Monocyte Chemoattractant Protein-1 Is an Independent Predictor of Coronary Artery Ectasia in Patients with Acute Coronary Syndrome
Abstract
1. Introduction
2. Methods
2.1. Patients and Study Design
2.2. Coronary Angiogram Analysis
2.3. Outcome and Follow-Up
2.4. Ethics Statement
2.5. Biomarker and Analytical Studies
2.6. Statistical Analysis
3. Results
3.1. Predictors of CAE
3.2. Prognostic Factors Associated with AAE during Follow-Up
4. Discussion
4.1. Predictors of CAE
4.2. CAE and Outcome
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antoniadis, A.P.; Chatzizisis, Y.S.; Giannoglou, G.D. Pathogenetic mechanisms of coronary ectasia. Int. J. Cardiol. 2008, 130, 335–343. [Google Scholar] [CrossRef]
- Giannoglou, G.D.; Antoniadis, A.P.; Chatzizisis, Y.S.; Damvopoulou, E.; Parcharidis, G.E.; Louridas, G.E. Prevalence of Ectasia in Human Coronary Arteries in Patients in Northern Greece Referred for Coronary Angiography. Am. J. Cardiol. 2006, 98, 314–318. [Google Scholar] [CrossRef]
- Markis, J.E.; Joffe, C.; Cohn, P.F.; Feen, D.J.; Herman, M.V.; Gorlin, R. Clinical significance of coronary arterial ectasia. Am. J. Cardiol. 1976, 37, 217–222. [Google Scholar] [CrossRef]
- Hartnell, G.G.; Parnell, B.M.; Pridie, R.B. Coronary artery ectasia. Its prevalence and clinical significance in 4993 patients. Br. Heart J. 1985, 54, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S. Coronary artery ectasia: From diagnosis to treatment. Hell. J. Cardiol. 2010, 51, 158–163. [Google Scholar]
- Swanton, R.H.; Thomas, M.L.; Coltart, D.J.; Jenkins, B.S.; Webb-Peploe, M.M.; Williams, B.T. Coronary artery ectasia—A variant of occlusive coronary arteriosclerosis. Heart 1978, 40, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Lamendola, C.L.; Culliford, A.T.; Harris, L.J.; Amendo, M.T. Multiple aneurysms of the coronary arteries in a patient with systemic aneurysmal disease. Ann. Thorac. Surg. 1990, 49, 1009–1010. [Google Scholar] [CrossRef]
- Papadakis, M.C.; Leontiadis, E.; Manginas, A.; Voudris, V.; Pavlides, G.; Karatasakis, G.; Foussas, S.G.; Mihalis, A.S.; Cokkinos, D.V. Frequency of coronary artery ectasia in patients undergoing surgery for ascending aortic aneurysms. Am. J. Cardiol. 2004, 94, 1433–1435. [Google Scholar] [CrossRef]
- Newburger, J.W.; Burns, J.C. Kawasaki disease. Vasc. Med. 1999, 4, 187–202. [Google Scholar] [CrossRef]
