Individuals with Peripheral Artery Disease (PAD) and Type 1 Diabetes Are More Likely to Undergo Limb Amputation than Those with PAD and Type 2 Diabetes
Abstract
1. Background
2. Methods
2.1. Data Source
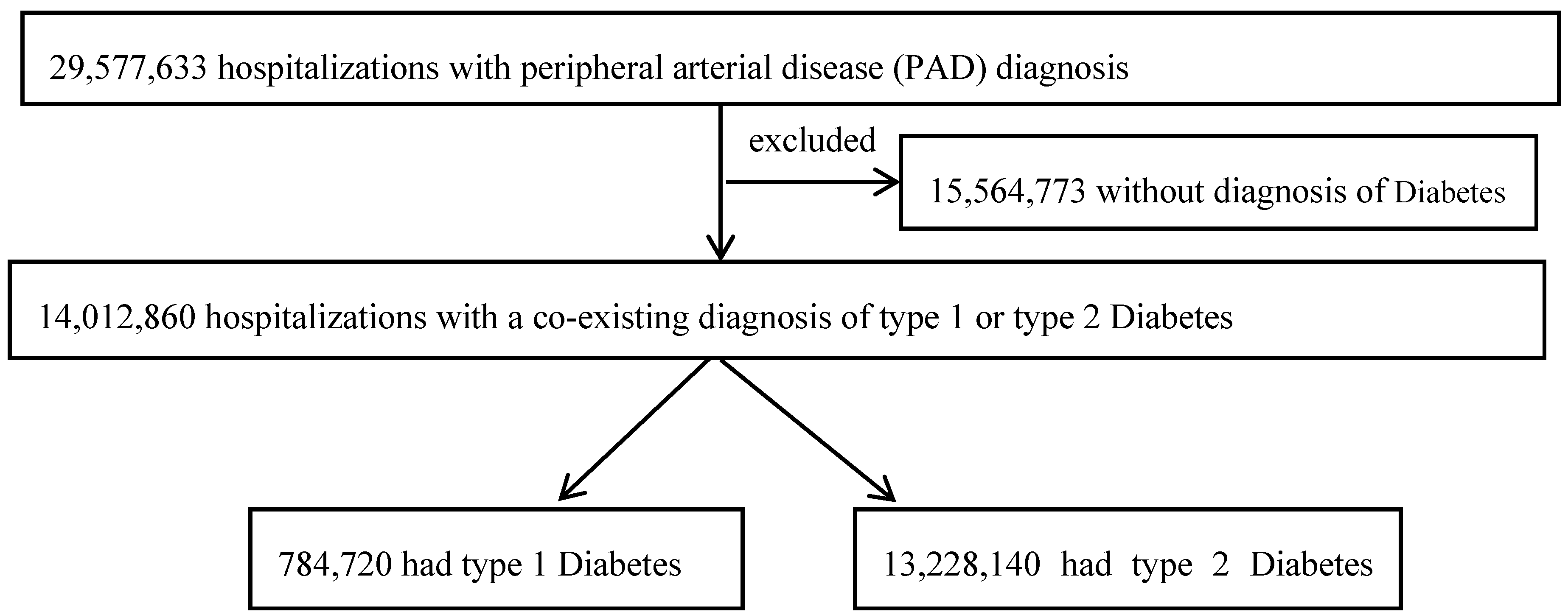
2.2. Study Population
2.3. Definition of PAD-Related Procedures
2.4. Definition of PAD-Related Disease Severity
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics According to DM Status
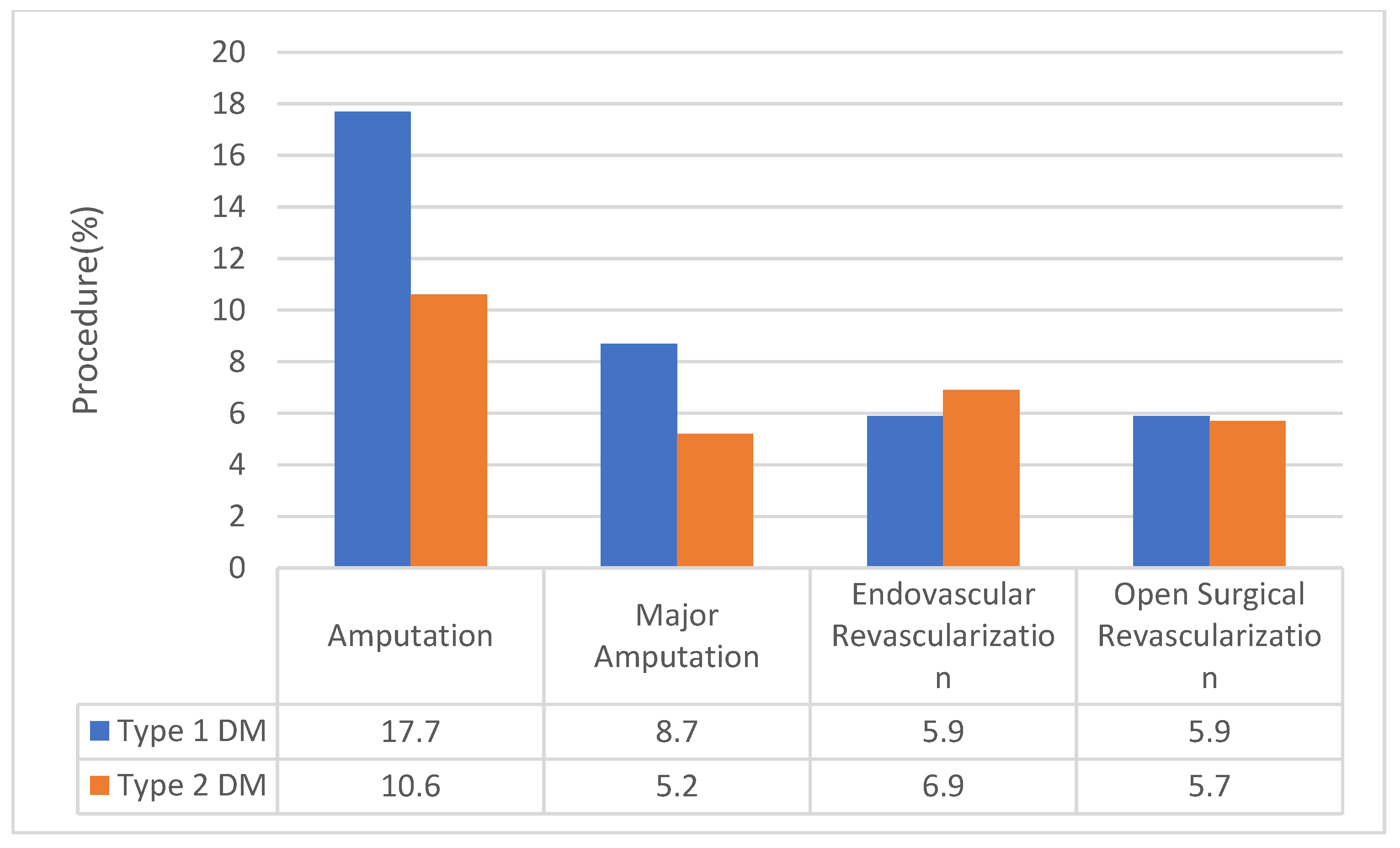
3.2. Vascular Procedure According to DM Status
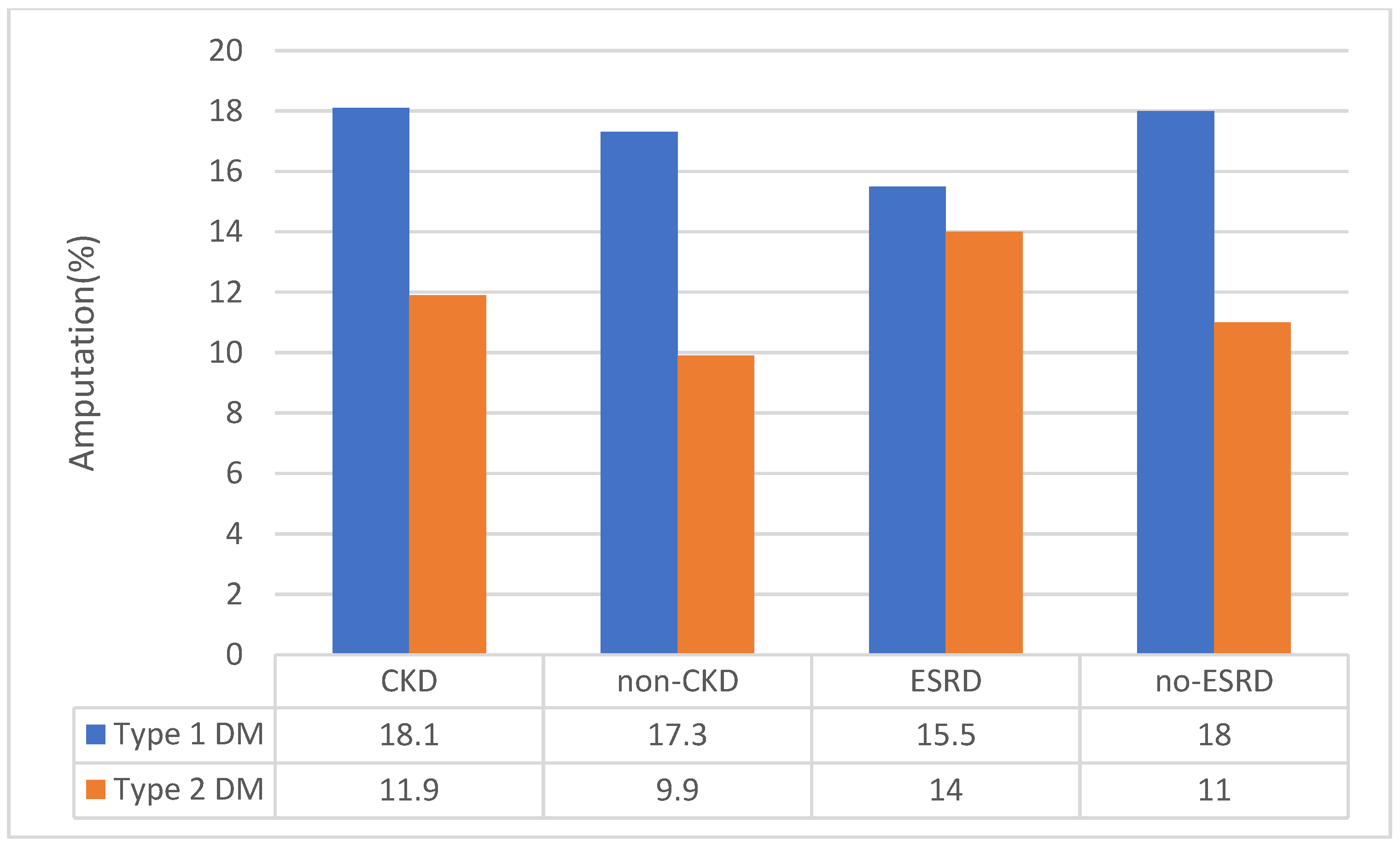
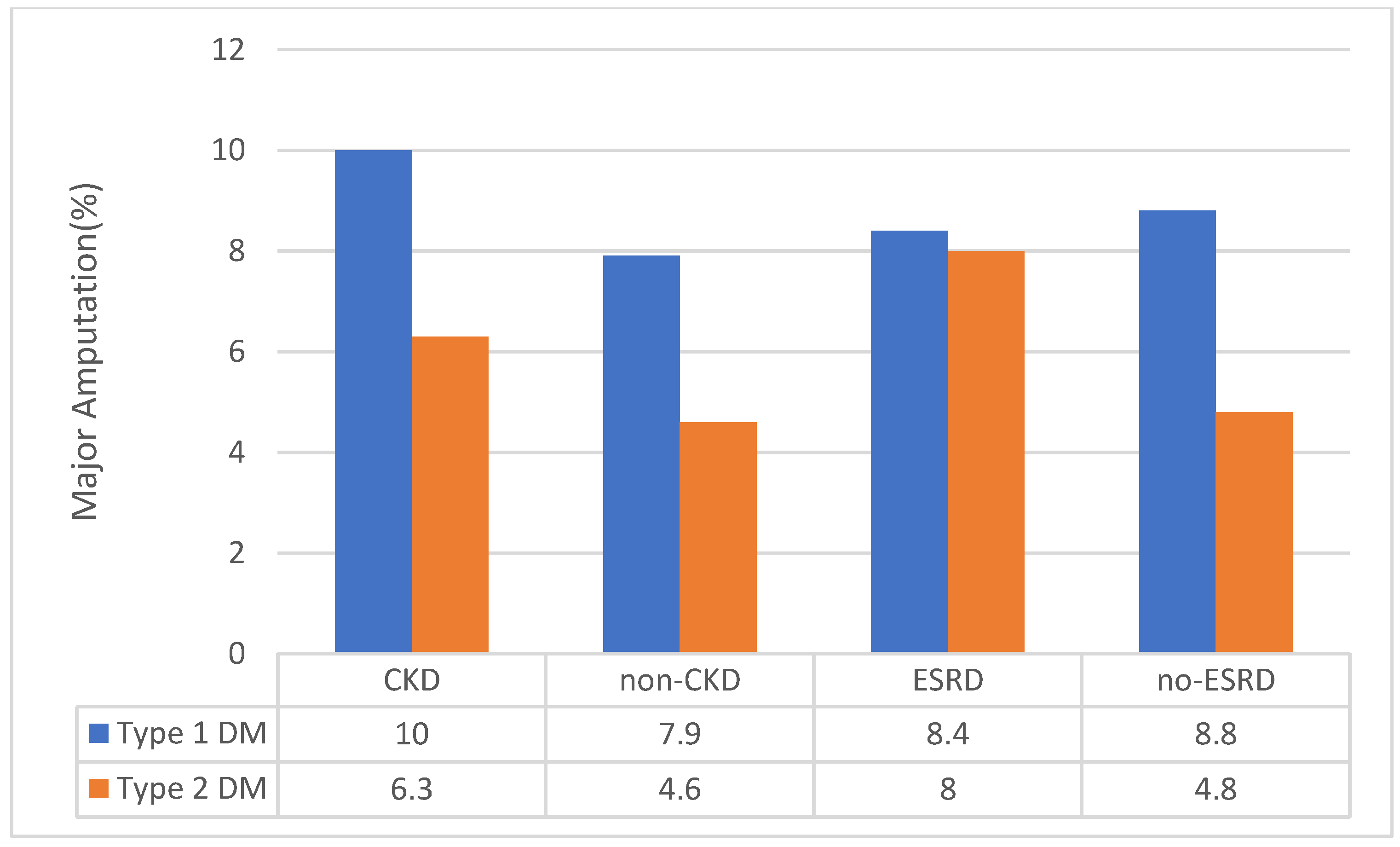
3.3. Sensitivity Analysis for CKD Status
3.4. Other Outcomes According to DM Status
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fowkes, F.G.R. Peripheral vascular disease: A public health perspective. J. Public Health 1990, 12, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Norgren, L.; Hiatt, W.; Dormandy, J.; Nehler, M.; Harris, K.; Fowkes, F.; Bell, K.; Caporusso, J.; Durand-Zaleski, I.; Komori, K.; et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur. J. Vasc. Endovasc. Surg. 