CT Findings in Pulmonary and Abdominal Sarcoidosis. Implications for Diagnosis and Classification
Abstract
:1. Introduction
2. Sarcoidosis, the Chameleon Disease
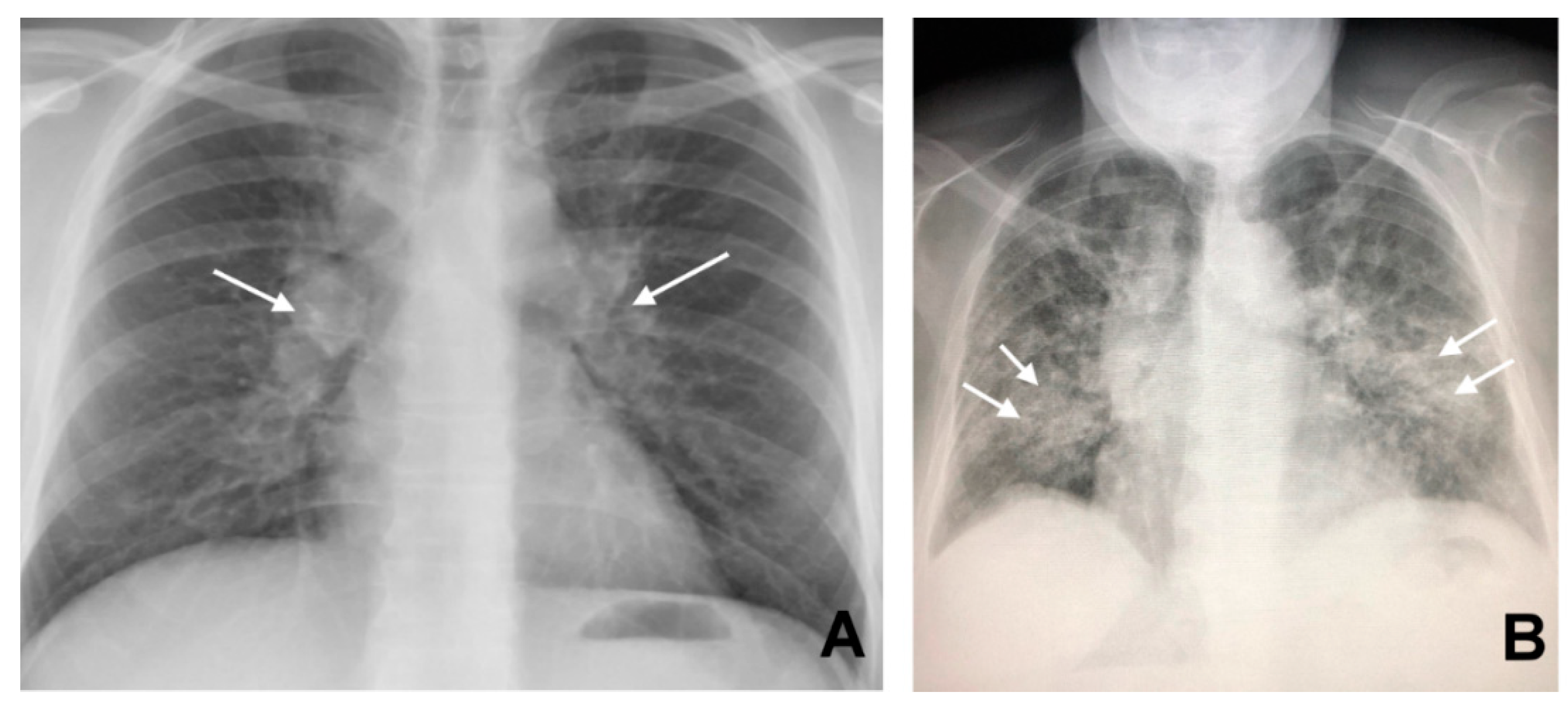
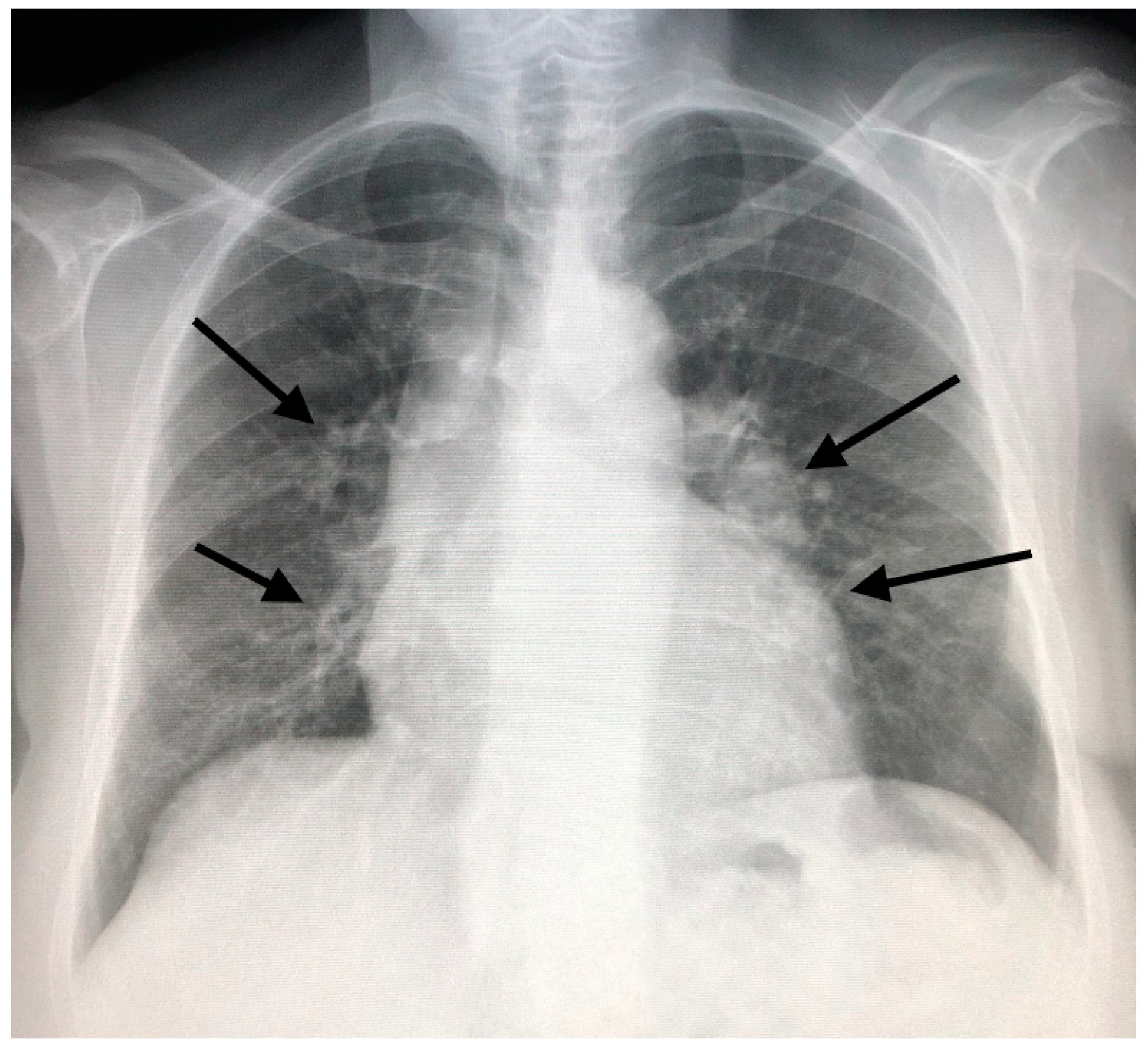
3. CT in Pulmonary Sarcoidosis, Imaging Findings and Classification
The Integration of 18-Fluoro-Deoxyglucose (FDG) Positron Emission Computed Tomography (PET/CT)
4. CT Features of Abdominal Sarcoidosis
4.1. The Involvement of Liver, Spleen and Abdominal Lymph Nodes
4.2. Gastrointestinal Tract, Peritoneum, and Pancreas
4.3. Renal Involvement from Sarcoidosis
5. Clinical Issues about the Extrapulmonary Involvement and Integrated Approaches for the Diagnosis of Sarcoidosis
5.1. Suspected Pulmonary Sarcoidosis
5.2. Screening for Extrapulmonary (Abdominal) Disease
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Judson, M.A. Environmental Risk Factors for Sarcoidosis. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Yadav, D.; Puranik, N.; Guleria, R.; Jin, J.-O. Sarcoidosis: Causes, Diagnosis, Clinical Features, and Treatments. J. Clin. Med. 2020, 9, 1081. [Google Scholar] [CrossRef]
- Tana, C.; Giamberardino, M.; Di Gioacchino, M.; Mezzetti, A.; Schiavone, C. Immunopathogenesis of Sarcoidosis and Risk of Malignancy: A Lost Truth? Int. J. Immunopathol. Pharm. 2013, 26, 305–313. [Google Scholar] [CrossRef] [Green Version]
- James, D.G. Sarcoidosis. Postgrad. Med. J. 1984, 60, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Iannuzzi, M.C.; Rybicki, B.A.; Teirstein, A.S. Sarcoidosis. N. Engl. J. Med. 2007, 357, 2153–2165. [Google Scholar] [CrossRef]
- Tana, C.; Tchernev, G.; Chokoeva, A.A.; Wollina, U.; Lotti, T.; Fioranelli, M.; Roccia, M.G.; Maximov, G.K.; Silingardi, M. Pulmonary and abdominal sarcoidosis, the great imitators on imaging? J. Boil. Regul. Homeost. Agents 2016, 30 (Suppl. 2), 45–48. [Google Scholar]
- Tchernev, G.; Chokoeva, A.A.; Tana, C.; Patterson, J.W.; Wollina, U.; Lotti, T. Sarcoid sine sarcoidosis? A classificative, semantic and therapeutic dilemma. J. Boil. Regul. Homeost. Agents 2015, 29 (Suppl. 2), 33–34. [Google Scholar]
- Chokoeva, A.A.; Tchernev, G.; Tana, M.; Tana, C. Exclusion criteria for sarcoidosis: A novel approach for an ancient disease? Eur. J. Intern. Med. 2014, 25, e120. [Google Scholar] [CrossRef] [PubMed]
- Vahid, B.; Spodik, M.; Braun, K.N.; Ghazi, L.J.; Esmaili, A. Sarcoidosis of Gastrointestinal Tract: A Rare Disease. Dig. Dis. Sci. 2007, 52, 3316–3320. [Google Scholar] [CrossRef] [PubMed]