- Mattern, A.L.; Baker, W.P.; McHale, J.J.; Lee, D.E. Congenital coronary aneurysms with angina pectoris and myocardial infarction treated with saphenous vein bypass graft. Am. J. Cardiol. 1972, 30, 906–909. [Google Scholar] [CrossRef]
- Adiloglu, A.K.; Can, R.; Nazli, C.; Ocal, A.; Ergene, O.; Tinaz, G.; Kisioglu, N. Ectasia and severe atherosclerosis: Relationships with chlamydia pneumoniae, helicobacterpylori, and inflammatory markers. Tex. Heart Inst. J. 2005, 32, 21–27. [Google Scholar] [PubMed]
- Li, J.-J.; Nie, S.-P.; Qian, X.-W.; Zeng, H.-S.; Zhang, C.-Y. Chronic inflammatory status in patients with coronary artery ectasia. Cytokine 2009, 46, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.E.; Villatoro, M.A.R.; Marquez, R.; Cardoso-Saldaña, G.; Posadas-Sánchez, R.; Juárez-Orozco, L.E. Evaluation and Analysis of Plasma Soluble Adhesion Molecules in Patients with Coronary Ectasia and Atherosclerotic Coronary Artery Disease. Arch. Med. Res. 2014, 45, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Akyel, A.; Sahinarslan, A.; Kiziltunc, E.; Yıldız, U.; Alsancak, Y.; Akboga, M.K.; Yayla, Ç.; Topal, S.; Bukan, N.; Özdemir, M. Neutrophil Gelatinase-Associated Lipocalin Levels in Isolated Coronary Artery Ectasia. Can. J. Cardiol. 2011, 27, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Turhan, H.; Erbay, A.R.; Yasar, A.S.; Aksoy, Y.; Bicer, A.; Yetkin, G.; Yetkin, E. Plasma soluble adhesion molecules; intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and E-selectin levels in patients with isolated coronary artery ectasia. Coron. Artery Dis. 2005, 16, 45–50. [Google Scholar] [CrossRef]
- Demir, Ş.; Karakoyun, G.; Kanadasi, M. Elevated high sensitivity C-reactive protein and uric acid levels in coronary artery ectasia. Acta Biochim. Pol. 2014, 61. [Google Scholar] [CrossRef]
- Gu, L.; Okada, Y.; Clinton, S.K.; Gerard, C.; Sukhova, G.K.; Libby, P.; Rollins, B.J. Absence of Monocyte Chemoattractant Protein-1 Reduces Atherosclerosis in Low Density Lipoprotein Receptor–Deficient Mice. Mol. Cell 1998, 2, 275–281. [Google Scholar] [CrossRef]
- Gosling, J.; Slaymaker, S.; Gu, L.; Tseng, S.; Zlot, C.H.; Young, S.G.; Rollins, B.J.; Charo, I.F. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J. Clin. Investig. 1999, 103, 773–778. [Google Scholar] [CrossRef]
- Tuñón, J.; Blanco-Colio, L.M.; Cristóbal, C.; Tarín, N.; Higueras, J.; Huelmos, A.; Alonso, J.J.; Egido, J.; Asensio, D.; Lorenzo, Ó.; et al. Usefulness of a Combination of Monocyte Chemoattractant Protein-1, Galectin-3, and N-Terminal Probrain Natriuretic Peptide to Predict Cardiovascular Events in Patients With Coronary Artery Disease. Am. J. Cardiol. 2014, 113, 434–440. [Google Scholar] [CrossRef]
