Abstract
Strigahermonthica (Del.) Benth is a parasitic weed that devastates cereals in Sub-Saharan Africa. Several control measures have been proposed for the parasite, of these, host plant resistance is considered the most cost-effective for poor farmers. Some tolerant/resistant lines have been developed and these lines display tolerance/resistance mechanisms to the parasite. A series of studies was done to investigate some of the mechanisms through which a resistant (TZISTR1108) and a susceptible (5057) maize line responds to S. hermonthica infestation, as well as the effects of parasitism on these lines. In this study, TZISTR1108 stimulated the germination and attachment of fewer S. hermonthica plants than 5057, both in the laboratory and on the field. In TZISTR1108, the growth of the S. hermonthica plants, that successfully attached, was slowed. When compared to the un-infested plants, the infested resistant plants showed fewer effects of parasitism than the infested susceptible plants. The infested TZISTR1108 plants were more vigorous, taller and resembled their un-infected counterparts. There were substantial reductions in the stomatal conductance and nitrogen content of the 5057 upon infestation. The resistant inbred line showed multiple mechanisms of resistance to S. hermonthica infestation. It thrives better than the susceptible line by reducing the attachment of S. hermonthica and it delays the parasite’s development.
1. Introduction
Striga hermonthica (Del.) Benth is a parasitic weed that has a deleterious impact on the growth and performance of all its grass (Gramineae (Poaceae)) hosts. These hosts include maize (Zea mays L.), sorghum [Sorghum bicolour (L.) Moench], sugar cane (Saccharum officinarum L.) and rice (Oryza sativa L) [1]. A single S. hermonthica plant can inflict approximately 5% loss in yield on a host plant [2], and high infestations can lead to total crop failure. The parasitic weed is very fecund, with a single plant producing up to 10,000–200,000 tiny seeds with very high viability under optimal conditions [2,3]. Thus, leading to high numbers of S. hermonthica seeds in the soil seed bank in areas where the plant is endemic. The growth and development of maize are negatively impacted by S. hermonthica, and this impact has been attributed to a combination of factors. These factors include resource withdrawal, changes in host allometry and a low rate of canopy carbon fixation in infected hosts [4]. The host plant loses water, carbon and nitrogen to the attached parasite, while experiencing elevated respiratory demand and a higher incidence of self-shading [5]. Striga hermonthica-infected plants have lower total biomass accumulation, lower grain yield and are noticeably shorter (due to lower internode elongation) than their un-infected counterparts [6,7]. In addition to lower biomass accumulation, the pattern of biomass partitioning in infected hosts is often disrupted such that biomass is preferentially allocated to the roots of the host rather than to the shoots. The infested host show reduction in photosynthesis and, chlorotic blotching and wilting of its foliage [8]. Even before the parasite emerges from the soil, the deleterious effects of the parasite can be apparent [8]. The severity of the impact of S. hermonthica on its hosts depends on the host and parasite lines, host nutrition, infection time and infection density [9].
Control measures currently being applied against S. hermonthica include crop rotation, intercropping, herbicide treatment [10] use of high nitrogen fertilizer, uprooting by hand [11], planting of catch and trap crops [12], herbicide seed dressing [13] and growing resistant/tolerant varieties [14,15]. However, when used individually, these methods are not wholly effective in controlling S. hermonthica infestations [1]. It is therefore necessary to combine some of these different methods while acknowledging their technical and practical limitations [3]. For any integrated Striga control strategy to be effective, host crop varieties that are tolerant or resistant to the parasite must be a component of the control strategy, which must then be adapted to the local farming system [16]. These host crop varieties that are tolerant or resistant to Striga are the most practical S. hermonthica control approach for resource-poor smallholder farmers [16].