- Tana, C.; Dietrich, C.F.; Schiavone, C. Hepatosplenic Sarcoidosis: Contrast-Enhanced Ultrasound Findings and Implications for Clinical Practice. Biomed. Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Rubio-Rivas, M.; Corbella, X. Clinical phenotypes and prediction of chronicity in sarcoidosis using cluster analysis in a prospective cohort of 694 patients. Eur. J. Intern. Med. 2020, 77, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Scadding, J.G. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years observation. Br. Med. J. 1961, 2, 1165–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, M.; Lee, K.S.; Itoh, H.; Hatabu, H. The spectrum of pulmonary sarcoidosis: Variations of high-resolution CT findings and clues for specific diagnosis. Eur. J. Radiol. 2010, 73, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Criado, E.; Sánchez, M.; Ramírez, J.; Arguis, P.; De Caralt, T.M.; Perea, R.J.; Xaubet, A. Pulmonary Sarcoidosis: Typical and Atypical Manifestations at High-Resolution CT with Pathologic Correlation 1. Radiographics 2010, 30, 1567–1586. [Google Scholar] [CrossRef] [Green Version]
- Raoof, S.; Amchentsev, A.; Vlahos, I.; Goud, A.; Naidich, D.P. Pictorial essay: Multinodular disease: A high-resolution CT scan diagnostic algorithm. Chest 2006, 129, 805–815. [Google Scholar] [CrossRef]
- Nunes, H.; Uzunhan, Y.; Gille, T.; Lamberto, C.; Valeyre, D.; Brillet, P.-Y. Imaging of sarcoidosis of the airways and lung parenchyma and correlation with lung function. Eur. Respir. J. 2012, 40, 750–765. [Google Scholar] [CrossRef]
- Guidry, C.; Fricke, R.G.; Ram, R.; Pandey, T.; Jambhekar, K. Imaging of Sarcoidosis. Radiol. Clin. N. Am. 2016, 54, 519–534. [Google Scholar] [CrossRef]
- Honda, O.; Johkoh, T.; Ichikado, K.; Yoshida, S.; Mihara, N.; Higashi, M.; Tomiyama, N.; Maeda, M.; Hamada, S.; Naito, H.; et al. Comparison of High Resolution CT Findings of Sarcoidosis, Lymphoma, and Lymphangitic Carcinoma: Is There Any Difference of Involved Interstitium? J. Comput. Assist. Tomogr. 1999, 23, 374–379. [Google Scholar] [CrossRef]
- Gross, B.; Schneider, H.; Proto, A. Eggshell calcification of lymph nodes: An update. Am. J. Roentgenol. 1980, 135, 1265–1268. [Google Scholar] [CrossRef]
- Geerts, S.; Wuyts, W.; Langhe, E.; Lenaerts, J.; Yserbyt, J. Connective tissue disease associated interstitial pneumonia: A challenge for both rheumatologists and pulmonologists. Sarcoidosis Vasc. Diffuse Lung Dis. 2017, 34, 326–335. [Google Scholar]
- Gawne-Cain, M.; Hansell, D. The pattern and distribution of calcified mediastinal lymph nodes in sarcoidosis and tuberculosis: A CT study. Clin. Radiol. 1996, 51, 263–267. [Google Scholar] [CrossRef]