- uñón, J.; Cristóbal, C.; Vicente, M.N.T.; Aceña, Á.; Gonzalez-Casaus, M.L.; Huelmos, A.; Alonso, J.J.; Lorenzo, Ó.; González-Parra, E.; Mahíllo-Fernández, I.; et al. Coexistence of Low Vitamin D and High Fibroblast Growth Factor-23 Plasma Levels Predicts an Adverse Outcome in Patients with Coronary Artery Disease. PLoS ONE 2014, 9, e95402. [Google Scholar] [CrossRef]
- Demir, M.; Demir, C.; Keçeoğlu, S. The relationship between vitamin D deficiency and coronary artery ectasia. Adv. Interv. Cardiol. 2014, 10, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Egashira, K.; Ni, W.; Kitamoto, S.; Usui, M.; Otani, K.; Ishibashi, M.; Hiasa, K.-I.; Nishida, K.-I.; Takeshita, A. Anti-Monocyte Chemoattractant Protein-1 Gene Therapy Limits Progression and Destabilization of Established Atherosclerosis in Apolipoprotein E–Knockout Mice. Circulation 2002, 106, 2700–2706. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Presa, M.; Bustos, C.; Ortego, M.; Tuñon, J.; Renedo, G.; Ruiz-Ortega, M.; Egido, J. Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-kappa B activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation 1997, 95, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Bustos, C.; Hernández-Presa, M.A.; Ortego, M.; Tuñón, J.; Ortega, L.; Pérez, F.; Díaz, C.; Hernández, G.; Egido, J. HMG-CoA reductase inhibition by atorvastatin reduces neointimal inflammation in a rabbit model of atherosclerosis. J. Am. Coll. Cardiol. 1998, 32, 2057–2064. [Google Scholar] [CrossRef]
- De Lemos, J.A.; Morrow, D.A.; Sabatine, M.S.; Murphy, S.A.; Gibson, C.M.; Antman, E.M.; McCabe, C.H.; Cannon, C.P.; Braunwald, E. Association Between Plasma Levels of Monocyte Chemoattractant Protein-1 and Long-Term Clinical Outcomes in Patients with Acute Coronary Syndromes. Circulation 2003, 107, 690–695. [Google Scholar] [CrossRef]
- Boles, U.; Rakhit, R.; Shiu, M.F.; Patel, K.; Henein, M. Coronary artery ectasia as a culprit for acute myocardial infarction: Review of pathophysiology and management. Anadolu Kardiyol. Derg. Anatol. J. Cardiol. 2013, 13, 695–701. [Google Scholar] [CrossRef][Green Version]
- Dahhan, A. Coronary Artery Ectasia in Atherosclerotic Coronary Artery Disease, Inflammatory Disorders, and Sickle Cell Disease. Cardiovasc. Ther. 2015, 33, 79–88. [Google Scholar] [CrossRef]
