High Prevalence of Intestinal Pathogens in Indigenous in Colombia
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethical Approval
2.2. General Information
2.3. Nucleic Acid Extractions and Polymerase Chain Reactions (PCRs)
2.4. Demographic Data
2.5. Statistical Methods
3. Results
3.1. Demographic Results
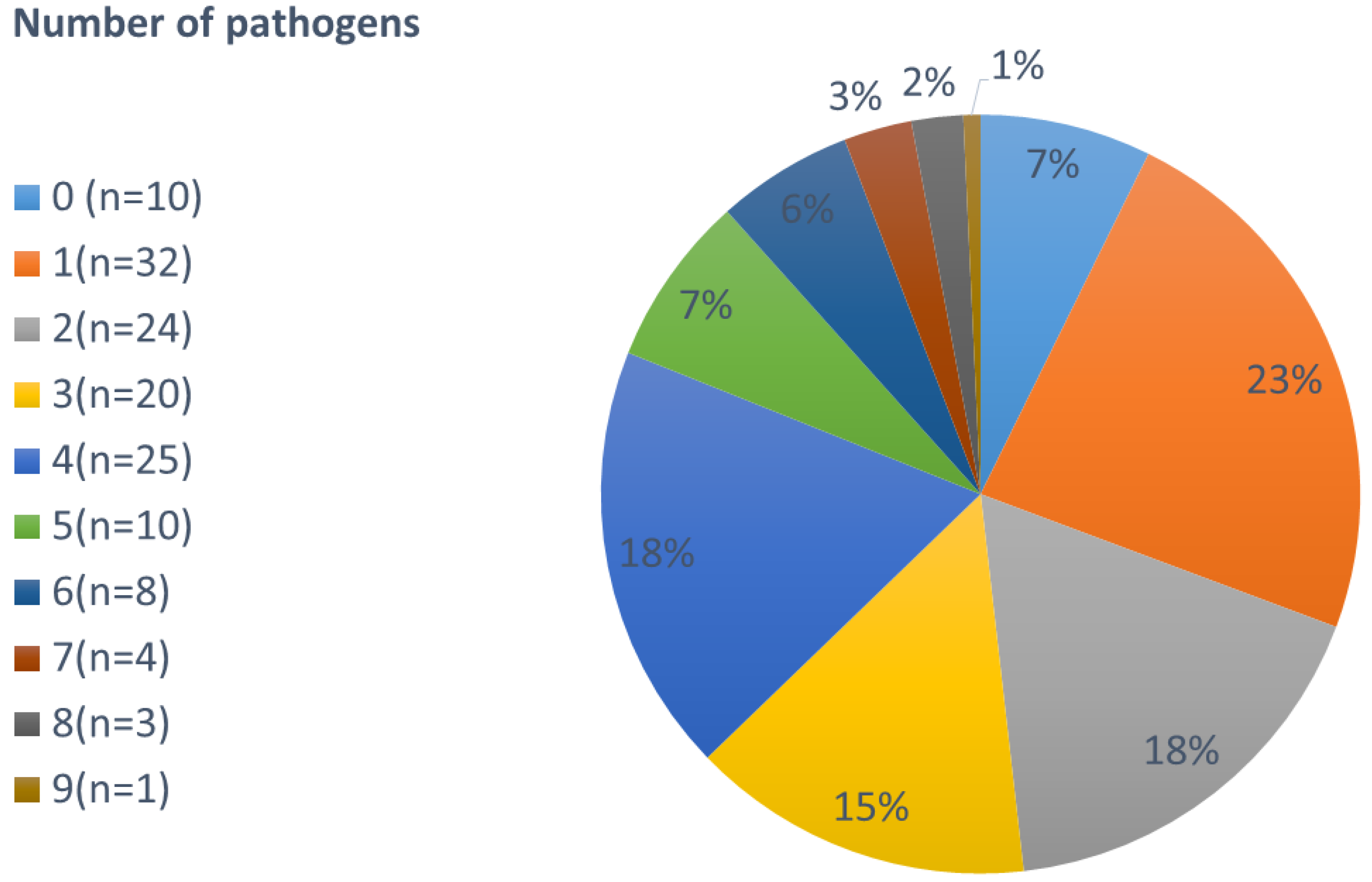
3.2. Co-Infections
3.3. PCR Based Results
3.3.1. Protozoa
3.3.2. Bacteria
3.3.3. Helminths
3.4. Microscopy Based Results
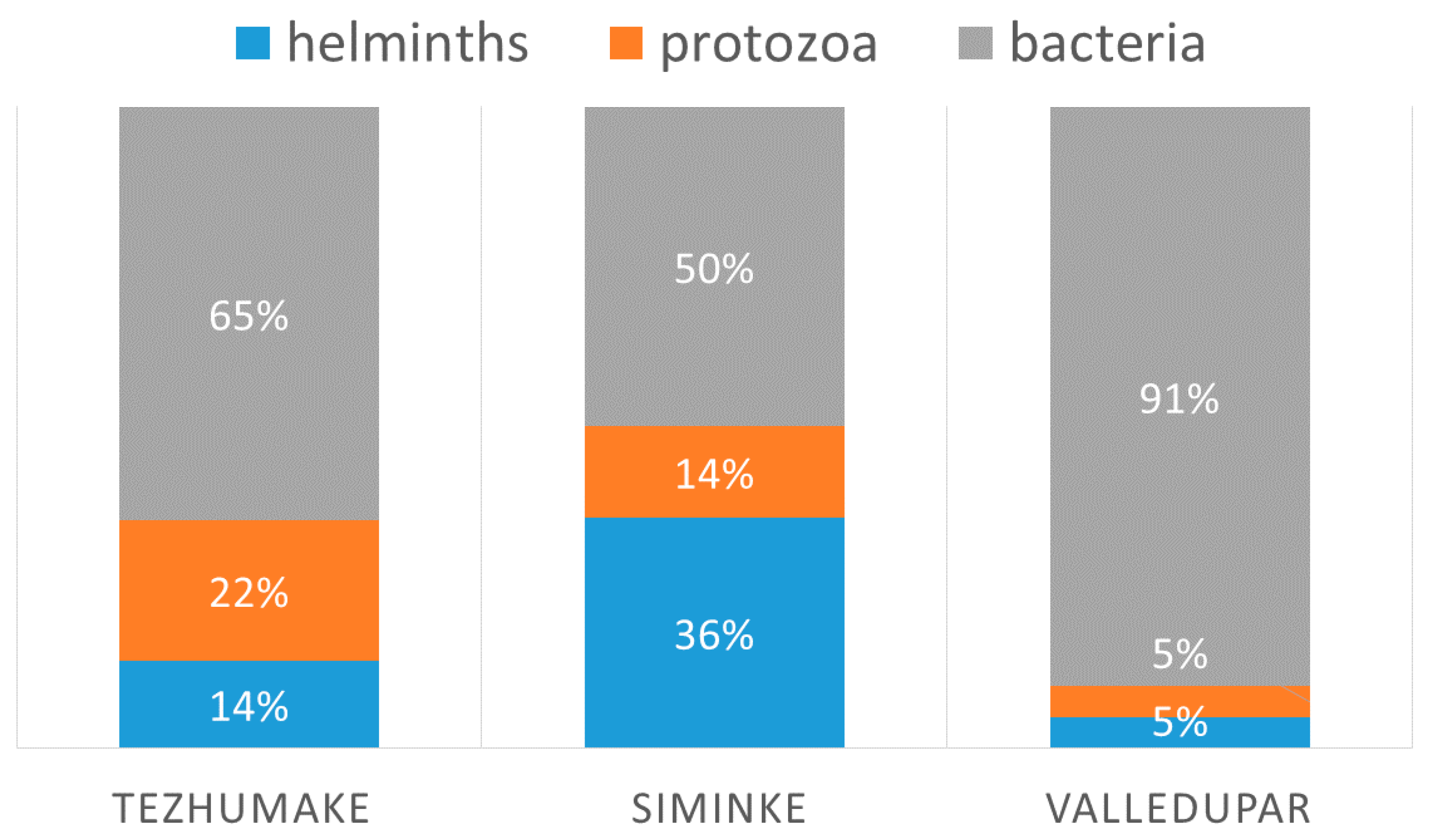
3.5. Villages
3.6. Demographic Data
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hawash, Y.A.; Ismail, K.A.; Almehmadi, M. High Frequency of Enteric Protozoan, Viral, and Bacterial Potential Pathogens in Community-Acquired Acute Diarrheal Episodes: Evidence Based on Results of Luminex Gastrointestinal Pathogen Panel Assay. Korean J. Parasitol. 2017, 55, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Soil-Transmitted Helminth Infections. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 19 June 2020).
