Resistance Exercise, Electrical Muscle Stimulation, and Whole-Body Vibration in Older Adults: Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
- Population (P): Male or female older adults. The criterion for inclusion was mean sample age ≥ 65.0 years. Patients with sarcopenia were included if they met this criterion (age ≥ 65.0 years); however, sarcopenia was not an inclusion criterion.
- Intervention (I): RT, EMS, or WBW interventional programs of any duration. Studies exploring multimodal interventional programs (e.g., RT programs combined with stretching exercise) were excluded.
- Comparisons (C): Control groups, receiving no intervention or placebo intervention. Groups that received cognitive training or other non-physical interventions were also accepted as control groups. Studies in which control groups received any type of exercise, vibration intervention, electrical stimulation, or nutritional supplementation were excluded.
- Outcomes (O): (a) Muscular strength or power, not limited to type of testing or body part; (b) body composition and muscle architecture (including body fat, fat free mass, muscle mass, regional muscle mass, skeletal muscle mass, cross-sectional muscle area, circumference measures, and sarcopenia index) and (c) functional mobility outcomes (timed up-and-go test, stepping tests, sit-stand tests, functional reach tests, etc.).
- Study design (S): Only randomized controlled trials (RCT) that included at least one intervention group (RT, EMS, or WBV) and control group.
2.2. Search Strategy
2.3. Data Extraction
2.4. Assessment of Study Quality
2.5. Data Analysis and Synthesis
3. Results
3.1. Summary of Search Results
3.2. Study Quality Assessment
3.3. Participant Data and Intervention Characteristics
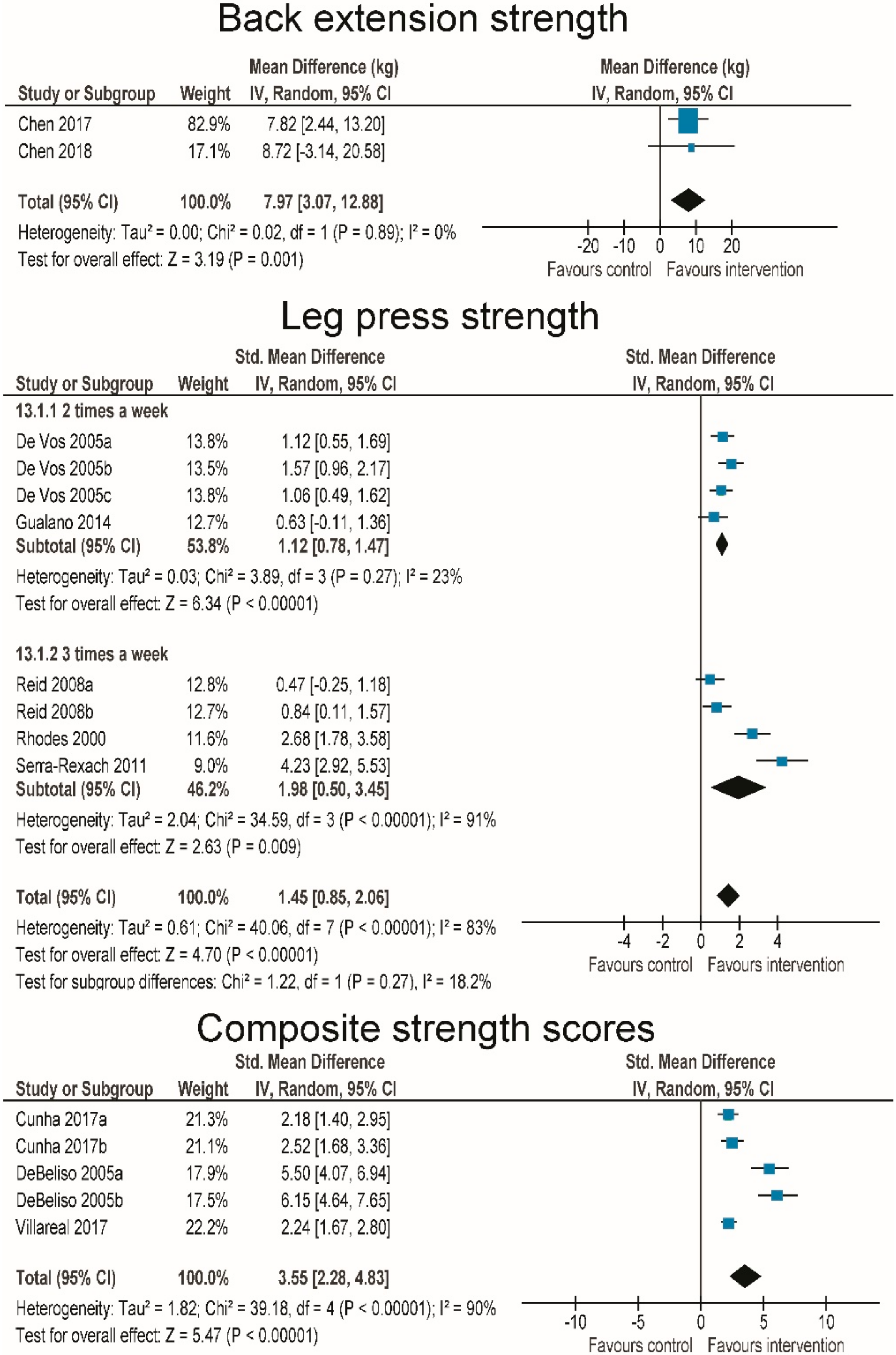
3.4. Effects of RT and WBV on Muscle Strength
3.5. Effects of RT on Body Composition
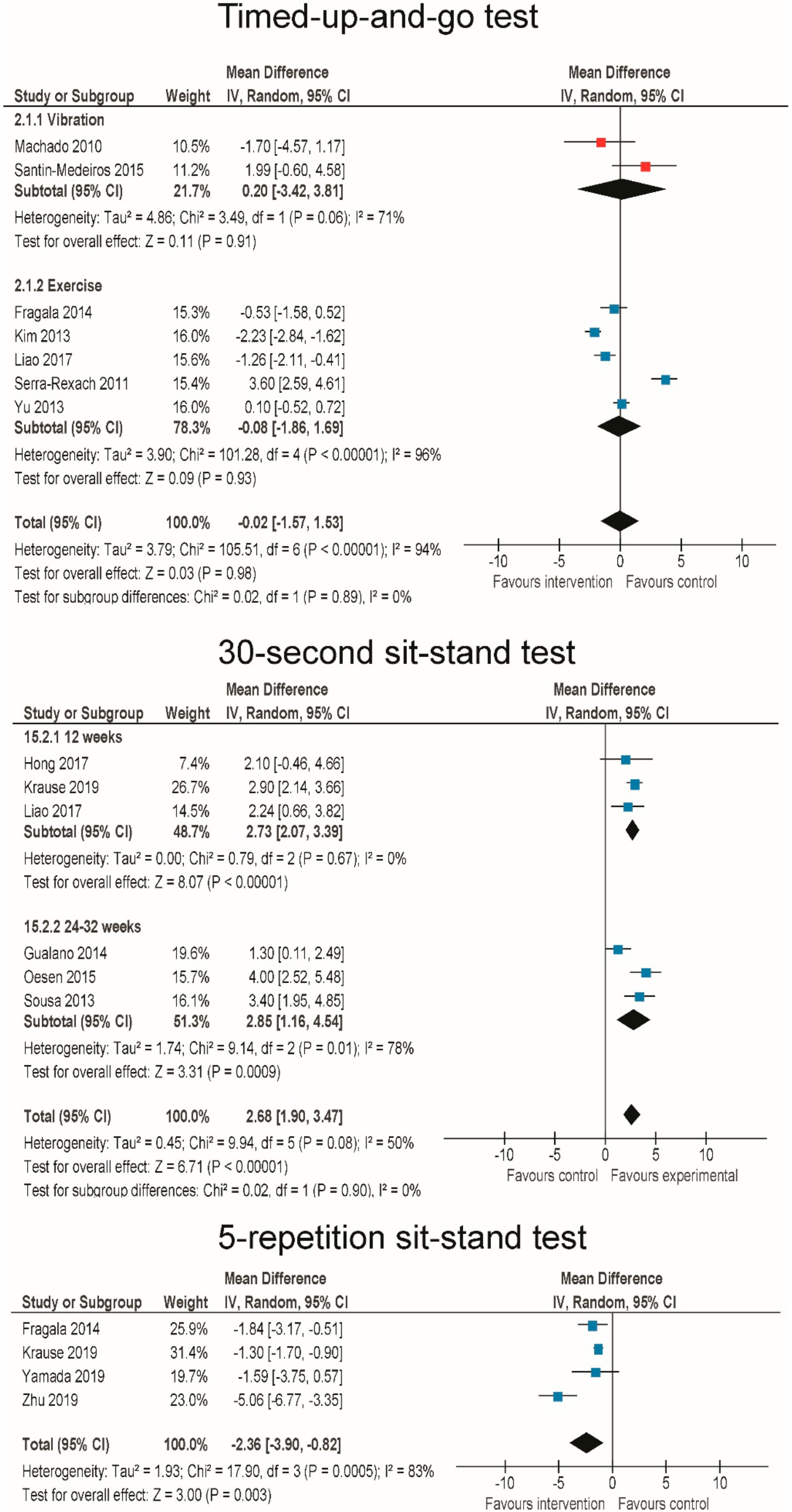
3.6. Effects of RT and WBV on Body Functional Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, X.; Yang, Y.; Schwebel, D.C.; Liu, Z.; Li, L.; Cheng, P.; Ning, P.; Hu, G. Population ageing and mortality during 1990-2017: A global decomposition analysis. PLoS Med. 2020, 17, e1003138. [Google Scholar] [CrossRef] [PubMed]
- Lutz, W.; Sanderson, W.; Scherbov, S. The coming acceleration of global population ageing. Nature 2008, 451, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Galloza, J.; Castillo, B.; Micheo, W. Benefits of Exercise in the Older Population. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 659–669. [Google Scholar] [CrossRef] [PubMed]
- De Labra, C.; Guimaraes-Pinheiro, C.; Maseda, A.; Lorenzo, T.; Millán-Calenti, J.C. Effects of physical exercise interventions in frail older adults: A systematic review of randomized controlled trials. BMC Geriatr. 2015, 15, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, P.; Pinto, R.S.; Radaelli, R.; Rech, A.; Grazioli, R.; Izquierdo, M.; Cadore, E.L. Benefits of resistance training in physically frail elderly: A systematic review. Aging Clin. Exp. Res. 2018, 30, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Barreto, P.D.S.; Rolland, Y.; Vellas, B.; Maltais, M. Association of Long-term Exercise Training with Risk of Falls, Fractures, Hospitalizations, and Mortality in Older Adults: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 394–405. [Google Scholar] [CrossRef] [Green Version]
- Cvecka, J.; Tirpakova, V.; Sedliak, M.; Kern, H.; Mayr, W.; Hamar, D. Physical activity in elderly. Eur. J. Transl. Myol. 2015, 25, 249–252. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Phu, S.; Boersma, D.; Duque, G. Exercise and Sarcopenia. J. Clin. Densitom. 2015, 18, 488–492. [Google Scholar] [CrossRef]