- Sudhir, K.; Ports, T.A.; Amidon, T.M.; Goldberger, J.J.; Bhushan, V.; Kane, J.P.; Yock, P.; Malloy, M.J. Increased Prevalence of Coronary Ectasia in Heterozygous Familial Hypercholesterolemia. Circulation 1995, 91, 1375–1380. [Google Scholar] [CrossRef]
- Balin, M.; Celik, A.; Kobat, M.A. The association between soluble lectin-like oxidized low-density lipoprotein receptor-1 levels and patients with isolated coronary artery ectasia. J. Thromb. Thrombolysis 2012, 33, 239–245. [Google Scholar] [CrossRef]
- Naoumova, R.P.; Tosi, I.; Patel, D.; Neuwirth, C.; Horswell, S.; Marais, A.D.; Van Heyningen, C.; Soutar, A.K. Severe Hypercholesterolemia in Four British Families with the D374Y Mutation in the PCSK9 Gene. Arter. Thromb. Vasc. Boil. 2005, 25, 2654–2660. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Nozue, T. Lipid Lowering Therapy and Circulating PCSK9 Concentration. J. Atheroscler. Thromb. 2017, 24, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Parra, E.; Rojas-Rivera, J.; Tuñón, J.; Praga, M.; Ortiz, A.; Egido, J. Vitamin D receptor activation and cardiovascular disease. Nephrol. Dial. Transplant. 2012, 27, iv17–iv21. [Google Scholar] [CrossRef]
- Lavie, C.J.; Lee, J.H.; Milani, R.V. Vitamin D and Cardiovascular Disease. J. Am. Coll. Cardiol. 2011, 58, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- González-Parra, E.; Tuñón, J.; Egido, J.; Ortiz, A. Phosphate: A stealthier killer than previously thought? Cardiovasc. Pathol. 2012, 21, 372–381. [Google Scholar] [CrossRef]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.-C.; Sloan, A.; Isakova, T.; Gutierrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.D.; Schurgers, L.J.; Brandenburg, V.M.; Christenson, R.H.; Vermeer, C.; Ketteler, M.; Shlipak, M.G.; Whooley, M.A.; Ix, J.H. The Associations of Fibroblast Growth Factor 23 and Uncarboxylated Matrix Gla Protein with Mortality in Coronary Artery Disease: The Heart and Soul Study. Ann. Intern. Med. 2010, 152, 640–648. [Google Scholar] [CrossRef]
- Boles, U.; Eriksson, P.; Zhao, Y.; Henein, M.Y. Coronary artery ectasia: Remains a clinical dilemma. Coron. Artery Dis. 2010, 21, 318–320. [Google Scholar] [CrossRef]
- Huang, Q.-J.; Liu, J.; Chen, M.-H.; Li, J. Relation of diabetes to coronary artery ectasia: A meta-analysis study. Anadolu Kardiyol. Derg. Anatol. J. Cardiol. 2014, 14, 322–327. [Google Scholar] [CrossRef]