- Arar, O.; Boni, F.; Meschi, T.; Tana, C. Pulmonary Sarcoidosis Presenting with Miliary Opacities. Curr. Med. Imaging Rev. 2019, 15, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Abehsera, M.; Valeyre, D.; Grenier, P.; Jaillet, H.; Battesti, J.P.; Brauner, M.W. Sarcoidosis with Pulmonary Fibrosis. Am. J. Roentgenol. 2000, 174, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Ziora, D.; Jastrzębski, D.; Łukasz, L. Advances in diagnosis of pulmonary sarcoidosis. Pneumonol. I Alergol. Pol. 2012, 80, 355–364. [Google Scholar]
- Heuvel, D.A.F.V.D.; De Jong, P.A.; Zanen, P.; Van Es, H.W.; Van Heesewijk, J.P.; Spee, M.; Grutters, J.C. Chest Computed Tomography-Based Scoring of Thoracic Sarcoidosis: Inter-rater Reliability of CT Abnormalities. Eur. Radiol. 2015, 25, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.L.; Wells, A.U.; Sverzellati, N.; Keir, G.J.; Calandriello, L.; Antoniou, K.M.; Copley, S.J.; Devaraj, A.; Maher, T.M.; Renzoni, E.A.; et al. An integrated clinicoradiological staging system for pulmonary sarcoidosis: A case-cohort study. Lancet Respir. Med. 2014, 2, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Pena, T.; Soubani, A.O.; Samavati, L. Aspergillus Lung Disease in Patients with Sarcoidosis: A Case Series and Review of the Literature. Lung 2011, 189, 167–172. [Google Scholar] [CrossRef]
- Tana, C. FDG-PET Imaging in Sarcoidosis. Curr. Med. Imaging Former. Curr. Med. Imaging Rev. 2019, 15, 2–3. [Google Scholar] [CrossRef]
- Ricci, F.; Mantini, C.; Grigoratos, C.; Bianco, F.; Bucciarelli, V.; Tana, C.; Mastrodicasa, D.; Caulo, M.; Aquaro, G.D.; Cotroneo, A.R.; et al. The Multi-modality Cardiac Imaging Approach to Cardiac Sarcoidosis. Curr. Med. Imaging Rev. 2019, 15, 10–20. [Google Scholar] [CrossRef]
- Mañá, J.; Salazar, A.; Manresa, F. Clinical Factors Predicting Persistence of Activity in Sarcoidosis: A Multivariate Analysis of 193 Cases. Respiration 1994, 61, 219–225. [Google Scholar] [CrossRef]
- Tana, M.; Di Carlo, S.; Romano, M.; Alessandri, M.; Schiavone, C.; Montagnani, A. FDG-PET/CT Assessment of Pulmonary Sarcoidosis: A Guide for Internists. Curr. Med. Imaging Rev. 2019, 15, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Mostard, R.L.; Van Kroonenburgh, M.J.; Drent, M. The role of the PET scan in the management of sarcoidosis. Curr. Opin. Pulm. Med. 2013, 19, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Tchernev, G.; Tana, C.; Schiavone, C.; Cardoso, J.-C.; Ananiev, J.; Wollina, U. Sarcoidosis vs. Sarcoid-like reactions: The Two Sides of the same Coin? Wien. Med. Wochenschr. 