- Landi, F.; Marzetti, E.; Martone, A.M.; Bernabei, R.; Onder, G. Exercise as a remedy for sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 25–31. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Broccatelli, M.; Savera, G.; D’Elia, M.; Pahor, M.; et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Trisan, R. Balance Problems and Fall Risks in the Elderly. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Thomas, S.M.; Veroniki, A.A.; Hamid, J.S.; Cogo, E.; Strifler, L.; Khan, P.A.; Robson, R.; Sibley, K.M.; MacDonald, H.; et al. Comparisons of interventions for preventing falls in older adults: A systematic review and meta-analysis. JAMA J. Am. Med. Assoc. 2017, 318, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.; Michaleff, Z.A.; Fairhall, N.; Paul, S.S.; Tiedemann, A.; Whitney, J.; Cumming, R.G.; Herbert, R.D.; Close, J.C.T.; Lord, S.R. Exercise to prevent falls in older adults: An updated systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1749–1757. [Google Scholar] [CrossRef]
- Aguirre, L.E.; Villareal, D.T. Physical Exercise as Therapy for Frailty. Nestle Nutr. Inst. Workshop Ser. 2015, 83, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Csapo, R.; Alegre, L.M. Effects of resistance training with moderate vs heavy loads on muscle mass and strength in the elderly: A meta-analysis. Scand. J. Med. Sci. Sports 2016, 26, 995–1006. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tanimoto, M.; Oba, N.; Sanada, K.; Miyachi, M.; Ishii, N. Effect of resistance training using bodyweight in the elderly: Comparison of resistance exercise movement between slow and normal speed movement. Geriatr. Gerontol. Int. 2015, 15, 1270–1277. [Google Scholar] [CrossRef]
- Lacroix, A.; Hortobágyi, T.; Beurskens, R.; Granacher, U. Effects of Supervised vs. Unsupervised Training Programs on Balance and Muscle Strength in Older Adults: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 2341–2361. [Google Scholar] [CrossRef]
- Šarabon, N.; Smajla, D.; Kozinc, Ž.; Kern, H. Speed-power based training in the elderly and its potential for daily movement function enhancement. Eur. J. Transl. Myol. 2020, 30, 8898. [Google Scholar] [CrossRef] [Green Version]
- Yarmohammadi, S.; Saadati, H.M.; Ghaffari, M.; Ramezankhani, A. A systematic review of barriers and motivators to physical activity in elderly adults in Iran and worldwide. Epidemiol. Health 2019, 41, e2019049. [Google Scholar] [CrossRef]
- Freiberger, E.; Kemmler, W.; Siegrist, M.; Sieber, C. Frailty und Trainingsinterventionen: Evidenz und Barrieren für Bewegungsprogramme. Z. Gerontol. Geriatr. 2016, 49, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Rogan, S.; Taeymans, J.; Radlinger, L.; Naepflin, S.; Ruppen, S.; Bruelhart, Y.; Hilfiker, R. Effects of whole-body vibration on postural control in elderly: An update of a systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2017, 73, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Rogan, S.; de Bruin, E.D.; Radlinger, L.; Joehr, C.; Wyss, C.; Stuck, N.J.; Bruelhart, Y.; de Bie, R.A.; Hilfiker, R. Effects of whole-body vibration on proxies of muscle strength in old adults: A systematic review and meta-analysis on the role of physical capacity level. Eur. Rev. Aging Phys. Act. 2015, 12, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jepsen, D.B.; Thomsen, K.; Hansen, S.; Jørgensen, N.R.; Masud, T.; Ryg, J. Effect of whole-body vibration exercise in preventing falls and fractures: A systematic review and meta-analysis. BMJ Open 2017, 7, e018342. [Google Scholar] [CrossRef]
- Roelants, M.; Delecluse, C.; Verschueren, S.M. Whole-body-vibration training increases knee-extension strength and speed of movement in older women. J. Am. Geriatr. Soc. 2004, 52, 901–908. [Google Scholar] [CrossRef]
- Bogaerts, A.; Delecluse, C.; Claessens, A.; Coudyzer, W.; Boonen, S.; Verschueren, S. Impact of Whole-Body Vibration Training Versus Fitness Training on Muscle Strength and Muscle Mass in Older Men: A 1-Year Randomized Controlled Trial. J. Gerontol. Ser. A 2007, 62, 630–635. [Google Scholar] [CrossRef] [Green Version]
