Setting up a Virtual Calprotectin Clinic in Inflammatory Bowel Diseases: Literature Review and Nancy Experience
Abstract
:1. Introduction
1.1. Efficacy of Patient Care Based on the e-Health
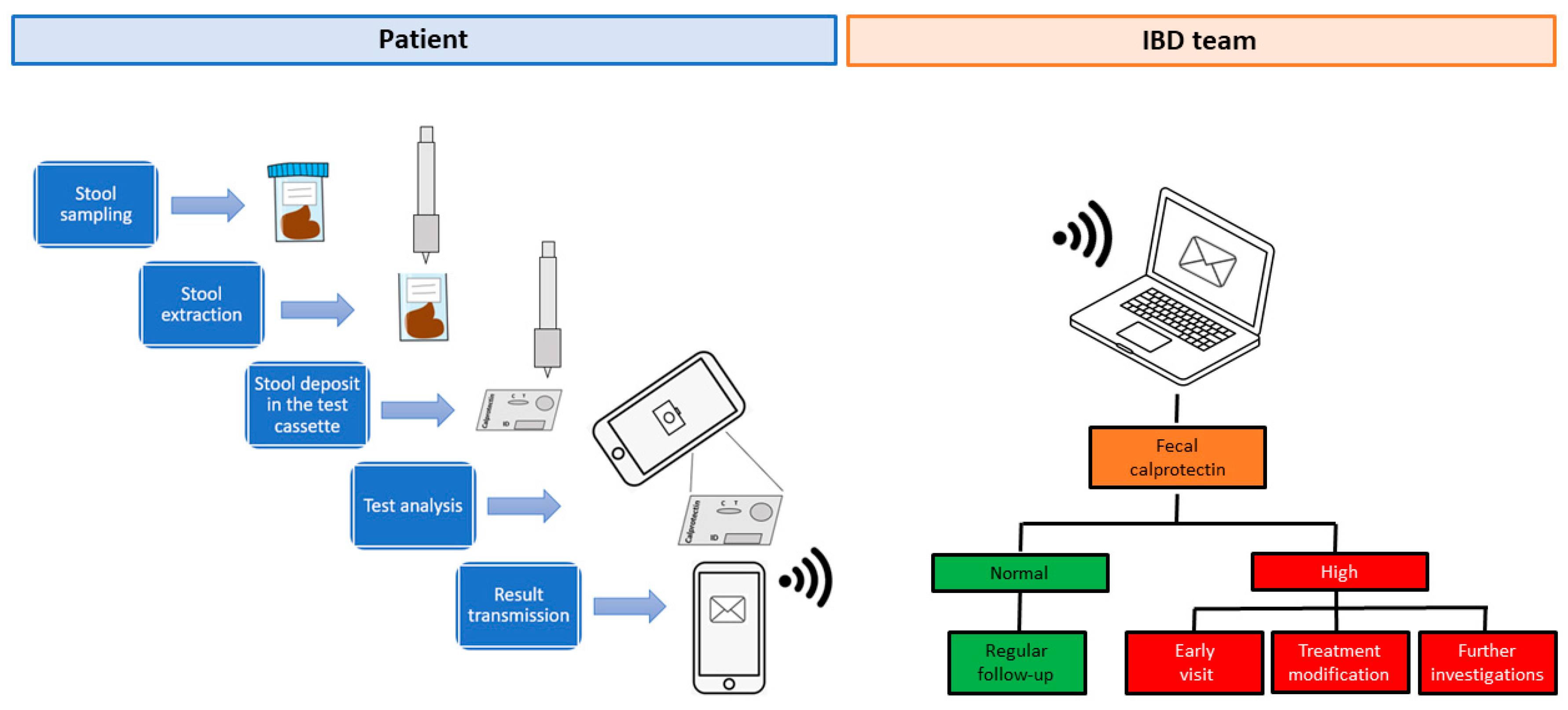
1.2. A System for Monitoring Fecal Calprotectin at Home
1.3. Fecal Calprotectin Home Testing
1.4. The Nancy Experience
2. Methods
Statistical Analysis
3. Results
3.1. Information about Fecal Calprotectin and IBDoc® Test
3.2. Information about IBDoc® Usability
3.3. Information about Patients’ Satisfaction with IBDoc®
3.4. Fecal Calprotectin Values and Profile of Patients Satisfied with IBDoc®
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Torres, J.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Acosta, M.B.-D.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohns Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Pouillon, L.; Ferrante, M.; Van Assche, G.; Rutgeerts, P.; Noman, M.; Sabino, J.; Casteele, N.V.; Gils, A.; Vermeire, S. Mucosal Healing and Long-term Outcomes of Patients With Inflammatory Bowel Diseases Receiving Clinic-Based vs Trough Concentration-Based Dosing of Infliximab. Clin. Gastroenterol. Hepatol. 2018, 16, 1276–1283.e1. [Google Scholar] [CrossRef]
- Colombel, J.-F.; Panaccione, R.; Bossuyt, P.; Lukas, M.; Baert, F.; Vaňásek, T.; Danalioglu, A.; Novacek, G.; Armuzzi, A.; Hébuterne, X.; et al. Effect of tight control management on Crohn’s disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet 2018, 390, 2779–2789. [Google Scholar] [CrossRef]
- Van Der Valk, M.E.; Mangen, M.-J.J.; Leenders, M.; Dijkstra, G.; Van Bodegraven, A.A.; Fidder, H.H.; De Jong, D.J.; Pierik, M.; Van Der Woude, C.J.; Romberg-Camps, M.J.L.; et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: Results from the COIN study. Gut 2012, 63, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Burisch, J.; Vardi, H.; Pedersen, N.; Brinar, M.; Cukovic-Cavka, S.; Kaimakliotis, I.; Duricova, D.; Bortlik, M.; Shonová, O.; Vind, I.; et al. Costs and Resource Utilization for Diagnosis and Treatment During the Initial Year in a European Inflammatory Bowel Disease Inception Cohort. Inflamm. Bowel Dis. 2015, 21, 121–131. [Google Scholar] [CrossRef]