- Köller, T.; Hahn, A.; Altangerel, E.; Verweij, J.J.; Landt, O.; Kann, S.; Dekker, D.; May, J.; Loderstädt, U.; Podbielski, A.; et al. Comparison of commercial and in-house real-time PCR platforms for 15 parasites and microsporidia in human stool samples without a gold standard. Acta Trop. 2020, 207, 105516. [Google Scholar] [CrossRef] [PubMed]
- Wiemer, D.; Loderstaedt, U.; von Wulffen, H.; Priesnitz, S.; Fischer, M.; Tannich, E.; Hagen, R.M. Real-time multiplex PCR for simultaneous detection of Campylobacter jejuni, Salmonella, Shigella and Yersinia species in fecal samples. Int. J. Med. Microbiol. 2011, 301, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.; Luetgehetmann, M.; Landt, O.; Schwarz, N.G.; Frickmann, H. Comparison of one commercial and two in-house TaqMan multiplex real-time PCR assays for detection of enteropathogenic, enterotoxigenic and enteroaggregative Escherichia coli. Trop. Med. Int. Health 2017, 22, 1371–1376. [Google Scholar] [CrossRef] [Green Version]
- Fenollar, F.; Laouira, S.; Lepidi, H.; Rolain, J.M.; Raoult, D. Value of Tropheryma whipplei quantitative polymerase chain reaction assay for the diagnosis of Whipple disease: Usefulness of saliva and stool specimens for first-line screening. Clin. Infect. Dis. 2008, 47, 659–667. [Google Scholar] [CrossRef] [Green Version]
- Frickmann, H.; Hanke, M.; Hahn, A.; Schwarz, N.G.; Landt, O.; Moter, A.; Kikhney, J.; Hinz, R.; Rojak, S.; Dekker, D.; et al. Detection of Tropheryma whipplei in stool samples by one commercial and two in-house real-time PCR assays. Trop. Med. Int. Health 2019, 24, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Niesters, H.G. Quantitation of viral load using real-time amplification techniques. Methods 2001, 25, 419–429. [Google Scholar] [CrossRef]
- Frickmann, H.; Tenner-Racz, K.; Eggert, P.; Schwarz, N.G.; Poppert, S.; Tannich, E.; Hagen, R.M. Influence of parasite density and sample storage time on the reliability of Entamoeba histolytica-specific PCR from formalin-fixed and paraffin-embedded tissues. Diagn. Mol. Pathol. 2013, 22, 236–244. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Sabry, M.A.; Taher, E.S.; Meabed, E.M.H. Prevalence and genotyping of zoonotic Giardia from Fayoum Governate Egypt. Res. J. Parasitol. 2009, 4, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.C.; Hopkins, R.M.; Homan, W.L. Nomenclature and genetic groupings of Giardia infecting mammals. Parasitol. Today 2000, 16, 210–213. [Google Scholar] [CrossRef]
- Foronda, P.; Bargues, M.D.; Abreu-Acosta, N.; Periago, M.V.; Valero, M.A.; Valladares, B.; Mas-Coma, S. Identification of genotypes of Giardia intestinalis of human isolates in Egypt. Parasitol. Res. 2008, 103, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, S.M.; el-Bahy, M.M.; Abd Elaziz, J.; El-Dardiry, M.A. Detection of protozoal parasites in the stools of diarrhoeic patients using different techniques. J. Egypt Soc. Parasitol. 2006, 36, 487–516. [Google Scholar] [PubMed]
- Jensen, L.A.; Marlin, J.W.; Dyck, D.D.; Laubach, H.E. Prevalence of multi-gastrointestinal infections with helminth, protozoan and Campylobacter spp. in Guatemalan children. J. Infect. Dev. Ctries. 2009, 3, 229–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, A.S.; Bendik, J.M.; Alliance, J.Y.; Roberts, J.M.; da Silva, A.J.; Moura, I.N.; Arrowood, M.J.; Eberhard, M.L.; Herwaldt, B.L. Epidemiology of Cyclospora cayetanensis and other intestinal parasites in a community in Haiti. J. Clin. Microbiol. 2003, 41, 2047–2054. [Google Scholar] [CrossRef] [Green Version]