2007, 33, S1–S75. [Google Scholar] [CrossRef] [PubMed]
- Dokun, A.O.; Annex, B. Genetic Polymorphisms in Peripheral Arterial Disease role of genomic methodologies. In Genomic and Personalized Medicine; Ginsburg, H.W.A.G., Ed.; Elsavier: Amsterdam, The Netherlands, 2008; Volumes 1–2. [Google Scholar]
- Criqui, M.H.; Aboyans, V. Epidemiology of Peripheral Artery Disease. Circ. Res. 2015, 116, 1509. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.R.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.A.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Criqui, M.; Fronek, A.; Barrett-Connor, E.; Klauber, M.; Gabriel, S.; Goodman, D. The prevalence of peripheral arterial disease in a defined population. Circulation 1985, 71, 510–515. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef]
- Peravali, R.; Gunnels, L.; Dhanabalan, K.; Ariganjoye, F.; Gerling, I.C.; Dokun, A.O. In Experimental Peripheral Arterial Disease, Type 2 Diabetes alters Post-Ischemic gene expression. J. Clin. Transl. Endocrinol. 2019. [Google Scholar] [CrossRef]
- Dormandy, J.; Heeck, L.; Vig, S. The fate of patients with critical leg ischemia. Semin. Vasc. Surg. 1999, 12, 142–147. [Google Scholar]
- Dormandy, J.; Heeck, L.; Vig, S. Predicting which patients will develop chronic critical leg ischemia. Semin. Vasc. Surg. 1999, 12, 138–141. [Google Scholar]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J. Vasc. Surg. 2019, 69, S1–S109. [Google Scholar] [CrossRef]
- Beks, P.J.; Mackaay, A.J.C.; De Neeling, J.N.D.; De Vries, H.; Bouter, L.M.; Heine, R.J. Peripheral arterial disease in relation to glycaemic level in an elderly Caucasian population: The Hoorn study. Diabetologia 1995, 38, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Welborn, T.A.; Knuiman, M.; McCann, V.; Stanton, K.; Constable, I.J. Clinical macrovascular disease in Caucasoid diabetic subjects: Logistic regression