2014, 164, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Gezer, N.S.; Başara, I.; Altay, C.; Harman, M.; Rocher, L.; Karabulut, N.; Seçil, M. Abdominal sarcoidosis: Cross-sectional imaging findings. Diagn Interv. Radiol. 2015, 21, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Karagiannidis, A.; Karavalaki, M.; Koulaouzidis, A. Hepatic sarcoidosis. Ann. Hepatol. 2006, 5, 251–256. [Google Scholar] [CrossRef]
- Sedki, M.; Fonseca, N.; Santiago, P.; Diaz, L.; Garcia-Buitrago, M.; Mirsaeidi, M. Levy C. Hepatic Sarcoidosis: Natural History and Management Implications. Front. Med. 2019, 6, 232. [Google Scholar] [CrossRef] [PubMed]
- Fetzer, D.T.; Rees, M.A.; Dasyam, A.K.; Tublin, M.E. Hepatic sarcoidosis in patients presenting with liver dysfunction: Imaging appearance, pathological correlation and disease evolution. Eur. Radiol. 2016, 26, 3129–3137. [Google Scholar] [CrossRef]
- Tadros, M.; Forouhar, F.; George, Y. Wu Hepatic Sarcoidosis. J. Clin. Transl. Hepatol. 2013, 1, 87–93. [Google Scholar]
- Palmucci, S.; Torrisi, S.; Caltabiano, D.C.; Puglisi, S.; Lentini, V.; Grassedonio, E.; Vindigni, V.; Reggio, E.; Giuliano, R.; Micali, G.; et al. Clinical and radiological features of extra-pulmonary sarcoidosis: A pictorial essay. Insights Into Imaging 2016, 7, 571–587. [Google Scholar] [CrossRef] [Green Version]
- Warshauer, D.M.; Lee, J.K.T. Imaging Manifestations of Abdominal Sarcoidosis. Am. J. Roentgenol. 2004, 182, 15–28. [Google Scholar] [CrossRef]
- Folz, S.J.; Johnson, C.D.; Swensen, S.J. Abdominal Manifestations of Sarcoidosis in CT Studies. J. Comput. Assist. Tomogr. 1995, 19, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Warshauer, D.M.; Molina, P.L.; Hamman, S.M.; Koehler, R.E.; Paulson, E.K.; Bechtold, R.E.; Perlmutter, M.L.; Hiken, J.N.; Francis, I.R.; Cooper, C.J. Nodular sarcoidosis of the liver and spleen: Analysis of 32 cases. Radiology 1995, 195, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Souto, M.M.; Tempes, B.C.; Lambert, B.F.; Trindade, E.N.; Trindade, M.R.M. Laparoscopic Splenectomy for Isolated Splenic Sarcoidosis. JSLS J. Soc. Laparoendosc. Surg. 2014, 18, 155–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britt, A.R.; Francis, I.R.; Glazer, G.M.; Ellis, J.H. Sarcoidosis: Abdominal manifestations at CT. Radiology 1991, 178, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Ali, M.A.; Borum, M.L. Gastric Sarcoidosis: A Case Report and Review of the Literature. South. Med. J. 2007, 100, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Chinitz, M.A.; Brandt, L.J.; Frank, M.S.; Frager, D.; Sablay, L. Symptomatic sarcoidosis of the stomach. Dig. Dis. Sci. 1985, 30, 682–688. [Google Scholar] [CrossRef]