- Mayr, W. Neuromuscular electrical stimulation for mobility support of elderly. Eur. J. Transl. Myol. 2015, 25, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Pette, D.; Vrbová, G. The contribution of neuromuscular stimulation in elucidating muscle plasticity revisited. Eur. J. Transl. Myol. 2017, 27, 33–39. [Google Scholar] [CrossRef]
- Taylor, M.J.; Schils, S.; Ruys, A.J. Home FES: An exploratory review. Eur. J. Transl. Myol. 2019, 29, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Kern, H.; Barberi, L.; Löfler, S.; Sbardella, S.; Burggraf, S.; Fruhmann, H.; Carraro, U.; Mosole, S.; Sarabon, N.; Vogelauer, M.; et al. Electrical stimulation (ES) counteracts muscle decline in seniors. Front. Aging Neurosci. 2014, 6. [Google Scholar] [CrossRef] [Green Version]
- Zampieri, S.; Mosole, S.; Löfler, S.; Fruhmann, H.; Burggraf, S.; Cvečka, J.; Hamar, D.; Sedliak, M.; Tirptakova, V.; Šarabon, N.; et al. Physical exercise in Aging: Nine weeks of leg press or electrical stimulation training in 70 years old sedentary elderly people. Eur. J. Transl. Myol. 2015, 25, 237–242. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Brennan, L.; Caulfield, B. The use of neuromuscular electrical stimulation (NMES) for managing the complications of ageing related to reduced exercise participation. Maturitas 2018, 113, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions|Cochrane Training. Available online: https://training.cochrane.org/handbook/current (accessed on 15 May 2020).
- Kemmler, W.; von Stengel, S. Whole-body electromyostimulation as a means to impact muscle mass and abdominal body fat in lean, sedentary, older female adults: Subanalysis of the TEST-III trial. Clin. Interv. Aging 2013, 8, 1353–1364. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, P.; Zhou, S.; Crowley, Z.; Davie, A.; Baglin, R. Effects of electromyostimulation on knee extensors and flexors strength and steadiness in older adults. J. Mot. Behav. 2011, 43, 413–421. [Google Scholar] [CrossRef]
- Bogaerts, A.C.; Delecluse, C.; Claessens, A.L.; Troosters, T.; Boonen, S.; Verschueren, S.M. Effects of whole body vibration training on cardiorespiratory fitness and muscle strength in older individuals (a 1-year randomised controlled trial). Age Ageing 2009, 38. [Google Scholar] [CrossRef] [Green Version]
- Marín-Cascales, E.; Rubio-Arias, J.A.; Romero-Arenas, S.; Alcaraz, P.E. Effect of 12 weeks of whole-body vibration versus multi-component training in post-menopausal women. Rejuvenation Res. 2015, 18, 508–516. [Google Scholar] [CrossRef]
- Verschueren, S.M.; Bogaerts, A.; Delecluse, C.; Claessens, A.L.; Haentjens, P.; Vanderschueren, D.; Boonen, S. The effects of whole-body vibration training and vitamin D supplementation on muscle strength, muscle mass, and bone density in institutionalized elderly women: A 6-month randomized, controlled trial. J. Bone Miner. Res. 2011, 26, 42–49. [Google Scholar] [CrossRef]
- Zheng, A.; Sakari, R.; Cheng, S.M.; Hietikko, A.; Moilanen, P.; Timonen, J.; Fagerlund, K.M.; Kärkkäinen, M.; Alèn, M.; Cheng, S. Effects of a low-frequency sound wave therapy programme on functional capacity, blood circulation and bone metabolism in frail old men and women. Clin. Rehabil. 2009, 23, 897–908. [Google Scholar] [CrossRef]
- Wei, N.; Pang, M.Y.C.; Ng, S.S.M.; Ng, G.Y.F. Optimal frequency/time combination of whole-body vibration training for improving muscle size and strength of people with age-related muscle loss (sarcopenia): A randomized controlled trial. Geriatr. Gerontol. Int. 2017, 17, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Bunout, D.; Barrera, G.; de la Maza, P.; Avendaño, M.; Gattas, V.; Petermann, M.; Hirsch, S. The impact of nutritional supplementation and resistance training on the health functioning of free-living Chilean elders: Results of 18 months of follow-up. J. Nutr. 2001, 131, 2441S–2446S. [Google Scholar] [CrossRef] [PubMed]