analysis of risk variables. Diabetologia 1984, 27, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J., 3rd; Macken, K.M.; Palumbo, P.J.; Elveback, L.R. Incidence and prevalence of clinical peripheral vascular disease in a population-based cohort of diabetic patients. Diabetes Care 1980, 3, 650–654. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32 (Suppl. 1), S62–S67. [Google Scholar] [CrossRef]
- Shanik, M.H.; Xu, Y.; Škrha, J.; Dankner, R.; Zick, Y.; Roth, J. Insulin Resistance and Hyperinsulinemia. Diabetes Care 2008, 31, S262. [Google Scholar] [CrossRef]
- Peravali, R.; Gunnels, L.; Alleboina, S.; Gerling, I.C.; Dokun, A.O. Type 1 diabetes alters ischemia-induced gene expression. J. Clin. Transl. Endocrinol. 2018, 15, 19–24. [Google Scholar] [CrossRef]
- Agarwal, M.A.; Aggarwal, A.; Rastogi, S.; Ventura, H.O.; Lavie, C.J. Cardiovascular disease burden in cancer patients from 2003 to 2014. Eur. Heart J. Qual. Care Clin. Outcomes 2018, 4, 69–70. [Google Scholar] [CrossRef]
- Agarwal, S.; Sud, K.; Shishehbor, M.H. Nationwide Trends of Hospital Admission and Outcomes Among Critical Limb Ischemia Patients: From 2003–2011. J. Am. Coll. Cardiol. 2016, 67, 1901–1913. [Google Scholar] [CrossRef]
- Lo, R.C.; Bensley, R.P.; Dahlberg, S.E.; Matyal, R.; Hamdan, A.D.; Wyers, M.; Chaikof, E.L.; Schermerhorn, M.L. Presentation, treatment, and outcome differences between men and women undergoing revascularization or amputation for lower extremity peripheral arterial disease. J. Vasc. Surg. 2014, 59, 409–418.e3. [Google Scholar] [CrossRef]
- HCUP. Healthcare Cost and Utilization Project (HCUP) Overview of the National (Nationwide) Inpatient Sample (NIS); Agency for Healthcare Research and Quality: Rockville, MD, USA. Available online: http://www.hcup-us.ahrq.gov/nisoverview.jsp (accessed on 24 March 2017).
- NIS. Data Elements of the National (Nationwide) Inpatient Sample (NIS). Available online: https://www.hcup-us.ahrq.gov/db/nation/nis/nisdde.jsp (accessed on 24 March 2017).