- Cross, R.K.; Jambaulikar, G.; Langenberg, P.; Tracy, J.K.; Collins, J.F.; Katz, J.; Regueiro, M.; Schwartz, D.A.; Quinn, C.C. TELEmedicine for Patients with Inflammatory Bowel Disease (TELE-IBD): Design and implementation of randomized clinical trial. Contemp. Clin. Trials 2015, 42, 132–144. [Google Scholar] [CrossRef]
- Zand, A.; Kim, B.J.; Van Deen, W.K.; Stokes, Z.; Platt, A.; O’Hara, S.; Khong, H.; Hommes, D.W. The effects of inflammatory bowel disease on caregivers: Significant burden and loss of productivity. BMC Health Serv. Res. 2020, 20, 556. [Google Scholar] [CrossRef]
- Bossuyt, P.; Pouillon, L.; Bonnaud, G.; Danese, S.; Peyrin-Biroulet, L. E-health in inflammatory bowel diseases: More challenges than opportunities? Dig. Liver Dis. 2017, 49, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.K.; Langenberg, P.; Regueiro, M.; Schwartz, D.A.; Tracy, J.K.; Collins, J.F.; Katz, J.; Ghazi, L.; Patil, S.A.; Quezada, S.M.; et al. A Randomized Controlled Trial of TELEmedicine for Patients with Inflammatory Bowel Disease (TELE-IBD). Am. J. Gastroenterol. 2019, 114, 472–482. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Nancey, S.; Danese, S.; Peyrin-Biroulet, L. A Practical Guide for Faecal Calprotectin Measurement: Myths and Realities. J. Crohns Colitis 2020. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, C.; Aimone-Gastin, I.; Baumann, C.; Dirrenberger, B.; Guéant, J.-L.; Peyrin-Biroulet, L. Compliance with the faecal calprotectin test in patients with inflammatory bowel disease. United Eur. Gastroenterol. J. 2017, 5, 702–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puolanne, A.; Kolho, K.-L.; Alfthan, H.; Farkkila, M. Is home monitoring of inflammatory bowel disease feasible? A randomized controlled study. Scand. J. Gastroenterol. 2019, 54, 849–854. [Google Scholar] [CrossRef]
- George, L.A.; Dominic, M.R.; Cross, R.K. Integration of telemedicine into clinical practice for inflammatory bowel disease. Curr. Opin. Gastroenterol. 2020, 36, 304–309. [Google Scholar] [CrossRef]