- Iranzo, M.A.C.; Balasch-Bernat, M.; Tortosa-Chuliá, M.; Balasch-Parisi, S. Effects of resistance training of peripheral muscles versus respiratory muscles in older adults with sarcopenia who are institutionalized: A randomized controlled trial. J. Aging Phys. Act. 2018, 26, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Chung, Y.C.; Chen, Y.J.; Ho, S.Y.; Wu, H.J. Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity. J. Am. Geriatr. Soc. 2017, 65, 827–832. [Google Scholar] [CrossRef] [PubMed]
- De Vos, N.J.; Singh, N.A.; Ross, D.A.; Stavrinos, T.M.; Orr, R.; Singh, M.A.F. Optimal load for increasing muscle power during explosive resistance training in older adults. J. Gerontol. Ser. A 2005, 60, 638–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontera, W.R.; Hughes, V.A.; Krivickas, L.S.; Kim, S.K.; Foldvari, M.; Roubenoff, R. Strength training in older women: Early and late changes in whole muscle and single cells. Muscle Nerve 2003, 28, 601–608. [Google Scholar] [CrossRef]
- Giné-Garriga, M.; Guerra, M.; Pagès, E.; Manini, T.M.; Jiménez, R.; Unnithan, V.B. The effect of functional circuit training on physical frailty in frail older adults: A randomized controlled trial. J. Aging Phys. Act. 2010, 18, 401–424. [Google Scholar] [CrossRef] [Green Version]
- Ikezoe, T.; Tsutou, A.; Asakawa, Y.; Tsuboyama, T. Low Intensity Training for Frail Elderly Women: Long-term Effects on Motor Function and Mobility. J. Phys. Ther. Sci. 2005, 17, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.S.; Kim, Y.Y.; Park, H.Y. Circuit Training Improvements in Korean Women with Sarcopenia. Percept. Mot. Skills 2019, 126, 828–842. [Google Scholar] [CrossRef]
- Kim, H.K.; Suzuki, T.; Saito, K.; Yoshida, H.; Kobayashi, H.; Kato, H.; Katayama, M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. J. Am. Geriatr. Soc. 2012, 60, 16–23. [Google Scholar] [CrossRef]
- Kim, H.; Suzuki, T.; Saito, K.; Yoshida, H.; Kojima, N.; Kim, M.; Sudo, M.; Yamashiro, Y.; Tokimitsu, I. Effects of exercise and tea catechins on muscle mass, strength and walking ability in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. Geriatr. Gerontol. Int. 2013, 13, 458–465. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Kojima, N.; Fujino, K.; Hosoi, E.; Kobayashi, H.; Somekawa, S.; Niki, Y.; Yamashiro, Y.; Yoshida, H. Exercise and Nutritional Supplementation on Community-Dwelling Elderly Japanese Women With Sarcopenic Obesity: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2016, 17, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Kryger, A.I.; Andersen, J.L. Resistance training in the oldest old: Consequences for muscle strength, fiber types, fiber size, and MHC isoforms. Scand. J. Med. Sci. Sports 2007, 17, 422–430. [Google Scholar] [CrossRef] [PubMed]
- De Liao, C.; Tsauo, J.Y.; Lin, L.F.; Huang, S.W.; Ku, J.W.; Chou, L.C.; Liou, T.H. Effects of elastic resistance exercise on body composition and physical capacity in older women with sarcopenic obesity. Medicine 2017, 96, e7115. [Google Scholar] [CrossRef]
- Martins, W.R.; Safons, M.P.; Bottaro, M.; Blasczyk, J.C.; Diniz, L.R.; Fonseca, R.M.C.; Bonini-Rocha, A.C.; De Oliveira, R.J. Effects of short term elastic resistance training on muscle mass and strength in untrained older adults: A randomized clinical trial. BMC Geriatr. 2015, 15, 99. [Google Scholar] [CrossRef] [Green Version]