- Agarwal, M.; Agrawal, S.; Garg, L.; Mohananey, D.; Garg, A.; Bhatia, N.; Lavie, C.J. National Trends in the Incidence, Management, and Outcomes of Heart Failure Complications in Patients Hospitalized for ST-Segment Elevation Myocardial Infarction. Mayo Clin. Proc. Innov. Qual. Outcomes 2017, 1, 26–36. [Google Scholar] [CrossRef]
- Agarwal, M.A.; Jain, N.; Podila, P.S.B.; Varadarajan, V.; Patel, B.; Shah, M.; Garg, L.; Khouzam, R.N.; Ibebuogu, U.; Reed, G.L.; et al. Association of history of heart failure with hospital outcomes of hyperglycemic crises: Analysis from a University hospital and national cohort. J. Diabetes Complicat. 2020, 34, 107466. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Agrawal, S.; Garg, L.; Lavie, C.J. Relation Between Obesity and Survival in Patients Hospitalized for Pulmonary Arterial Hypertension (from a Nationwide Inpatient Sample Database 2003 to 2011). Am. J. Cardiol. 2017, 120, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.A.; Garg, L.; Shah, M.; Patel, B.; Jain, N.; Jain, S.; Kabra, R.; Kovesdy, C.; Reed, G.L.; Lavie, C.J. Relation of Obesity to Outcomes of Hospitalizations for Atrial Fibrillation. Am. J. Cardiol. 2019, 123, 1448–1452. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Agrawal, S.; Garg, L.; Garg, A.; Bhatia, N.; Kadaria, D.; Reed, G. Effect of Chronic Obstructive Pulmonary Disease on In-Hospital Mortality and Clinical Outcomes After ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2017, 119, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Garimella, P.S.; Hart, P.D.; O’Hare, A.; DeLoach, S.; Herzog, C.A.; Hirsch, A.T. Peripheral artery disease and CKD: A focus on peripheral artery disease as a critical component of CKD care. Am. J. Kidney Dis. 2012, 60, 641–654. [Google Scholar] [CrossRef]
- Mahler, R.J.; Adler, M.L. Type 2 Diabetes Mellitus: Update on Diagnosis, Pathophysiology, and Treatment. J. Clin. Endocrinol. Metab. 1999, 84, 1165–1171. [Google Scholar] [CrossRef]
- Zaccardi, F.; Webb, D.R.; Yates, T.; Davies, M.J. Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective. Postgrad. Med. J. 2016, 92, 63. [Google Scholar] [CrossRef]
- Pottecher, J.; Adamopoulos, C.; Lejay, A.; Bouitbir, J.; Charles, A.L.; Meyer, A.; Singer, M.; Wolff, V.; Diemunsch, P.; Laverny, G.; et al. Diabetes Worsens Skeletal Muscle Mitochondrial Function, Oxidative Stress, and Apoptosis After Lower-Limb Ischemia-Reperfusion: Implication of the RISK and SAFE Pathways? Front. Physiol. 2018, 9, 579. [Google Scholar] [CrossRef]
- Hopley, C.W.; Kavanagh, S.; Patel, M.R.; Ostrom, C.; Baumgartner, I.; Berger, J.S.; Blomster, J.I.; Fowkes, F.G.R.; Jones, W.S.; Katona, B.G.; et al. Chronic kidney disease and risk for cardiovascular and limb outcomes in patients with symptomatic peripheral artery disease: The EUCLID trial. Vasc. Med. 2019, 24, 422–430. [Google Scholar] [CrossRef]