- Erra, P.; Crusco, S.; Nugnes, L.; Pollio, A.M.; Di Pilla, G.; Biondi, G.; Vigliardi, G. Colonic sarcoidosis: Unusual onset of a systemic disease. World J. Gastroenterol. 2015, 21, 3380–3387. [Google Scholar] [CrossRef]
- Zissin, R.; Gayer, G.; Bernheim, J.; Kots, E.; Shapiro-Feinberg, M.; Hertz, M. Granulomatous appendicitis presenting as right lower quadrant pain: CT findings. Abdom. Imaging 2003, 28, 280–283. [Google Scholar] [CrossRef]
- Nicolini, A.; Vita, M.; Lanata, S. Peritoneal sarcoidosis: An unusual presentation and a brief review of the literature. Monaldi Arch. Chest Dis. 2011, 75. [Google Scholar] [CrossRef]
- Gorkem, U.; Gungor, T.; Bas, Y.Y.; Toğrul, C. Abdominal Sarcoidosis May Mimic Peritoneal Carcinomatosis. Case Rep. Obs. Gynecol. 2015, 2015, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Chokoeva, A.A.; Tchernev, G.; Tana, C.; Ananiev, J.; Wollina, U. Sarcoid-like pattern in a patient with tuberculosis. J. Boil. Regul. Homeost. Agents 2015, 28, 783–788. [Google Scholar]
- MacArthur, K.L.; Forouhar, F.; Wu, G.Y. Intra-abdominal Complications of Sarcoidosis. J. Med. Assoc. 2010, 109, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Shukla, M.; Hassan, M.F.; Toor, V.; Kaur, J.; Solomon, C.; Cohen, H. Symptomatic pancreatic sarcoidosis. Case report and review of literature. JOP J. Pancreas 2007, 8, 770–774. [Google Scholar]
- Roudenko, A.; Murillo, P.; Akers, S.; Koo, C.W. Renal sarcoid: Pseudotumoral radiologic manifestations and pathologic correlation. Radiol. Case Rep. 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Heldmann, M.; Behm, W.; Reddy, M.P.; Bozeman, C.; Welman, G.; Abreo, F.; Minagar, A. Pseudotumoral Renal Sarcoid: MRI, PET, and MDCT Appearance with Pathologic Correlation. Am. J. Roentgenol. 2005, 185, 697–699. [Google Scholar] [CrossRef]
- Akaike, G.; Itani, M.; Shah, H.; Ahuja, J.; Gunes, B.Y.; Assaker, R.; Behnia, S. PET/CT in the Diagnosis and Workup of Sarcoidosis: Focus on Atypical Manifestations. Radiographics 2018, 38, 1536–1549. [Google Scholar] [CrossRef] [Green Version]
- Statement on Sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) Adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am. J. Respir. Crit. Care Med. 1999, 160, 736–755. [Google Scholar] [CrossRef]
- Judson, M.A.; Baughman, R.P.; Teirstein, A.S.; Terrin, M.L.; Yeager, H. Defining organ involvement in sarcoidosis: The ACCESS proposed instrument. ACCESS Research Group. A Case Control Etiologic Study of Sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. Wasog 1999, 16, 75–86. [Google Scholar]
