smoker | Ex-Smoker | Statistic Difference | ||
|---|---|---|---|---|---|
| Exacerbated * | Not Exacerbated | Exacerbated * | Not Exacerbated | ||
| Number of Patients | 221 | 296 | 236 | 272 | N.S. |
| Number of Males <%> | 161 <72.9> | 208 <70.5> | 172 <72.9> | 200 <73.5> | N.S. |
| Age (At Baseline Measurement) | 61.6 (7.6) | 63.3 (7.8) | 65.8 (8.2) | 66.7 (6.5) | <0.001 |
| Height (cm) | 169 (9.5) | 169 (8.9) | 170 (8.8) | 170 (8.7) | N.S. |
| BMI | 28.3 (5.2) | 27.7 (5.3) | 29.4 (4.9) | 30.0 (5.2) | <0.001 |
| mMRC Score | 2.25 (0.5) | 2.19 (0.4) | 2.28 (0.5) | 2.21 (0.4) | N.S. |
| Baseline %FEV1 (%) | 58.7 (6.6) | 58.8 (7.8) | 59.4 (7.7) | 59.5 (7.2) | N.S. |
| Smoking Pack-Years | 37.8 (20.8) | 40.0 (20.2) | 35.7 (23.1) | 38.4 (22.3) | N.S. |
| Smoking Years | 38.1 (10.4) | 40.3 (10.2) | 32.5 (11.4) | 34.1 (10.8) | <0.001 |
| Estimated Disease Duration ** | 14.6 (6.3) | 15.3 (5.8) | 21.3 (7.3) | 21.4 (7.7) | <0.001 |
| Estimated Age at COPD Onset | 47.0 (9.9) | 47.9 (10.0) | 44.6 (10.9) | 45.3 (9.7) | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawamatsu, S.; Jin, R.; Araki, S.; Yoshioka, H.; Sato, H.; Sato, Y.; Hisaka, A. Scores of Health-Related Quality of Life Questionnaire Worsen Consistently in Patients of COPD: Estimating Disease Progression over 30 Years by SReFT with Individual Data Collected in SUMMIT Trial. J. Clin. Med. 2020, 9, 2676. https://doi.org/10.3390/jcm9082676
Kawamatsu S, Jin R, Araki S, Yoshioka H, Sato H, Sato Y, Hisaka A. Scores of Health-Related Quality of Life Questionnaire Worsen Consistently in Patients of COPD: Estimating Disease Progression over 30 Years by SReFT with Individual Data Collected in SUMMIT Trial. Journal of Clinical Medicine. 2020; 9(8):2676. https://doi.org/10.3390/jcm9082676
Chicago/Turabian StyleKawamatsu, Shinya, Ryota Jin, Shogo Araki, Hideki Yoshioka, Hiromi Sato, Yasunori Sato, and Akihiro Hisaka. 2020. "Scores of Health-Related Quality of Life Questionnaire Worsen Consistently in Patients of COPD: Estimating Disease Progression over 30 Years by SReFT with Individual Data Collected in SUMMIT Trial" Journal of Clinical Medicine 9, no. 8: 2676. https://doi.org/10.3390/jcm9082676
APA StyleKawamatsu, S., Jin, R., Araki, S., Yoshioka, H., Sato, H., Sato, Y., & Hisaka, A. (2020). Scores of Health-Related Quality of Life Questionnaire Worsen Consistently in Patients of COPD: Estimating Disease Progression over 30 Years by SReFT with Individual Data Collected in SUMMIT Trial. Journal of Clinical Medicine, 9(8), 2676. https://doi.org/10.3390/jcm9082676