- Mills Sr, J.L.; Conte, M.S.; Armstrong, D.G.; Pomposelli, F.B.; Schanzer, A.; Sidawy, A.N.; Andros, G. Society for Vascular Surgery Lower Extremity Guidelines, Committee: The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk stratification based on wound, ischemia, and foot infection (WIfI). J. Vasc. Surg. 2014, 59, 220–234. [Google Scholar] [CrossRef]
| Variable | Overall (n = 14,012,860) | Type 1 DM (n = 784,720) | Type 2 DM (n = 13,228,140) | p-Value |
|---|---|---|---|---|
| Age, mean ± SD (years) | 69.0 + 12.3 | 60.0 + 14.2 | 69.6 + 12.0 | <0.001 |
| Age group (years) | <0.001 | |||
| 18–40 | 1.5 (1.5 to 1.6) | 9.8 (9.4 to 10.2) | 10.0 (9.9 to 10.1) | |
| 41–60 | 22.9(22.6 to 23.1) | 41.2 (40.5 to 42.0) | 21.8 (21.5 to 22.1) | |
| 61–75 | 42.0 (41.9 to 42.2) | 32.2(31.6 to 32.9) | 42.6 (42.4 to 42.8) | |
| >75 | 33.6 (33.3 to 33.9) | 16.8 (16.2 to 17.3) | 34.6 (34.3 to 34.9) | |
| Females | 43.3(43.1 to 43.5) | 43.2(42.7 to 43.7) | 43.3(43.1 to 43.5) | 0.71 |
| Race/Ethnicity | <0.001 | |||
| Caucasian | 65.7 (64.5 to 66.8) | 65.6 (63.5 to 67.6) | 65.7 (64.5 to 66.8) | |
| African American | 17.7 (16.9 to 18.6) | 19.8 (18.3 to 21.3) | 17.6 (16.8 to 18.5) | |
| Others * | 16.6(15.6 to 17.6) | 14.6 (13.3 to 16.1) | 16.7 (15.7 to 17.8) | |
| Primary expected payer | <0.001 | |||
| Public insurance | 81.9 (81.6 to 82.2) | 76.0 (75.4 to 76.6) | 82.2 (81.9 to 82.5) | |
| Private insurance | 14.5(14.2 to 14.7) | 19.9 (19.3 to 20.4) | 14.1 (13.9 to 14.4) | |
| Others @ | 3.6 (3.5 to 3.9) | 4.1(3.9 to 4.4) | 3.7 (3.5 to 3.9) | |
| Comorbidities | ||||
| Smoker | 23.9 (23.5 to 24.4) | 16.6 (16.0 to17.2) | 24.4 (23.9 to 24.8) | <0.001 |
| Hypertension | 74.7 (74.4 to 75.0) | 66.3(65.6 to 66.9) | 75.2 (74.9 to 75.4) | <0.001 |
| Dyslipidemia | 44.6 (44.0 to 45.2) | 30.2 (29.4 to 30.1) | 45.4 (44.9 to 46.0) | <0.001 |
| Prior myocardial infarction | 11.5 (11.3 to 11.8) | 9.4 (9.1 to9.8) | 11.6 (11.4 to 11.9) | <0.001 |
| Prior percutaneous coronary intervention | 9.1 (8.9 to 9.3) | 6.0 (5.7 to 6.3) | 9.3(9.1 to 9.5) | <0.001 |
| Prior coronary artery bypass grafting | 15.7 (15.5 to 16.0) | 12.8(12.4 to 13.2) | 15.9 (15.7 to 16.1) | <0.001 |
| Carotid Artery Disease | 4.4 (4.3 to 4.5) | 2.1(1.9 to 2.2) | 4.5 (4.4 to 4.7) | <0.001 |
| Prior cerebrovascular vascular accident | 10.5 (10.3 to 10.7) | 6.1 (5.8 to 6.3) | 10.8 (10.5 to 11.0) | <0.001 |
| Atrial fibrillation | 17.3 (17.1 to 17.5) | 9.2 (8.9 to 9.5) | 17.8(17.6 to 18.0) | <0.001 |
| Congestive Heart failure | 19.4 (19.1 to19.6) | 17.7(17.2 to 18.1) | 19.5 (19.2 to 9.7) | <0.001 |
| Chronic Pulmonary Disease | 24.5 (24.2 to 24.8) | 16.3(15.9 to 16.8) | 25.0 (24.7 to 25.3) | <0.001 |
| Chronic kidney disease | 33.8(33.4 to 34.2) | 38.3(37.6 to 39.1) | 33.5 (33.1 to 33.9) | <0.001 |