- Matsufuji, S.; Shoji, T.; Yano, Y.; Tsujimoto, Y.; Kishimoto, H.; Tabata, T.; Emoto, M.; Inaba, M. Effect of Chair Stand Exercise on Activity of Daily Living: A Randomized Controlled Trial in Hemodialysis Patients. J. Ren. Nutr. 2015, 25, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Breil, F.A.; Vogt, M.; Steiner, R.; Klossner, S.; Hoppeler, H.; Däpp, C.; Lippuner, K.; Popp, A. Different response to eccentric and concentric training in older men and women. Eur. J. Appl. Physiol. 2009, 107, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Oesen, S.; Halper, B.; Hofmann, M.; Jandrasits, W.; Franzke, B.; Strasser, E.M.; Graf, A.; Tschan, H.; Bachl, N.; Quittan, M.; et al. Effects of elastic band resistance training and nutritional supplementation on physical performance of institutionalised elderly—A randomized controlled trial. Exp. Gerontol. 2015, 72, 99–108. [Google Scholar] [CrossRef]
- Reid, K.F.; Callahan, D.M.; Carabello, R.J.; Phillips, E.M.; Frontera, W.R.; Fielding, R.A. Lower extremity power training in elderly subjects with mobility limitations: A randomized controlled trial. Aging Clin. Exp. Res. 2008, 20, 337–343. [Google Scholar] [CrossRef]
- Rhodes, E.C.; Martin, A.D.; Taunton, J.E.; Donnelly, M.; Warren, J.; Elliot, J. Effects of one year of resistance training on the relation between muscular strength and bone density in elderly women. Br. J. Sports Med. 2000, 34, 18–22. [Google Scholar] [CrossRef]
- Scanlon, T.C.; Fragala, M.S.; Stout, J.R.; Emerson, N.S.; Beyer, K.S.; Oliveira, L.P.; Hoffman, J.R. Muscle architecture and strength: Adaptations to short-term resistance training in older adults. Muscle Nerve 2014, 49, 584–592. [Google Scholar] [CrossRef]
- Su, Z.; Zhao, J.; Wang, N.; Chen, Y.; Guo, Y.; Tian, Y. Effects of weighted tai chi on leg strength of older adults. J. Am. Geriatr. Soc. 2015, 63, 2208–2210. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, K.S.S.; Dias, J.M.D.; Araújo, M.C.; Pinheiro, A.C.; Moreira, B.S.; Dias, R.C. Effects of a progressive resistance exercise program with high-speed component on the physical function of older women with sarcopenic obesity: A randomized controlled trial. Brazilian J. Phys. Ther. 2016, 20, 432–440. [Google Scholar] [CrossRef]
- Yamada, M.; Kimura, Y.; Ishiyama, D.; Nishio, N.; Otobe, Y.; Tanaka, T.; Ohji, S.; Koyama, S.; Sato, A.; Suzuki, M.; et al. Synergistic effect of bodyweight resistance exercise and protein supplementation on skeletal muscle in sarcopenic or dynapenic older adults. Geriatr. Gerontol. Int. 2019, 19, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Lee, M.J.; Lee, H.M.; Lee, J.S. Effect of low-intensity resistance training with heat stress on the HSP72, anabolic hormones, muscle size, and strength in elderly women. Aging Clin. Exp. Res. 2017, 29, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Y.; Chan, R.; Kwok, T.; Cheng, K.C.C.; Ha, A.; Woo, J. Effects of exercise and nutrition supplementation in community-dwelling older Chinese people with sarcopenia: A randomized controlled trial. Age Ageing 2019, 48, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Macedo, A.R.; Alves, C.R.R.; Roschel, H.; Benatti, F.B.; Takayama, L.; de Sá Pinto, A.L.; Lima, F.R.; Pereira, R.M.R. Creatine supplementation and resistance training in vulnerable older women: A randomized double-blind placebo-controlled clinical trial. Exp. Gerontol. 2014, 53, 7–15. [Google Scholar] [CrossRef]
- Serra-Rexach, J.A.; Bustamante-Ara, N.; Hierro Villarán, M.; González Gil, P.; Sanz Ibáñez, M.J.; Blanco Sanz, N.; Ortega Santamaría, V.; Gutiérrez Sanz, N.; Marín Prada, A.B.; Gallardo, C.; et al. Short-term, light- to moderate-intensity exercise training improves leg muscle strength in the oldest old: A randomized controlled trial. J. Am. Geriatr. Soc. 2011, 59, 594–602. [Google Scholar] [CrossRef]
- Chen, H.T.; Wu, H.J.; Chen, Y.J.; Ho, S.Y.; Chung, Y.C. Effects of 8-week kettlebell training on body composition, muscle strength, pulmonary function, and chronic low-grade inflammation in elderly women with sarcopenia. Exp. Gerontol. 2018, 112, 112–118. [Google Scholar] [CrossRef]