- Davies, A.P.; Campbell, B.; Evans, M.R.; Bone, A.; Roche, A.; Chalmers, R.M. Asymptomatic carriage of protozoan parasites in children in day care centers in the United kingdom. Pediatr. Infect. Dis. J. 2009, 28, 838–840. [Google Scholar] [CrossRef]
- Efunshile, M.A.; Ngwu, B.A.; Kurtzhals, J.A.; Sahar, S.; König, B.; Stensvold, C.R. Molecular Detection of the Carriage Rate of Four Intestinal Protozoa with Real-Time Polymerase Chain Reaction: Possible Overdiagnosis of Entamoeba histolytica in Nigeria. Am. J. Trop. Med. Hyg. 2015, 93, 257–262. [Google Scholar] [CrossRef] [Green Version]
- McElligott, J.T.; Naaktgeboren, C.; Makuma-Massa, H.; Summer, A.P.; Deal, J.L. Prevalence of intestinal protozoa in communities along the Lake Victoria region of Uganda. Int. J. Infect. Dis. 2013, 17, e658–e659. [Google Scholar] [CrossRef] [Green Version]
- Utzinger, J.; Botero-Kleiven, S.; Castelli, F.; Chiodini, P.L.; Edwards, H.; Köhler, N.; Gulletta, M.; Lebbad, M.; Manser, M.; Matthys, B.; et al. Microscopic diagnosis of sodium acetate-acetic acid-formalin-fixed stool samples for helminths and intestinal protozoa: A comparison among European reference laboratories. Clin. Microbiol. Infect. 2010, 16, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Nazeer, J.T.; El Sayed Khalifa, K.; von Thien, H.; El-Sibaei, M.M.; Abdel-Hamid, M.Y.; Tawfik, R.A.; Tannich, E. Use of multiplex real-time PCR for detection of common diarrhea causing protozoan parasites in Egypt. Parasitol. Res. 2013, 112, 595–601. [Google Scholar] [CrossRef]
- Krumkamp, R.; Sarpong, N.; Schwarz, N.G.; Adlkofer, J.; Loag, W.; Eibach, D.; Hagen, R.M.; Adu-Sarkodie, Y.; Tannich, E.; May, J. Gastrointestinal infections and diarrheal disease in Ghanaian infants and children: An outpatient case-control study. PLoS Negl. Trop. Dis. 2015, 9, e0003568. [Google Scholar] [CrossRef]
- Rivero, Z.; Bracho, A.; Calchi, M.; Díaz, I.; Acurero, E.; Maldonado, A.; Chourio, G.; Arráiz, N.; Corzo, G. Detection and differentiation of Entamoeba histolytica and Entamoeba dispar by polymerase chain reaction in a community in Zulia State, Venezuela. Cad. Saude Publica 2009, 25, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.; Morán, P.; González, E.; García, G.; Ramiro, M.; Gómez, A.; de León Mdel, C.; Melendro, E.I.; Valadez, A.; Ximénez, C. Entamoeba histolytica and Entamoeba dispar: Prevalence infection in a rural Mexican community. Exp. Parasitol. 2005, 110, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Botero-Garcés, J.; Montoya-Palacio, M.N.; Barguil, J.I.; Castaño-González, A. An outbreak of Cyclospora cayetanensis in Medellín, Colombia. Rev. Salud Publica (Bogota) 2006, 8, 258–268. [Google Scholar] [CrossRef]