| End-Stage Renal Disease | 11.6 (11.5 to 11.6) | 13.4 (13.1 to 13.6) | 11.5(11.4 to 11.5) | <0.001 |
| Disease severity | ||||
| Intermittent Claudication | 3.6(3.5 to 3.6) | 2.2 (2.1 to 2.3) | 3.6(3.6 to 3.7) | <0.001 |
| CLTI | 32.7 (32.4 to 33.1) | 45.2(44.5 to 45.8) | 32.0 (31.6 to 32.4) | <0.001 |
| Ulcer | 18.1 (17.9 to 18.3) | 25.9(25.4 to 26.4) | 17.7(17.4 to 17.9) | <0.001 |
| Complicated Ulcer # | 10.9 (10.7 to 11.0) | 16.6 (16.2 to 17.0) | 10.5 (10.4 to 10.7) | <0.001 |
| Outcomes | ||||
| Length of stay | 6.6 (6.5 to 6.6) | 7.8 (7.7 to 8.0) | 6.5 (6.4 to 6.6) | <0.001 |
| In-hospital mortality | 3.1 (3.0 to 3.1) | 3.0 (2.9 to 3.1) | 3.1 (3.0 to 3.1) | 0.23 |
| Major or Minor Amputation | 11.0 (10.8 to 11.2) | 17.7 (17.2 to 18.1) | 10.6 (10.4 to 10.8) | <0.001 |
| Major Amputation | 5.4 (5.3 to 5.5) | 8.7 (8.5 to 9.0) | 5.2(5.1 to 5.3) | <0.001 |
| Endovascular revascularization | 6.8 (6.6 to 7.1) | 5.9 (5.6 to 6.2) | 6.9(6.6 to 7.1) | <0.001 |
| Open surgical revascularization | 5.7 (5.5 to 5.8) | 5.6 (5.6 to 6.2) | 5.7 (5.5 to 5.8) | 0.12 |
| AOR for Vascular Procedure in Type 1 DM * | ||||
|---|---|---|---|---|
| Vascular Procedure | Model 1 | Model 2 | Model 3 | Model 4 |
| Amputation (major or minor) | 1.81 (1.77 to 1.87) | 1.43 (1.39 to 1.48) | 1.19 (1.16 to 1.23) | 1.12 (1.08 to 1.16) |
| Major Amputation | 1.77 (1.70 to 1.81) | 1.54 (1.48 to 1.59) | 1.22 (1.18 to 1.27) | 1.15 (1.11 to 1.20) |
| Endovascular revascularization | 0.85 (0.81 to 0.89) | 0.79 (0.75 to 0.85) | 0.81 (0.76 to 0.85) | 0.84 (0.79 to 0.88) |
| Open revascularization | 1.04 (0.99 to 1.09) | 0.94 (0.89 to 0.99) | 0.92 (0.87 to 0.96) | 0.92 (0.88 to 0.96) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, N.; Agarwal, M.A.; Jalal, D.; Dokun, A.O. Individuals with Peripheral Artery Disease (PAD) and Type 1 Diabetes Are More Likely to Undergo Limb Amputation than Those with PAD and Type 2 Diabetes. J. Clin. Med. 2020, 9, 2809. https://doi.org/10.3390/jcm9092809
Jain N, Agarwal MA, Jalal D, Dokun AO. Individuals with Peripheral Artery Disease (PAD) and Type 1 Diabetes Are More Likely to Undergo Limb Amputation than Those with PAD and Type 2 Diabetes. Journal of Clinical Medicine. 2020; 9(9):2809. https://doi.org/10.3390/jcm9092809
Chicago/Turabian StyleJain, Nidhi, Manyoo A. Agarwal, Diana Jalal, and Ayotunde O. Dokun. 2020. "Individuals with Peripheral Artery Disease (PAD) and Type 1 Diabetes Are More Likely to Undergo Limb Amputation than Those with PAD and Type 2 Diabetes" Journal of Clinical Medicine 9, no. 9: 2809. https://doi.org/10.3390/jcm9092809
APA StyleJain, N., Agarwal, M. A., Jalal, D., & Dokun, A. O. (2020). Individuals with Peripheral Artery Disease (PAD) and Type 1 Diabetes Are More Likely to Undergo Limb Amputation than Those with PAD and Type 2 Diabetes. Journal of Clinical Medicine, 9(9), 2809. https://doi.org/10.3390/jcm9092809