- Cunha, P.M.; Ribeiro, A.S.; Tomeleri, C.M.; Schoenfeld, B.J.; Silva, A.M.; Souza, M.F.; Nascimento, M.A.; Sardinha, L.B.; Cyrino, E.S. The effects of resistance training volume on osteosarcopenic obesity in older women. J. Sports Sci. 2018, 36, 1564–1571. [Google Scholar] [CrossRef]
- DeBeliso, M.; Harris, C.; Spitzer-Gibson, T.; Adams, K.J. A comparison of periodised and fixed repetition training protocol on strength in older adults. J. Sci. Med. Sport 2005, 8, 190–199. [Google Scholar] [CrossRef]
- Villareal, D.T.; Aguirre, L.; Gurney, A.B.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N. Engl. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Z.J.; Zhang, H.L.; Yin, L.X. Effects of intradialytic resistance exercise on systemic inflammation in maintenance hemodialysis patients with sarcopenia: A randomized controlled trial. Int. Urol. Nephrol. 2019, 51, 1415–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fragala, M.S.; Fukuda, D.H.; Stout, J.R.; Townsend, J.R.; Emerson, N.S.; Boone, C.H.; Beyer, K.S.; Oliveira, L.P.; Hoffman, J.R. Muscle quality index improves with resistance exercise training in older adults. Exp. Gerontol. 2014, 53, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Crognale, D.; Cogan, K.; Contarelli, S.; Egan, B.; Newsholme, P.; De Vito, G. The effects of a combined bodyweight-based and elastic bands resistance training, with or without protein supplementation, on muscle mass, signaling and heat shock response in healthy older people. Exp. Gerontol. 2019, 115, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, T.; Von Stengel, S.; Sieber, C.; Kemmler, W. The favorable effects of a high-intensity resistance training on sarcopenia in older community-dwelling men with osteosarcopenia: The randomized controlled frost study. Clin. Interv. Aging 2019, 14, 2173–2186. [Google Scholar] [CrossRef] [Green Version]
- Piastra, G.; Perasso, L.; Lucarini, S.; Monacelli, F.; Bisio, A.; Ferrando, V.; Gallamini, M.; Faelli, E.; Ruggeri, P. Effects of Two Types of 9-Month Adapted Physical Activity Program on Muscle Mass, Muscle Strength, and Balance in Moderate Sarcopenic Older Women. BioMed Res. Int. 2018, 2018, 5095673. [Google Scholar] [CrossRef]
- Tsuzuku, S.; Kajioka, T.; Sakakibara, H.; Shimaoka, K. Slow movement resistance training using body weight improves muscle mass in the elderly: A randomized controlled trial. Scand. J. Med. Sci. Sports 2018, 28, 1339–1344. [Google Scholar] [CrossRef]
- Hong, J.; Kim, J.; Kim, S.W.; Kong, H.J. Effects of home-based tele-exercise on sarcopenia among community-dwelling elderly adults: Body composition and functional fitness. Exp. Gerontol. 2017, 87, 33–39. [Google Scholar] [CrossRef]
- Huang, S.W.; Ku, J.W.; Lin, L.F.; Liao, C.D.; Chou, L.C.; Liou, T.H. Body composition influenced by progressive elastic band resistance exercise of sarcopenic obesity elderly women: A pilot randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 556–563. [Google Scholar] [CrossRef]
- Strasser, E.M.; Hofmann, M.; Franzke, B.; Schober-Halper, B.; Oesen, S.; Jandrasits, W.; Graf, A.; Praschak, M.; Horvath-Mechtler, B.; Krammer, C.; et al. Strength training increases skeletal muscle quality but not muscle mass in old institutionalized adults: A randomized, multi-arm parallel and controlled intervention study. Eur. J. Phys. Rehabil. Med. 2018, 54, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cabello, A.; González-Agüero, A.; Ara, I.; Casajús, J.A.; Vicente-Rodríguez, G. Efectos de una intervención de vibración corporal total sobre la masa magra en personas ancianas. Nutr. Hosp. 2013, 28, 1255–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemmler, W.; Bebenek, M.; Engelke, K.; Von Stengel, S. Impact of whole-body electromyostimulation on body composition in elderly women at risk for sarcopenia: The Training and ElectroStimulation Trial (TEST-III). Age (Omaha) 2014, 36, 395–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, N.; Mendes, R.; Abrantes, C.; Sampaio, J.; Oliveira, J. Is once-weekly resistance training enough to prevent sarcopenia? J. Am. Geriatr. Soc. 2013, 61, 1423–1424. [Google Scholar] [CrossRef]