- Karanja, R.M.; Gatei, W.; Wamae, N. Cyclosporiasis: An emerging public health concern around the world and in Africa. Afr. Health Sci. 2007, 7, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Frickmann, H.; Schwarz, N.G.; Rakotozandrindrainy, R.; May, J.; Hagen, R.M. PCR for enteric pathogens in high-prevalence settings. What does a positive signal tell us? Infect. Dis. (Lond. Engl.) 2015, 47, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; DuPont, H.L.; Ramsey, D.J. Global etiology of travelers’ diarrhea: Systematic review from 1973 to the present. Am. J. Trop. Med. Hyg. 2009, 80, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.A.; Elias, W.P.; Scaletsky, I.C.; Guth, B.E.; Rodrigues, J.F.; Piazza, R.M.; Ferreira, L.C.; Martinez, M.B. Diarrheagenic Escherichia coli. Braz. J. Microbiol. 2016, 47 (Suppl. 1), 3–30. [Google Scholar] [CrossRef] [Green Version]
- Crofts, A.A.; Giovanetti, S.M.; Rubin, E.J.; Poly, F.M.; Gutiérrez, R.L.; Talaat, K.R.; Porter, C.K.; Riddle, M.S.; DeNearing, B.; Brubaker, J.; et al. Enterotoxigenic E. coli virulence gene regulation in human infections. Proc. Natl. Acad. Sci. USA 2018, 115, E8968–E8976. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Torres, A.G. Enteropathogenic Escherichia coli: Foe or innocent bystander? Clin. Microbiol. Infect. 2015, 21, 729–734. [Google Scholar] [CrossRef] [Green Version]
- van den Beld, M.J.C.; Warmelink, E.; Friedrich, A.W.; Reubsaet, F.A.G.; Schipper, M.; de Boer, R.F.; Notermans, D.W.; Petrignani, M.W.F.; van Zanten, E.; Rossen, J.W.A.; et al. Incidence, clinical implications and impact on public health of infections with Shigella spp. and entero-invasive Escherichia coli (EIEC): Results of a multicenter cross-sectional study in the Netherlands during 2016–2017. BMC Infect. Dis. 2019, 19, 1037. [Google Scholar] [CrossRef] [Green Version]
- Young, K.T.; Davis, L.M.; Dirita, V.J. Campylobacter jejuni: Molecular biology and pathogenesis. Nat. Rev. Microbiol. 2007, 5, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Jaakkonen, A.; Kivistö, R.; Aarnio, M.; Kalekivi, J.; Hakkinen, M. Persistent contamination of raw milk by Campylobacter jejuni ST-883. PLoS ONE 2020, 15, e0231810. [Google Scholar] [CrossRef] [PubMed]
- Vinnemeier, C.D.; Klupp, E.M.; Krumkamp, R.; Rolling, T.; Fischer, N.; Owusu-Dabo, E.; Addo, M.M.; Adu-Sarkodie, Y.; Käsmaier, J.; Aepfelbacher, M.; et al. Tropheryma whipplei in children with diarrhoea in rural Ghana. Clin. Microbiol. Infect. 2016, 22, 65.e1–65.e3. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Bethony, J.; Bottazzi, M.E.; Brooker, S.; Buss, P. Hookworm: “The great infection of mankind”. PLoS Med. 2005, 2, e67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pullan, R.L.; Smith, J.L.; Jasrasaria, R.; Brooker, S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites Vectors 2014, 7, 37. [Google Scholar] [CrossRef]
- Moser, W.; Schindler, C.; Keiser, J. Efficacy of recommended drugs against soil transmitted helminths: Systematic review and network meta-analysis. BMJ 2017, 358, j4307. [Google Scholar] [CrossRef] [Green Version]
- Basuni, M.; Muhi, J.; Othman, N.; Verweij, J.J.; Ahmad, M.; Miswan, N.; Rahumatullah, A.; Aziz, F.A.; Zainudin, N.S.; Noordin, R. A pentaplex real-time polymerase chain reaction assay for detection of four species of soil-transmitted helminths. Am. J. Trop. Med. Hyg. 2011, 84, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Parasites—Soil-Transmitted Helminths. Available online: https://www.cdc.gov/parasites/sth/index.html (accessed on 9 June 2020).