- Elkjaer, M.; Shuhaibar, M.; Burisch, J.; Bailey, Y.; Scherfig, H.; Laugesen, B.; Langholz, E.; O’Morain, C.; Lynge, E.; Munkholm, P.; et al. E-health empowers patients with ulcerative colitis: A randomised controlled trial of the web-guided ‘Constant-care’ approach. Gut 2010, 59, 1652–1661. [Google Scholar] [CrossRef]
- Del Hoyo, J.; Nos, P.; Faubel, R.; Muñoz, D.; Domínguez, D.; Bastida, G.; Valdivieso, B.; Correcher, M.; Aguas, M.; Salcedo, V.T.; et al. A Web-Based Telemanagement System for Improving Disease Activity and Quality of Life in Patients With Complex Inflammatory Bowel Disease: Pilot Randomized Controlled Trial. J. Med Internet Res. 2018, 20, e11602. [Google Scholar] [CrossRef]
- De Jong, M.J.; van der Meulen-de Jong, A.E.; Romberg-Camps, M.J.; Becx, M.C.; Maljaars, J.P.; Cilissen, M.; van Bodegraven, A.A.; Mahmmod, N.; Markus, T.; Hameeteman, W.M.; et al. Telemedicine for management of inflammatory bowel disease (myIBDcoach): A pragmatic, multicentre, randomised controlled trial. Lancet 2017, 390, 959–968. [Google Scholar] [CrossRef]
- De Jong, M.J.; Boonen, A.; van der Meulen-de Jong, A.E.; Romberg-Camps, M.J.; van Bodegraven, A.A.; Mahmmod, N.; Markus, T.; Dijkstra, G.; Winkens, B.; van Tubergen, A.; et al. Cost-effectiveness of Telemedicine-directed Specialized vs Standard Care for Patients With Inflammatory Bowel Diseases in a Randomized Trial. Clin. Gastroenterol. Hepatol. 2020, 18, 1744–1752. [Google Scholar] [CrossRef]
- IBDoc® Calprotectin Home Test Kit Procedure; Calprotectin. Available online: https://www.calprotectin.co.uk/calprotectin-products/calprotectin-products-patient-self-testing/ibdoc-calprotectin-home-test-kit-procedure/ (accessed on 28 April 2020).
- Step-by-Step Instructions for the QuantOn Cal® App|QuantOn Cal®. Available online: https://quantoncal.com/en/app (accessed on 28 April 2020).
- Calprosmart—Home Testkit. Available online: https://calpro.no/products/calprosmart-home-testkit (accessed on 28 April 2020).
- Calprotectin Home Test for Patient Self Testing of Their IBD Status; Calprotectin. Available online: https://www.calprotectin.co.uk/calprotectin-products/calprotectin-products-patient-self-testing/ (accessed on 28 April 2020).
- QuantOn Cal. Available online: https://www.biohithealthcare.co.uk/Diagnostics/Item/QuantOn-Cal (accessed on 28 April 2020).