- Yip, H.-K.; Chen, M.-C.; Wu, C.-J.; Hang, C.-L.; Hsieh, K.Y.-K.; Fang, C.-Y.; Yeh, K.-H.; Fu, M. Clinical features and outcome of coronary artery aneurysm in patients with acute myocardial infarction undergoing a primary percutaneous coronary intervention. Cardiology 2002, 98, 132–140. [Google Scholar] [CrossRef]
- Doi, T.; Kataoka, Y.; Noguchi, T.; Shibata, T.; Nakashima, T.; Kawakami, S.; Nakao, K.; Fujino, M.; Nagai, T.; Kanaya, T.; et al. Coronary Artery Ectasia Predicts Future Cardiac Events in Patients With Acute Myocardial InfarctionHighlights. Arter. Thromb. Vasc. Boil. 2017, 37, 2350–2355. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, V.L.; Davis, M.J.; Bove, A.A. Current knowledge and significance of coronary artery ectasia: A chronologic review of the literature, recommendations for treatment, possible etiologies, and future considerations. Clin. Cardiol. 1998, 21, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Krüger, D.; Stierle, U.; Herrmann, G.; Simon, R.; Sheikhzadeh, A. Exercise induced myocardial ischemia in isolated coronary artery ectasias and aneurysms (“dilated coronopathy”). J. Am. Coll. Cardiol. 1999, 34, 1461–1470. [Google Scholar] [CrossRef]
| Characteristics | Global Population (n = 270) | Non-CAE-Group (n = 247) | CAE-Group (n = 23) | p-Value a |
|---|---|---|---|---|
| Age (years) | 65.0 (54.0–76.0) | 66.0 (54.0–77.0) | 63.0 (57.0–73.0) | 0.563 |
| Male sex (%) | 66.7 | 64.4 | 91.3 | 0.004 |
| Body mass index (kg/m2) | 28.0 (25.6–30.9) | 27.9 (25.1–30.7) | 29.0 (27.0–33.6) | 0.027 |
| Diabetes mellitus (%) | 20.7 | 21.1 | 17.4 | 0.794 |
| Smoker (past or present) (%) | 68.9 | 68.4 | 73.9 | 0.581 |
| Hypertension (%) | 70.0 | 69.6 | 73.9 | 0.665 |
| Dyslipidemia (%) | 50.0 | 47.0 | 82.6 | 0.001 |
| Peripheral artery disease (%) | 1.5 | 1.6 | 0.0 | 1.000 |
| Cerebrovascular events (%) | 5.6 | 6.1 | 0.0 | 0.625 |
| Atrial fibrillation (%) | 6.3 | 6.1 | 8.7 | 0.645 |
| LV ejection fraction | 0.60 (0.50–0.67) | 0.60 (0.50–0.68) | 0.57 (0.47–0.60) | 0.431 |
| STEMI (%)/NSTEACS (%) | 46.7/53.3 | 47.0/53.0 | 43.5/56.5 | 0.748 |
| Full revascularization (%) | 70.0 | 71.3 | 56.5 | 0.152 |
| SYNTAX score | 14 (7–23) | 14 (6–23) | 19.5 (11–24.5) | 0.082 |
| ASA (%) | 91.1 | 91.1 | 91.3 | 1.000 |
| Clopidogrel (%) | 74.8 | 74.9 | 73.9 | 0.917 |
| OAC (%) | 5.9 | 6.1 | 4.3 | 1.000 |
| Statins (%) | 93.0 | 93.1 | 91.3 | 0.670 |
| ACEI (%) | 69.3 | 70.0 | 60.9 | 0.371 |
| ARB (%) | 21.1 | 20.6 | 26.1 | 0.593 |