- Judson, M.A.; Costabel, U.; Drent, M.; Wells, A.U.; Maier, L.A.; Koth, L.; Shigemitsu, H.; A Culver, D.; Gelfand, J.; Valeyre, D.; et al. The WASOG Sarcoidosis Organ Assessment Instrument: An update of a previous clinical tool. Sarcoidosis Vasc. Diffus. Lung Dis. 2014, 31, 19–27. [Google Scholar]
- Choy, G.; Khalilzadeh, O.; Michalski, M.; Synho, D.; Samir, A.E.; Pianykh, O.S.; Geis, J.R.; Pandharipande, P.V.; Brink, J.A.; Dreyer, K.J. Current Applications and Future Impact of Machine Learning in Radiology. Radiology 2018, 288, 318–328. [Google Scholar] [CrossRef]
- Kim, S.Y.; Diggans, J.; Pankratz, D.; Huang, J.; Pagán, M.; Sindy, N.; Tom, E.; Anderson, J.; Choi, Y.; A Lynch, D.; et al. Classification of usual interstitial pneumonia in patients with interstitial lung disease: Assessment of a machine learning approach using high-dimensional transcriptional data. Lancet Respir. Med. 2015, 3, 473–482. [Google Scholar] [CrossRef]
- Ungprasert, P.; Carmona, E.M.; Crowson, C.S.; Matteson, E.L. Diagnostic Utility of Angiotensin-Converting Enzyme in Sarcoidosis: A Population-Based Study. Lung 2015, 194, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Tana, C.; Tana, M.; Mezzetti, A.; Schiavone, C. Sarcoidosis: Old certainties and new perspectives. Ital. J. Med. 2012, 6, 186–194. [Google Scholar] [CrossRef]
- Eurelings, L.E.M.; Miedema, J.R.; Dalm, V.A.S.H.; Van Daele, P.L.A.; Van Hagen, P.M.; Van Laar, J.A.M.; Dik, W.A. Sensitivity and specificity of serum soluble interleukin-2 receptor for diagnosing sarcoidosis in a population of patients suspected of sarcoidosis. PLoS ONE 2019, 14, e0223897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crouser, E.D.; Maier, L.A.; Wilson, K.C.; Bonham, C.A.; Morgenthau, A.S.; Patterson, K.C.; Abston, E.; Bernstein, R.C.; Blankstein, R.; Chen, E.S.; et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 201, e26–e51. [Google Scholar] [CrossRef]
- Meyer, K.C.; Raghu, G.; Baughman, R.P.; Brown, K.K.; Costabel, U.; Du Bois, R.M.; Drent, M.; Haslam, P.L.; Kim, D.S.; Nagai, S.; et al. An Official American Thoracic Society Clinical Practice Guideline: The Clinical Utility of Bronchoalveolar Lavage Cellular Analysis in Interstitial Lung Disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1004–1014. [Google Scholar] [CrossRef]