- Haisma, S.-M.; Galaurchi, A.; Almahwzi, S.; Adekanmi Balogun, J.A.; Muller Kobold, A.C.; van Rheenen, P.F. Head-to-head comparison of three stool calprotectin tests for home use. PLoS ONE 2019, 14, e0214751. [Google Scholar] [CrossRef] [PubMed]
- McCombie, A.; Walmsley, R.; Barclay, M.; Ho, C.; Langlotz, T.; Regenbrecht, H.; Gray, A.; Visesio, N.; Inns, S.; Schultz, M. A Noninferiority Randomized Clinical Trial of the Use of the Smartphone-Based Health Applications IBDsmart and IBDoc in the Care of Inflammatory Bowel Disease Patients. Inflamm. Bowel Dis. 2019, 26, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Bello, C.; Roseth, A.; Guardiola, J.; Reenaers, C.; Ruiz-Cerulla, A.; Van Kemseke, C.; Arajol, C.; Reinhard, C.; Seidel, L.; Louis, E. Usability of a home-based test for the measurement of fecal calprotectin in asymptomatic IBD patients. Dig. Liver Dis. 2017, 49, 991–996. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Tung, C.-C.; Weng, M.-T.; Wong, J.-M. Experience of patients with inflammatory bowel disease in using a home fecal calprotectin test as an objective reported outcome for self-monitoring. Intest. Res. 2018, 16, 546–553. [Google Scholar] [CrossRef] [Green Version]
- Hejl, J.; Theede, K.; Møllgren, B.; Madsen, K.V.; Heidari, A.; á Steig, A.; Fenger, M. Point of care testing of fecal calprotectin as a substitute for routine laboratory analysis. Pract. Lab. Med. 2017, 10, 10–14. [Google Scholar] [CrossRef]
- Vinding, K.K.; Elsberg, H.; Thorkilgaard, T.; Bélard, E.; Pedersen, N.; Elkjaer, M.; Marker, D.; Carlsen, K.; Burisch, J.; Munkholm, P. Fecal Calprotectin Measured By Patients at Home Using Smartphones—A New Clinical Tool in Monitoring Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Ankersen, D.V.; Weimers, P.; Marker, D.; Bennedsen, M.; Saboori, S.; Paridaens, K.; Burisch, J.; Munkholm, P. Individualized home-monitoring of disease activity in adult patients with inflammatory bowel disease can be recommended in clinical practice: A randomized-clinical trial. World J. Gastroenterol. 2019, 25, 6158–6171. [Google Scholar] [CrossRef]
- Weber, J.; Ueberschlag, M.E.; Prica, M.; Kräuchi, S.; Reinhard, C.; Jermann, T. P273. Validation of a smartphone-based patient monitoring system measuring calprotectin as the therapy follow-up marker. J. Crohns Colitis 2015, 1, S212–S213. [Google Scholar]
- About IBDoc®. Available online: https://www.ibdoc.net/about-ibdoc/ (accessed on 23 July 2020).
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Nunes, P.B.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohns Colitis 2018, 13, 144–164K. [Google Scholar] [CrossRef] [Green Version]
- Buisson, A.; Gonzalez, F.; Poullenot, F.; Sollellis, E.; Flamant, M.; Bonnaud, G.; Thevenin, A.; Duruy, M.; Filippi, J.; L’Hopital, F.; et al. Comparative Acceptability and Perceived Clinical Utility of Monitoring Tools. Inflamm. Bowel Dis. 2017, 23, 1425–1433. [Google Scholar] [CrossRef] [Green Version]
- Heida, A.; Knol, M.; Kobold, A.C.M.; Bootsman, J.; Dijkstra, G.; Van Rheenen, P.F. Agreement Between Home-Based Measurement of Stool Calprotectin and ELISA Results for Monitoring Inflammatory Bowel Disease Activity. Clin. Gastroenterol. Hepatol. 2017, 15, 1742–1749.e2. [Google Scholar] [CrossRef] [Green Version]
- Coronavirus Disease (COVID-19) Situation Reports. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 29 June 2020).