- Kemmler, W.; Teschler, M.; Weissenfels, A.; Bebenek, M.; von Stengel, S.; Kohl, M.; Freiberger, E.; Goisser, S.; Jakob, F.; Sieber, C.; et al. Whole-body electromyostimulation to fight sarcopenic obesity in community-dwelling older women at risk. Resultsof the randomized controlled FORMOsA-sarcopenic obesity study. Osteoporos. Int. 2016, 27, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; García-López, D.; González-Gallego, J.; Garatachea, N. Whole-body vibration training increases muscle strength and mass in older women: A randomized-controlled trial. Scand. J. Med. Sci. Sports 2010, 20, 200–207. [Google Scholar] [CrossRef]
- Santin-Medeiros, F.; Rey-López, J.P.; Santos-Lozano, A.; Cristi-Montero, C.S.; Vallejo, N.G. Effects of Eight Months of Whole-Body Vibration Training on the Muscle Mass and Functional Capacity of Elderly Women. J. Strength Cond. Res. 2015, 29, 1863–1869. [Google Scholar] [CrossRef]
- Strandberg, E.; Ponsot, E.; Piehl-Aulin, K.; Falk, G.; Kadi, F. Resistance training alone or combined with N-3 PUFA-rich diet in older women: Effects on muscle Fiber hypertrophy. J. Gerontol. Ser. A 2019, 74, 489–494. [Google Scholar] [CrossRef]
- Yu, W.; An, C.; Kang, H. Effects of resistance exercise using Thera-band on balance of elderly adults: A randomized controlled trial. J. Phys. Ther. Sci. 2013, 25, 1471–1473. [Google Scholar] [CrossRef] [Green Version]
- Carraro, U.; Gava, K.; Baba, A.; Marcante, A.; Piccione, F. To contrast and reverse skeletal muscle atrophy by full-body in-bed gym, a mandatory lifestyle for older olds and borderline mobility-impaired persons. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2018; Volume 1088, pp. 549–560. [Google Scholar]
- Sajer, S.; Guardiero, G.S.; Scicchitano, B.M. Myokines in home-based functional electrical stimulation-induced recovery of skeletal muscle in elderly and permanent denervation. Eur. J. Transl. Myol. 2018, 28, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Oreská, Ľ.; Slobodová, L.; Vajda, M.; Kaplánová, A.; Tirpáková, V.; Cvečka, J.; Buzgó, G.; Ukropec, J.; Ukropcová, B.; Sedliak, M. The effectiveness of two different multimodal training modes on physical performance in elderly. Eur. J. Transl. Myol. 2020, 30, 8920. [Google Scholar] [CrossRef] [Green Version]
- Cristi, C.; Collado, P.S.; Márquez, S.; Garatachea, N.; Cuevas, M.J. Whole-body vibration training increases physical fitness measures without alteration of inflammatory markers in older adults. Eur. J. Sport Sci. 2014, 14, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Mazini Filho, M.L.; Aidar, F.J.; Gama De Matos, D.; Costa Moreira, O.; Patrocínio De Oliveira, C.E.; Rezende De Oliveira Venturini, G.; Magalhäes Curty, V.; Menezes Touguinha, H.; Caputo Ferreira, M.E. Circuit strength training improves muscle strength, functional performance and anthropometric indicators in sedentary elderly women. J. Sports Med. Phys. Fitness 2018, 58, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.B.; de Souza Bezerra, E.; da Rosa Orssatto, L.B.; de Paiva Vieira, E.; Picanço, L.A.A.; dos Santos, J.O.L. Functional resistance training can increase strength, knee torque ratio, and functional performance in elderly women. J. Exerc. Rehabil. 2018, 14, 654–659. [Google Scholar] [CrossRef]
- Bean, J.F.; Kiely, D.K.; Larose, S.; Goldstein, R.; Frontera, W.R.; Leveille, S.G. Are changes in leg power responsible for clinically meaningful improvements in mobility in older adults? J. Am. Geriatr. Soc. 2010, 58, 2363–2368. [Google Scholar] [CrossRef] [PubMed]