- Schär, F.; Trostdorf, U.; Giardina, F.; Khieu, V.; Muth, S.; Marti, H.; Vounatsou, P.; Odermatt, P. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl. Trop. Dis. 2013, 7, e2288. [Google Scholar] [CrossRef] [Green Version]
- Hernández, P.C.; Morales, L.; Chaparro-Olaya, J.; Sarmiento, D.; Jaramillo, J.F.; Ordoñez, G.A.; Cortés, F.; Sánchez, L.K. Intestinal parasitic infections and associated factors in children of three rural schools in Colombia. A cross-sectional study. PLoS ONE 2019, 14, e0218681. [Google Scholar] [CrossRef] [Green Version]
- Buret, A.G.; Motta, J.P.; Allain, T.; Ferraz, J.; Wallace, J.L. Pathobiont release from dysbiotic gut microbiota biofilms in intestinal inflammatory diseases: A role for iron? J. Biomed. Sci. 2019, 26, 1. [Google Scholar] [CrossRef]
- Hemphill, A.; Müller, N.; Müller, J. Comparative Pathobiology of the Intestinal Protozoan Parasites Giardia lamblia, Entamoeba histolytica, and Cryptosporidium parvum. Pathogens(Basel Switzerland) 2019, 8, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dettmann, E.; Becker, C.; Schmeißer, C. Distance functions for matching in small samples. Comput. Stat. Data Anal. 2011, 55, 1942–1960. [Google Scholar] [CrossRef]
- Hsieh, F.Y. Sample size tables for logistic regression. Stat. Med. 1989, 8, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Y.R.; Sanchez, R. Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin. Microbiol. Rev. 2010, 23, 218–234. [Google Scholar] [CrossRef] [Green Version]
- Halliez, M.C.; Buret, A.G. Extra-intestinal and long term consequences of Giardia duodenalis infections. World J. Gastroenterol. 2013, 19, 8974–8985. [Google Scholar] [CrossRef]
- Verweij, J.J.; Blangé, R.A.; Templeton, K.; Schinkel, J.; Brienen, E.A.; van Rooyen, M.A.; van Lieshout, L.; Polderman, A.M. Simultaneous detection of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum in fecal samples by using multiplex real-time PCR. J. Clin. Microbiol. 2004, 42, 1220–1223. [Google Scholar] [CrossRef] [Green Version]
- Haque, R.; Roy, S.; Siddique, A.; Mondal, U.; Rahman, S.M.; Mondal, D.; Houpt, E.; Petri, W.A., Jr. Multiplex real-time PCR assay for detection of Entamoeba histolytica, Giardia intestinalis, and Cryptosporidium spp. Am. J. Trop. Med. Hyg. 2007, 76, 713–717. [Google Scholar] [CrossRef] [Green Version]
- Stark, D.; Al-Qassab, S.E.; Barratt, J.L.; Stanley, K.; Roberts, T.; Marriott, D.; Harkness, J.; Ellis, J.T. Evaluation of multiplex tandem real-time PCR for detection of Cryptosporidium spp.; Dientamoeba fragilis, Entamoeba histolytica, and Giardia intestinalis in clinical stool samples. J. Clin. Microbiol. 2011, 49, 257–262. [Google Scholar] [CrossRef] [Green Version]