- Lees, C.W.; Regueiro, M.; Mahadevan, U. International Organization for the Study of Inflammatory Bowel Disease Innovation in IBD Care During the COVID-19 Pandemic: Results of a Global Telemedicine Survey by the International Organization for the Study of Inflammatory Bowel Disease. Gastroenterology 2020. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Sands, B.E.; Ng, S.C.; PeyrinBiroulet, L. The day after COVID-19 in IBD: How to go back to ‘normal’. Nat. Rev. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Bao, J.; Setiawan, I.M.A.; Saptono, A.; Parmanto, B.; Fairman, A.; Fazzino, T.; Kagen, S.; Apolinário-Hagen, J. The mHealth App Usability Questionnaire (MAUQ): Development and Validation Study. JMIR mHealth uHealth 2019, 7, e11500. [Google Scholar] [CrossRef] [PubMed]
| Patients’ Characteristics | n (%) |
|---|---|
| Patients | 20 |
| Female | 12 (60%) |
| Age | |
| <25 years | 4 (20%) |
| 25–44 years | 16 (80%) |
| Marital status: | |
| Married | 5 (25%) |
| Unmarried | 15 (75%) |
| Residence | |
| Urban area | 9 (45%) |
| Rural area | 11 (55%) |
| Educational level | |
| <Bachelor’s degree | 1 (5%) |
| Bachelor’s degree | 5 (25%) |
| >Bachelor’s degree | 14 (70%) |
| Disease | |
| Ulcerative colitis | 7 (35%) |
| Crohn’s disease | 13 (65%) |
| Disease duration | |
| <1 year | 2 (10%) |
| 1–5 years | 7 (35%) |
| 6–10 years | 5 (25%) |
| >11 years | 6 (30%) |
| CD location | |
| Ileitis | 3 (23%) |
| Colitis | 1 (8%) |
| Ileocolitis | 9 (69%) |
| UC location | |
| Extensive colitis | 5 (71%) |
| Left-side colitis | 2 (29%) |
| Proctitis | 0 |
| Smoking status | |
| Active smoker | 5 (25%) |
| Former smoker | 1 (5%) |
| Non-smoker | 14 (70%) |
| Perianal disease | 3 (15%) |
| Upper disease | 1 (5%) |
| Surgery | 6 (30%) |
| Clinical disease activity | |
| Harvey-Bradshaw Index, mean ± standard deviation | 2.15 ± 1.72 |
| Partial Mayo score, mean ± standard deviation | 2.42 ± 2.63 |
| Medications | |
| Local steroid | 6 (30%) |
| Systemic steroid | 12 (60%) |
| 5-ASA | 8 (40%) |
| Thiopurine | 9 (45%) |
| Methotrexate | 4 (20%) |
| Infliximab | 9 (45%) |
| Adalimumab | 11 (55%) |
| Vedolizumab | 4 (20%) |
| Ustekinumab | 4 (20%) |
| Current quality of life | |
| 7 Completely satisfactory | 4 (20%) |
| 6 Satisfactory | 3 (15%) |
| 5 Quite satisfactory | 3 (15%) |
| 4 Indifferent | 3 (15%) |
| 3 Quite unsatisfactory | 4 (20%) |
| 2 Not satisfactory | 3 (15%) |
| 1 Not at all satisfactory | 0 |
| Membership in an association for IBD patients | |
| Yes | 5 (25%) |
| No | 15 (75%) |
| Frequency of smartphone use | |
| 7 Completely regular | 11 (55%) |
| 6 Regular | 3 (15%) |
| 5 Quite regular | 6 (30%) |
| 4 Indifferent | 0 |
| 3 Quite not regular | 0 |
| 2 Not regular | 0 |
| 1 Not at all regular | 0 |
| Questionnaire | n (%) |
|---|---|
| Information about Fecal Calprotectin and IBDoc® | |
| (1) Is stool sampling a problem for you? | |