| Beta-blockers (%) | 70.7 | 69.6 | 82.6 | 0.171 |
| Hemoglobin (g/dL) | 14.3 (13.3–15.1) | 14.3 (13.3–15.1) | 14.6 (13.9–15.2) | 0.402 |
| Platelets count (per µL) | 239 (191–280) | 238 (191–279) | 250 (191–295) | 0.441 |
| Leucocytes count (per µL) | 7.2 (6.1–8.7) | 7.2 (6.0–8.6) | 7.8 (6.5–9.0) | 0.198 |
| % Neutrophils | 59.4 (52.3–64.7) | 59.4 (52.3–64.7) | 57.1 (51.0–65.2) | 0.746 |
| % Lymphocytes | 30.0 (24.1–36.3) | 30.1 (24.1–36.3) | 27.1 (24.1–36.9) | 0.912 |
| LDL cholesterol (mg/dL) | 75.5 (61.0–94.0) | 74.0 (61.0–93.0) | 87.0 (70.0–110.0) | 0.048 |
| HDL cholesterol (mg/dL) | 43.5 (36.0–51.0) | 44.0 (36.0–52.0) | 40.0 (35.0–48.0) | 0.339 |
| Triglycerides (mg/dL) | 102.0 (75.8–139.3) | 97.0 (72.0–137.0) | 137.0 (100.0–196.0) | 0.005 |
| GFR (mL/min/1.73 m2) | 75.4 (60.8–88.3) | 75.2 (60.2–87.5) | 80.9 (66.2–93.8) | 0.212 |
| Hs-CRP (mg/L) | 1.8 (0.8–3.8) | 1.7 (0.8–3.7) | 2.4 (1.4–4.7) | 0.154 |
| MCP-1 (pg/mL) | 142.5 (112.2–179.2) | 138.9 (111.9–176.9) | 170.1 (132.6–213.1) | 0.016 |
| NGAL (ng/mL) | 165.2 (127.4–219.2) | 164.2 (125.6–221.0) | 167.7 (130.1–191.7) | 0.982 |
| sTWEAK (pg/mL) | 198.1 (159.5–249.2) | 197.8 (156.3–248.3) | 206.8 (185.8–272.2) | 0.254 |
| Parathormone (pg/mL) | 65.2 (49.7–83.9) | 65.2 (50.2–84.3) | 64.1 (48.6–77.9) | 0.569 |
| Phosphate (mg/dL) | 3.3 (2.9–3.7) | 3.3 (2.9–3.7) | 3.3 (2.8–3.6) | 0.746 |
| FGF-23 (RU/mL) | 67.6 (54.3–88.3) | 68.1 (54.4–89.0) | 64.0 (41.8–87.2) | 0.370 |
| Calcidiol (ng/mL) | 18.4 (12.5–24.9) | 18.4 (12.7–25.1) | 18.4 (10.4–24.1) | 0.527 |
| PCSK-9 (ng/mL) | 52.1 (42.4–64.3) | 51.8 (42.0–64.3) | 54.5 (46.6–66.5) | 0.265 |
| Lp (a) (mg/dL) | 21.8 (7.0–48.4) | 21.6 (7.0–48.2) | 26.6 (12.9–67.1) | 0.219 |
| Variable | Univariate OR (95% CI) | p Value | Multivariate OR (95% CI) | p Value |
|---|---|---|---|---|
| Age | 0.99 (0.96–1.03) | 0.687 | - | - |
| Male gender | 5.81 (1.33–25.37) | 0.004 | 6.55 (1.40–30.73) | 0.003 |
| Hypertension | 1.24 (0.47–3.26) | 0.665 | - | - |
| Diabetes | 0.79 (0.26–2.42) | 0.673 | - | - |
| Dyslipidemia | 5.36 (1.77–16.23) | 0.001 | 7.39 (2.06–26.58) | <0.001 |
| Smoker | 1.31 (0.50–3.46) | 0.581 | - | - |
| BMI | 1.09 (1.01–1.17) | 0.037 | 1.08 (0.97–1.20) | 0.191 |
| LVEF | 0.99 (0.96–1.03) | 0.686 | - | - |
| ST-elevated ACS | 0.87 (0.37–2.06) | 0.748 | - | - |
| GFR | 1.02 (0.99–1.04) | 0.154 | 1.01 (0.98–1.03) | 0.581 |
| Hemoglobin | 1.11 (0.81–1.53) | 0.500 | - | - |
| Platelet count | 1.00 (0.99–1.01) | 0.577 | - | - |