- Tchernev, G.; Chokoeva, A.A.; Schiavone, C.; Erme, A.M.D.; Tana, C.; Darling, M.; Kaley, J.; Gianfaldoni, S.; Wollina, U.; Lotti, T.; et al. Sarcoidosis exclusion criteria: The “simple truth” for a “complicated diagnosis”. J. Boil. Regul. Homeost. Agents 2015, 29. [Google Scholar]
- Tana, C.; Iannetti, G.; Mezzetti, A.; Schiavone, C. Splenic sarcoidosis remains a diagnostic challenge. J. Clin. Ultrasound 2014, 42, 156. [Google Scholar] [CrossRef]
- Tchernev, G.; Cardoso, J.; Chokoeva, A.; Verma, S.; Tana, C.; Ananiev, J.; Gulubova, M.; Philipov, S.; Kanazawa, N.; Nenoff, P.; et al. The “Mystery” of Cutaneous Sarcoidosis: Facts and Controversies. Int. J. Immunopathol. Pharm. 2014, 27, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Tana, C.; Wegener, S.; Borys, E.; Pambuccian, S.; Tchernev, G.; Tana, M.; Giamberardino, M.A.; Silingardi, M. Challenges in the diagnosis and treatment of neurosarcoidosis. Ann. Med. 2015, 47, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Tana, C.; Iannetti, G.; D’Alessandro, P.; Tana, M.; Mezzetti, A.; Schiavone, C. Pitfalls of contrast-enhanced ultrasound (CEUS) in the diagnosis of splenic sarcoidosis. J. Ultrasound 2013, 16, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baughman, R.P.; Scholand, M.B.; Rahaghi, F.F. Clinical phenotyping: Role in treatment decisions in sarcoidosis. Eur. Respir. Rev. 2020, 29, 190145. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tana, C.; Donatiello, I.; Coppola, M.G.; Ricci, F.; Maccarone, M.T.; Ciarambino, T.; Cipollone, F.; Giamberardino, M.A. CT Findings in Pulmonary and Abdominal Sarcoidosis. Implications for Diagnosis and Classification. J. Clin. Med. 2020, 9, 3028. https://doi.org/10.3390/jcm9093028
Tana C, Donatiello I, Coppola MG, Ricci F, Maccarone MT, Ciarambino T, Cipollone F, Giamberardino MA. CT Findings in Pulmonary and Abdominal Sarcoidosis. Implications for Diagnosis and Classification. Journal of Clinical Medicine. 2020; 9(9):3028. https://doi.org/10.3390/jcm9093028
Chicago/Turabian StyleTana, Claudio, Iginio Donatiello, Maria Gabriella Coppola, Fabrizio Ricci, Marica Tina Maccarone, Tiziana Ciarambino, Francesco Cipollone, and Maria Adele Giamberardino. 2020. "CT Findings in Pulmonary and Abdominal Sarcoidosis. Implications for Diagnosis and Classification" Journal of Clinical Medicine 9, no. 9: 3028. https://doi.org/10.3390/jcm9093028
APA StyleTana, C., Donatiello, I., Coppola, M. G., Ricci, F., Maccarone, M. T., Ciarambino, T., Cipollone, F., & Giamberardino, M. A. (2020). CT Findings in Pulmonary and Abdominal Sarcoidosis. Implications for Diagnosis and Classification. Journal of Clinical Medicine, 9(9), 3028. https://doi.org/10.3390/jcm9093028