| Predictor Variables | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome variables (%) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| BACTERIA | 1. C. jejuni (n = 42) | 1 | 0 | 24 | 31 | 17 | 25 | 45 | 42 ** | 56 | 29 | 41 | 60 | 43 | 47 * | 55 ** | 0 | 33 |
| 2. Salmonella spp. (n = 7) | 0 | 1 | 7 | 12 * | 9 | 9 | 0 | 71 | 11 | 12 | 3 | 0 | 10 | 0 | 7 | 0 | 0 | |
| 3. EAEC (n = 93) | 69 | 86 | 1 | 84 ** | 87 * | 90 *** | 84 | 64 | 89 | 53 | 79 | 80 | 71 | 63 | 59 | 67 | 67 | |
| 4. ETEC (n = 53) | 29 | 86 * | 46 ** | 1 | 52 | 56 *** | 48 | 44 | 44 | 47 | 38 | 40 | 33 | 34 | 41 | 67 | 67 | |
| 5. EHEC (n = 23) | 1 | 29 | 19 | 22 * | 1 | 57 | 10 * | 15 | 11 | 6 | 12 | 0 | 19 | 13 | 10 | 33 | 17 | |
| 6. EPEC (n = 57) | 2 | 71 | 80 | 55 *** | 23 | 1 | 42 | 33 | 44 | 24 | 47 | 7 | 80 | 41 | 31 | 33 | 67 | |
| 7. EIEC/Shig. (n = 25) | 1 | 14 | 52 | 23 | 16 | 17.4 * | 1 | 17 | 22 | 18 | 47 *** | 80 ** | 52 | 31 * | 24 | 67 | 67 | |
| PROTOZOA | 8. Giardia (n = 66) | 67 ** | 8 | 38 * | 45 | 44 | 39 * | 42 | 1 | 44 | 53 | 41 * | 40 | 38 * | 44 | 76 *** | 33 | 50 |
| 9. E. histo (n = 9) | 12 | 14 | 5 | 9 | 5 | 7 | 7 | 6 | 1 | 0 | 9 | 20 | 5 | 6 | 21 *** | 0 | 0 | |
| 10. Cyclospora spp. (n = 17) | 12 | 29 | 29 * | 10 | 4 | 7 | 19 | 14 | 0 | 1 | 12 | 0 | 29 * | 22 | 17 | 0 | 0 | |
| HELMINTHS | 11. Necator/hookworm (n = 37) | 33 | 14 | 67 *** | 29 | 17 | 28 | 64 *** | 21 * | 33 | 24 | 1 | 80 * | 67 *** | 41 *** | 17 | 33 | 50 * |
| 12. S. stercoralis (n = 5) | 7 | 0 | 20 | 4 | 0 | 7 | 13 ** | 3 | 11 | 0 | 12 * | 1 | 20 | 6 | 3 | 0 | 17 | |
| 13. Ascaris (n = 21) | 21 | 29 | 1 | 16 | 9 | 19 | 52 | 12 | 11 | 35 ** | 41 *** | 4.8 | 1 | 41 *** | 14 | 33 | 10 | |
| 14. Trichuris (n = 32) | 36 ** | 0 | 62 *** | 22 | 17 | 23 | 40 * | 21.2 | 22 | 41 | 52 *** | 40 * | 62 *** | 1 | 38 * | 67 | 33 | |
| 15. H. nana (n = 29) | 38 ** | 29 | 19 | 18 | 13 | 16 | 28 | 33 ** | 67 *** | 29 | 20 | 20 | 19 | 34 * | 1 | 33 | 50 | |
| 16. Enterobius (n = 3) | 0 | 0 | 5 | 2 | 4 | 2 | 8 * | 2 | 0 | 6 | 4 | 0 | 5 | 6 | 3 | 1 | 17 * | |
| 17. Taenia (n = 6) | 5 | 0 | 10 | 4 | 4 | 7 | 16 ** | 5 | 0 | 0 | 12 * | 20 | 10 | 6 | 10 | 33 * | 1 | |
| Organism | Village | Age Group in Years | Overall (n = 137) | ||||
|---|---|---|---|---|---|---|---|
| Semink (n = 43) | Tezhumake (n = 81) | Valledupar (n = 13) | <6 (n = 21) | 6–18 (n = 42) | >18 (n = 72) | ||
| Bacteria | 49.8% | 64.5% | 90.5% | 64.3% | 64.4% | 75.5% | 60.2% |
| C. jejuni | 16.5% | 11.1% | 50% | 12.5% | 13.6% | 6.4% | 17.6% |
| Salmonella spp. | 1.7% | 2.9% | 0% | 1.4% | 1.5% | 1.7% | 2.2% |