| Leucocytes count | 1.13 (0.91–1.40) | 0.288 | - | - |
| Neutrophile (%) | 1.00 (0.96–1.04) | 0.892 | - | - |
| Lymphocyte (%) | 1.00 (0.95–1.05) | 0.937 | - | - |
| LDL 1 | 2.07 (1.07–4.02) | 0.040 | 1.94 (0.87–4.34) | 0.110 |
| HDL | 0.98 (0.94–1.02) | 0.331 | - | |
| Triglycerides 1 | 1.59 (1.19–2.13) | 0.003 | 1.18 (0.84–1.67) | 0.341 |
| Calcidiol | 0.96 (0.91–1.02) | 0.366 | - | - |
| Phosphate | 0.84 (0.39–1.81) | 0.657 | - | - |
| FGF-23 2 | 1.05 (0.80–1.38) | 0.721 | - | - |
| PTH | 1.00 (0.98–1.01) | 0.750 | - | - |
| MCP-1 3 | 1.74 (1.15–2.65) | 0.007 | 2.25 (1.35–3.76) | 0.001 |
| NGAL | 1.00 (0.99–1.01) | 0.648 | - | - |
| sTWEAK3 | 1.03 (0.82–1.30) | 0.796 | - | - |
| Hs-CRP | 1.01 (0.98–1.04) | 0.564 | - | - |
| PCSK-9 4 | 1.15 (0.92–1.43) | 0.234 | - | - |
| Lipoprotein (a) | 1.01 (0.99–1.03) | 0.205 | - | - |
| Variable | Univariate HR (95% CI) | p Value | Multivariate HR (95% CI) | p Value |
|---|---|---|---|---|
| Age | 1.02 (0.99–1.04) | 0.148 | 0.99 (0.96–1.04) | 0.958 |
| Male gender | 0.69 (0.37–1.29) | 0.248 | - | - |
| Hypertension | 2.57 (1.08–6.13) | 0.018 | 1.37 (0.53–3.53) | 0.502 |
| Diabetes | 1.24 (0.59–2.60) | 0.585 | - | - |
| Dyslipidemia | 1.45 (0.77–2.73) | 0.244 | - | - |
| Smoker | 0.54 (0.29–1.00) | 0.054 | 0.61 (0.28–1.33) | 0.217 |
| BMI | 1.05 (0.99–1.11) | 0.120 | 1.05 (0.98–1.11) | 0.209 |
| LVEF | 0.99 (0.97–1.02) | 0.691 | - | - |
| ST-elevated ACS | 0.61 (0.32–1.18) | 0.133 | 0.67 (0.33–1-37) | 0.268 |
| PVD | 0.67 (0.25–1.80) | 0.477 | - | - |
| Stroke | 1.83 (0.65–5.16) | 0.291 | - | - |
| Atrial fibrillation | 0.78 (0.46–1.30) | 0.369 | - | - |
| Full revascularization | 0.50 (0.27–0.93) | 0.033 | 0.65 (0.29–1.45) | 0.293 |
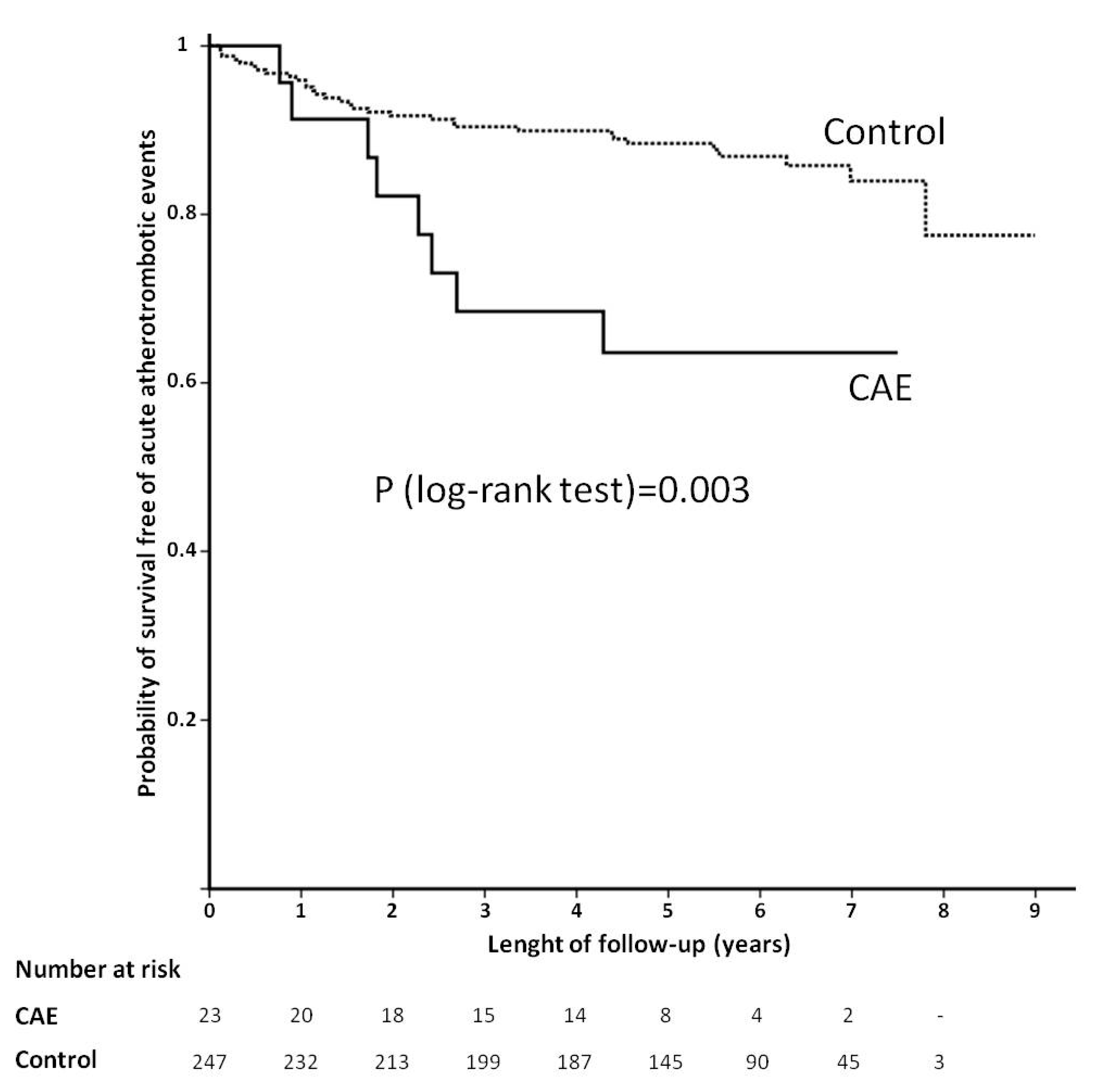
| Coronary artery ectasia | 3.08 (1.41–6.71) | 0.012 | 2.82 (1.29–6.15) | 0.019 |
| Syntax Score | 1.04 (1.01–1.06) | 0.009 | 1.04 (1.01–1.06) | 0.006 |
| Clopidogrel | 1.05 (0.74–1.49) | 0.788 | - | - |
| VKA | 0.70 (0.42–1.18) | 0.224 | - | - |
| Statins | 1.29 (0.80–2.06) | 0.324 | - | - |
| Betablockers | 0.84 (0.58–1.20) | 0.315 | - | - |
| ACEI/ARB | 1.24 (0.82–1.86) | 0.332 | - | - |
| PPI | 0.53 (0.33–0.85) | 0.002 | 0.56 (0.35–0.89) | 0.006 |
| GFR | 0.99 (0.97–1.00) | 0.082 | 1.00 (0.97–1.02) | 0.714 |
| Hemoglobin | 0.85 (0.69–1.05) | 0.138 | 1.01 (0.77–1.33) | 0.928 |
| Platelet count 1 | 1.73 (1.10–2.71) | 0.021 | 1.68 (1.04–2.71) | 0.038 |