| 7 Definitely simple | 12 (60%) |
| 6 Simple | 5 (25%) |
| 5 Quite simple | 0 |
| 4 Indifferent | 1 (5%) |
| 3 Quite difficult | 2 (10%) |
| 2 Difficult | 0 |
| 1 Definitely difficult | 0 |
| (2) Have you heard of the fecal calprotectin dosage? | |
| Yes | 10 (50%) |
| No | 10 (50%) |
| (3) Did you already have a prescription for fecal calprotectin dosage with traditional method? | |
| Yes | 8/10 (80%) |
| No | 2/10 (20%) |
| (4) Who told you about the IBDoc® test? | |
| Gastroenterologist | 20 (100%) |
| General practitioner | 0 |
| Pharmacist | 0 |
| Participation in information days | 0 |
| Newspapers/magazines | 0 |
| Internet | 0 |
| (5) What is the IBDoc® test for (multiple answers are allowed)? | |
| To avoid a colonoscopy | 9 (45%) |
| To detect early disease relapse | 9 (45%) |
| To improve therapy adaption | 6 (30%) |
| To manage fecal calprotectin test independently | 3 (15%) |
| To monitor disease activity | 1 (5%) |
| Information about IBDoc® usability | |
| (6) Is it the first time you use IBDoc®? | |
| Yes | 20 (100%) |
| No | 0 |
| (7) Do you think you have received adequate information for the use of IBDoc®? | |
| 7 Definitely adequate | 18 (90%) |
| 6 Adequate | 2 (10%) |
| 5 Quite adequate | 0 |
| 4 Indifferent | 0 |
| 3 Quite inadequate | 0 |
| 2 Inadequate | 0 |
| 1 Definitely inadequate | 0 |
| (8) How do you rate the application installation and connection? | |
| 7 Definitely easy | 8 (40%) |
| 6 Easy | 9 (45%) |
| 5 Quite easy | 2 (10%) |
| 4 Indifferent | 0 |
| 3 Quite difficult | 1 (5%) |
| 2 Difficult | 0 |
| 1 Definitely difficult | 0 |
| (9) How do you rate test preparation? | |
| 7 Definitely easy | 5 (25%) |
| 6 Easy | 10 (50%) |
| 5 Quite easy | 0 |
| 4 Indifferent | 3 (15%) |
| 3 Quite difficult | 1 (5%) |
| 2 Difficult | 0 |
| 1 Definitely difficult | 1 (5%) |
| (10) How do you rate stool sampling? | |
| 7 Definitely easy | 6 (30%) |
| 6 Easy | 7 (35%) |
| 5 Quite easy | 2 (10%) |
| 4 Indifferent | 0 |
| 3 Quite difficult | 2 (10%) |
| 2 Difficult | 3 (15%) |
| 1 Definitely difficult | 0 |
| (11) How do you rate stool preparation? | |
| 7 Definitely easy | 8 (40%) |
| 6 Easy | 8 (40%) |
| 5 Quite easy | 0 |
| 4 Indifferent | 0 |
| 3 Quite difficult | 3 (15%) |
| 2 Difficult | 1 (5%) |
| 1 Definitely difficult | 0 |
| (12) How do you rate the stool deposit in the test cassette? | |
| 7 Definitely easy | 11 (55%) |
| 6 Easy | 7 (35%) |
| 5 Quite easy | 1 (5%) |
| 4 Indifferent | 0 |
| 3 Quite difficult | 1 (5%) |
| 2 Difficult | 0 |
| 1 Definitely difficult | 0 |
| (13) How do you rate reading of the test cassette? | |
| 7 Definitely easy | 10 (50%) |
| 6 Easy | 7 (35%) |
| 5 Quite easy | 2 (10%) |
| 4 Indifferent | 1 (5%) |
| 3 Quite difficult | 0 |
| 2 Difficult | 0 |
| 1 Definitely difficult | 0 |
| (14) How do you rate recording of test results? | |
| 7 Definitely easy | 16 (80%) |
| 6 Easy | 4 (20%) |