| Shigella spp./EIEC | 12.2% | 6.4% | 0% | 9.7% | 5.3% | 4.7% | 7.7% |
| EHEC | 4.3% | 8.2% | 10.5% | 5.6% | 4.5% | 5.6% | 7.1% |
| ETEC | 16.5% | 18.1% | 2.6% | 9.7% | 13.6% | 11.1% | 15.7% |
| EAEC | 27.8% | 31.6% | 18.4% | 19.4% | 18.9% | 23.1% | 28.7% |
| EPEC | 19.1% | 17.5% | 13.2% | 9.7% | 9.8% | 15.8% | 17.6% |
| T. whipplei | 0.9% | 2.9% | 5.3% | 29.2% | 31.8% | 31.6% | 2.5% |
| Protozoa | 14.3% | 21.9% | 4.8% | 20.5% | 17.1% | 11.3% | 17.3% |
| G. intestinalis | 60.6% | 75.9% | 100% | 73.9% | 82.9% | 57.1% | 71% |
| E. histolytica | 6.1% | 12.1% | 0% | 4.3% | 2.9% | 20% | 9.7% |
| C. cayetanenis | 30.3% | 12.1% | 0% | 21.7% | 14.3% | 20% | 18.3% |
| Cryptosporidium spp. | 3% | 0% | 0% | 0% | 0% | 2.9% | 1.1% |
| Helminths | 35.9% | 13.6% | 4.8% | 15.2% | 18.5% | 13.2% | 22.5% |
| N. americanus | 21.7% | 19.4% | 0% | 23.5% | 15.8% | 36.6% | 20.7% |
| A. lumbricoides | 24.1% | 2.8% | 0% | 23.5% | 18.4% | 24.4% | 17.4% |
| S. stercoralis | 4.8% | 0% | 50% | 5.9% | 5.3% | 4.9% | 4.1% |
| T. trichiura | 28.9% | 19.4% | 50% | 17.6% | 39.5% | 34.1% | 26.4% |
| H. nana | 12% | 52.8% | 0% | 35.3% | 31.6% | 26.8% | 24% |
| E. vermicularis | 2.4% | 2.8% | 0% | 5.9% | 2.6% | 2.4% | 2.5% |
| Taenia spp. | 6% | 2.8% | 0% | 11.8% | 2.6% | 7.3% | 5% |
| Total | 42.9% | 49.3% | 7.8% | 17.9% | 32.7% | 49.4% | 100% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kann, S.; Bruennert, D.; Hansen, J.; Mendoza, G.A.C.; Gonzalez, J.J.C.; Quintero, C.L.A.; Hanke, M.; Hagen, R.M.; Backhaus, J.; Frickmann, H. High Prevalence of Intestinal Pathogens in Indigenous in Colombia. J. Clin. Med. 2020, 9, 2786. https://doi.org/10.3390/jcm9092786
Kann S, Bruennert D, Hansen J, Mendoza GAC, Gonzalez JJC, Quintero CLA, Hanke M, Hagen RM, Backhaus J, Frickmann H. High Prevalence of Intestinal Pathogens in Indigenous in Colombia. Journal of Clinical Medicine. 2020; 9(9):2786. https://doi.org/10.3390/jcm9092786
Chicago/Turabian StyleKann, Simone, Daniela Bruennert, Jessica Hansen, Gustavo Andrés Concha Mendoza, José José Crespo Gonzalez, Cielo Leonor Armenta Quintero, Miriam Hanke, Ralf Matthias Hagen, Joy Backhaus, and Hagen Frickmann. 2020. "High Prevalence of Intestinal Pathogens in Indigenous in Colombia" Journal of Clinical Medicine 9, no. 9: 2786. https://doi.org/10.3390/jcm9092786
APA StyleKann, S., Bruennert, D., Hansen, J., Mendoza, G. A. C., Gonzalez, J. J. C., Quintero, C. L. A., Hanke, M., Hagen, R. M., Backhaus, J., & Frickmann, H. (2020). High Prevalence of Intestinal Pathogens in Indigenous in Colombia. Journal of Clinical Medicine, 9(9), 2786. https://doi.org/10.3390/jcm9092786