| Leucocytes count | 1.06 (0.90–1.24) | 0.474 | - | - |
| LDL 2 | 1.33 (0.80–2.23) | 0.289 | - | - |
| HDL | 0.98 (0.95–1.01) | 0.132 | 0.98 (0.94–1.01) | 0.123 |
| Triglycerides 2 | 1.23 (0.98–1.54) | 0.088 | 0.93 (0.68–1.26) | 0.623 |
| Calcidiol | 0.97 (0.93–1.01) | 0.110 | 0.98 (0.94–1.03) | 0.403 |
| Phosphate | 1.17 (0.65–2.08) | 0.603 | - | - |
| FGF-23 3 | 1.13 (0.99–1.28) | 0.124 | 1.06 (0.90–1.24) | 0.510 |
| PTH | 1.01 (0.99–1.02) | 0.098 | 1.01 (0.99–1.02) | 0.426 |
| MCP-1 4 | 1.25 (0.95–1.66) | 0.168 | 0.96 (0.66–1.38) | 0.801 |
| NGAL | 1.00 (0.99–1.01) | 0.496 | - | - |
| Hs-CRP | 0.99 (0.96–1.03) | 0.496 | - | - |
| PCSK-9 5 | 0.92 (0.76–1.12) | 0.394 | - | - |
| Lipoprotein (a) | 1.00 (0.99–1.01) | 0.846 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco-Peláez, J.A.; Martín-Reyes, R.; Pello-Lázaro, A.M.; Aceña, Á.; Lorenzo, Ó.; Martín-Ventura, J.L.; Blanco-Colio, L.; González-Casaus, M.L.; Hernández-González, I.; Carda, R.; et al. Monocyte Chemoattractant Protein-1 Is an Independent Predictor of Coronary Artery Ectasia in Patients with Acute Coronary Syndrome. J. Clin. Med. 2020, 9, 3037. https://doi.org/10.3390/jcm9093037
Franco-Peláez JA, Martín-Reyes R, Pello-Lázaro AM, Aceña Á, Lorenzo Ó, Martín-Ventura JL, Blanco-Colio L, González-Casaus ML, Hernández-González I, Carda R, et al. Monocyte Chemoattractant Protein-1 Is an Independent Predictor of Coronary Artery Ectasia in Patients with Acute Coronary Syndrome. Journal of Clinical Medicine. 2020; 9(9):3037. https://doi.org/10.3390/jcm9093037
Chicago/Turabian StyleFranco-Peláez, Juan Antonio, Roberto Martín-Reyes, Ana María Pello-Lázaro, Álvaro Aceña, Óscar Lorenzo, José Luis Martín-Ventura, Luis Blanco-Colio, María Luisa González-Casaus, Ignacio Hernández-González, Rocío Carda, and et al. 2020. "Monocyte Chemoattractant Protein-1 Is an Independent Predictor of Coronary Artery Ectasia in Patients with Acute Coronary Syndrome" Journal of Clinical Medicine 9, no. 9: 3037. https://doi.org/10.3390/jcm9093037
APA StyleFranco-Peláez, J. A., Martín-Reyes, R., Pello-Lázaro, A. M., Aceña, Á., Lorenzo, Ó., Martín-Ventura, J. L., Blanco-Colio, L., González-Casaus, M. L., Hernández-González, I., Carda, R., Martín-Mariscal, M. L., Egido, J., & Tuñón, J. (2020). Monocyte Chemoattractant Protein-1 Is an Independent Predictor of Coronary Artery Ectasia in Patients with Acute Coronary Syndrome. Journal of Clinical Medicine, 9(9), 3037. https://doi.org/10.3390/jcm9093037