- Licurci, M.D.; de Almeida Fagundes, A.; Arisawa, E.A. Acute effects of whole body vibration on heart rate variability in elderly people. J. Bodyw. Mov. Ther. 2018, 22, 618–621. [Google Scholar] [CrossRef]
- Wei, N.; Ng, G.Y.F. The effect of whole body vibration training on quadriceps voluntary activation level of people with age-related muscle loss (sarcopenia): A randomized pilot study. BMC Geriatr. 2018, 18, 240. [Google Scholar] [CrossRef] [Green Version]
- Barberi, L.; Scicchitano, B.M.; Musarò, A. Molecular and cellular mechanisms of muscle aging and sarcopenia and effects of electrical stimulation in seniors. Eur. J. Transl. Myol. 2015, 25, 231. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.; Man, W.D.C.; Gao, W.; Higginson, I.J.; Wilcock, A.; Maddocks, M. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Syst. Rev. 2016, 2016, CD009419. [Google Scholar] [CrossRef] [Green Version]
- Evangelista, A.; Teixeira, C.; Barros, B.M.; de Azevedo, J.; Paunksnis, M.; Souza, C.; Wadhi, T.; Rica, R.; Braz, T.V.; Bocalini, D. Does Whole-Body Electrical Muscle Stimulation Combined With Strength Training Promote Morphofunctional Alterations? Clinics 2019, 74, e1334. [Google Scholar] [CrossRef] [Green Version]
- Langeard, A.; Bigot, L.; Chastan, N.; Gauthier, A. Does neuromuscular electrical stimulation training of the lower limb have functional effects on the elderly?: A systematic review. Exp. Gerontol. 2017, 91, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to endurance and strength training. Cold Spring Harb. Perspect. Med. 2018, 8, a029769. [Google Scholar] [CrossRef] [PubMed]
- Stewart, V.H.; Saunders, D.H.; Greig, C.A. Responsiveness of muscle size and strength to physical training in very elderly people: A systematic review. Scand. J. Med. Sci. Sports 2014, 24, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Ogborn, D.; Krieger, J.W. Effects of Resistance Training Frequency on Measures of Muscle Hypertrophy: A Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 1689–1697. [Google Scholar] [CrossRef]
- Ralston, G.W.; Kilgore, L.; Wyatt, F.B.; Buchan, D.; Baker, J.S. Weekly Training Frequency Effects on Strength Gain: A Meta-Analysis. Sports Med. Open 2018, 4, 36. [Google Scholar] [CrossRef]
- Grgic, J.; Schoenfeld, B.J.; Davies, T.B.; Lazinica, B.; Krieger, J.W.; Pedisic, Z. Effect of Resistance Training Frequency on Gains in Muscular Strength: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 1207–1220. [Google Scholar] [CrossRef]
- Lopes, J.S.S.; Machado, A.F.; Micheletti, J.K.; de Almeida, A.C.; Cavina, A.P.; Pastre, C.M. Effects of training with elastic resistance versus conventional resistance on muscular strength: A systematic review and meta-analysis. SAGE Open Med. 2019, 7, 205031211983111. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šarabon, N.; Kozinc, Ž.; Löfler, S.; Hofer, C. Resistance Exercise, Electrical Muscle Stimulation, and Whole-Body Vibration in Older Adults: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2020, 9, 2902. https://doi.org/10.3390/jcm9092902
Šarabon N, Kozinc Ž, Löfler S, Hofer C. Resistance Exercise, Electrical Muscle Stimulation, and Whole-Body Vibration in Older Adults: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2020; 9(9):2902. https://doi.org/10.3390/jcm9092902
Chicago/Turabian StyleŠarabon, Nejc, Žiga Kozinc, Stefan Löfler, and Christian Hofer. 2020. "Resistance Exercise, Electrical Muscle Stimulation, and Whole-Body Vibration in Older Adults: Systematic Review and Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 9, no. 9: 2902. https://doi.org/10.3390/jcm9092902
APA StyleŠarabon, N., Kozinc, Ž., Löfler, S., & Hofer, C. (2020). Resistance Exercise, Electrical Muscle Stimulation, and Whole-Body Vibration in Older Adults: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine, 9(9), 2902. https://doi.org/10.3390/jcm9092902