| 5 Quite easy | 0 |
| 4 Indifferent | 0 |
| 3 Quite difficult | 0 |
| 2 Difficult | 0 |
| 1 Definitely difficult | 0 |
| (15) How do you rate time analysis of stools? | |
| 7 Definitely easy | 13 (65%) |
| 6 Easy | 6 (30%) |
| 5 Quite easy | 0 |
| 4 Indifferent | 1 (5%) |
| 3 Quite difficult | 0 |
| 2 Difficult | 0 |
| 1 Definitely inappropriate | 0 |
| (16) How do you rate results’ transmission? | |
| 7 Definitely easy | 16 (80%) |
| 6 Easy | 4 (20%) |
| 5 Quite easy | 0 |
| 4 Indifferent | 0 |
| 3 Quite difficult | 0 |
| 2 Difficult | 0 |
| 1 Definitely difficult | 0 |
| (17) Do the IBDoc® results match the clinical status? | |
| 7 Definitely easy | 11 (55%) |
| 6 Easy | 1 (5%) |
| 5 Quite easy | 2 (10%) |
| 4 Indifferent | 1 (5%) |
| 3 Quite difficult | 4 (20%) |
| 2 Difficult | 1 (5%) |
| 1 Definitely unpaired | 0 |
| Information about patients’ satisfaction with IBDoc® | |
| (18) How satisfied are you with the use of IBDoc®? | |
| 7 Definitely easy | 11 (55%) |
| 6 Easy | 6 (30%) |
| 5 Quite easy | 2 (10%) |
| 4 Indifferent | 1 (5%) |
| 3 Quite difficult | 0 |
| 2 Difficult | 0 |
| 1 Completely unsatisfied | 0 |
| (19) Overall, is the IBDoc® test easy to use? | |
| 7 Definitely easy | 7 (35%) |
| 6 Easy | 8 (40%) |
| 5 Quite easy | 4 (20%) |
| 4 Indifferent | 1 (5%) |
| 3 Quite difficult | 0 |
| 2 Difficult | 0 |
| 1 Definitely difficult | 0 |
| (20) Would you like to use the IBDoc® test regularly? | |
| Yes | 19 (95%) |
| No | 1 (5%) |
| (21) Would you recommend the use of IBDoc® test to other patients? | |
| Yes | 20 (100%) |
| No | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amico, F.; Netter, P.; Baumann, C.; Veltin, M.; Zallot, C.; Aimone-Gastin, I.; Danese, S.; Peyrin-Biroulet, L. Setting up a Virtual Calprotectin Clinic in Inflammatory Bowel Diseases: Literature Review and Nancy Experience. J. Clin. Med. 2020, 9, 2697. https://doi.org/10.3390/jcm9092697
D’Amico F, Netter P, Baumann C, Veltin M, Zallot C, Aimone-Gastin I, Danese S, Peyrin-Biroulet L. Setting up a Virtual Calprotectin Clinic in Inflammatory Bowel Diseases: Literature Review and Nancy Experience. Journal of Clinical Medicine. 2020; 9(9):2697. https://doi.org/10.3390/jcm9092697
Chicago/Turabian StyleD’Amico, Ferdinando, Patrick Netter, Cedric Baumann, Muriel Veltin, Camille Zallot, Isabelle Aimone-Gastin, Silvio Danese, and Laurent Peyrin-Biroulet. 2020. "Setting up a Virtual Calprotectin Clinic in Inflammatory Bowel Diseases: Literature Review and Nancy Experience" Journal of Clinical Medicine 9, no. 9: 2697. https://doi.org/10.3390/jcm9092697
APA StyleD’Amico, F., Netter, P., Baumann, C., Veltin, M., Zallot, C., Aimone-Gastin, I., Danese, S., & Peyrin-Biroulet, L. (2020). Setting up a Virtual Calprotectin Clinic in Inflammatory Bowel Diseases: Literature Review and Nancy Experience. Journal of Clinical Medicine, 9(9), 2697. https://doi.org/10.3390/jcm9092697