In general terms, tolerance is defined as the potential of a host plant to function optimally in the presence of attached parasites. Resistance relates to the potential of a host to prevent the parasite from forming viable attachments to its roots [11]. Resistance by hosts can take different forms. These forms may involve both generic and specialized defense mechanisms mobilized simultaneously or independently, to disrupt important points during the parasite’s growth cycle [17]. Host plant resistance mechanisms are broadly divided into pre-attachment and post-attachment resistance [18,19]. Pre-attachment resistance is seen in a lack of or a decrease in the generation of germination stimulants, inhibition of germination, haustorium formation inhibition or reduction, partial inhibition of haustorium development, and formation of mechanical barriers to infection [18]. Post attachment resistance occurs following the successful connection of the parasite to the vascular system of the host. According to [18], post-attachment resistance can take several forms including abiosis and hypersensitive responses. Reports have shown that resistance to S. hermonthica in maize could be expressed through low stimulation of Striga seed germination, low haustorial induction, avoidance through root architecture, escape by early maturity, resistance to attachment, and failure to support attached parasites (incompatibility) [20].
Resistance to S. hermonthica is considered an important trait for maize cultivars that are specifically developed for Striga infested areas. Some potential sources of plants with resistance to S. hermonthica have been identified, including tropical and temperate maize inbred lines, African landrace collections, and Zea diploperennis-derived (a wild relative of maize) maize lines [21]. Under S. hermonthica infestation, these resistant maize plants consistently produced higher grain yields, supported fewer emerged parasites, and sustained minimal parasite damage across locations and seasons, when compared to susceptible maize plants [21]. Striga hermonthica infestation causes a plethora of deleterious effects in its host. These effects are expected to be reduced in the resistant plant when compared to those in the susceptible plant. This study investigated the responses of a resistant and a susceptible maize line to S. hermonthica parasitism.
2. Material and Methods
2.1. Plant Materials
The seeds of the two maize lines, TZISTR1108 (a resistant Zea diploperennis-derived maize line) and 5057 (susceptible), were obtained from the International Institute of Tropical Agriculture’s (IITA) maize improvement program. Detailed descriptions of the genetic background and development of TZISTR1108 have been published [22,23]. The S. hermonthica seeds used for this study were collected from locations within northern Nigeria and bulked. Both the maize and striga seeds were surface sterilized by immersion in 1% sodium hypochlorite solution containing three drops of Tween-20 (to break the surface tension) for five minutes. The surface-sterilized seeds were then rinsed four times with double-distilled water to remove the chlorine. The surface-sterilized S. hermonthica seeds were transferred into a Petri dish lined with 90 mm Whatman glass microfiber filter paper, and the filter paper was moistened about with two ml of distilled water. The Petri dish was wrapped with aluminum foil and kept in the dark at 28 °C for 20 days for preconditioning.
2.2. Preparation of Long-Ashton Solution
The Long Ashton solution was prepared to provide the plants with one mM NH4NO3 to ensure that S. hermonthica germination and attachment were not impeded by excessive nitrogen. Stock solutions of the macronutrients were prepared mixed at the concentrations listed in Supplementary Table S1. Working solutions were then prepared by mixing appropriate amounts of the stock solutions with water in a 140 L drum. The resulting solution was used to feed the plants throughout the experiments.
2.3. Germination-Inducing Activity of Root Exudates
The germination inducing ability of exudates from the roots of the maize plants was tested in two experiments: A test tube and a pot experiment.
2.3.1. Test Tube Experiment
Surface-sterilized seeds from the TZISTR1108 and 5057 maize lines were germinated in a Petri dish that was lined with moistened filter paper. Five previously germinated seeds of both lines were each fixed in a felt plug and suspended in a test tube that was filled with 45 mL of nutrient solution (40% Long Ashton solution containing 1 mol m−3 ammonium nitrate) prepared as described in Section 2.2. The tube was wrapped with aluminum foil and kept in the screen house and the nutrient solution topped up as was required. After 30 days, the nutrient solution was replaced with 30 mL of distilled water for 48 h. The maize plants were then gently removed from the tubes, and the solution was transferred into Falcon tubes to the 30 mL mark. The roots of the maize plants were removed, oven-dried, and weighed to determine the dry weight of the roots of each plant. The solution containing maize root exudates were pipetted into Petri dishes with 100 preconditioned Striga hermonthica seeds. The Petri dishes were wrapped in aluminum foil and kept in a controlled environment (incubator) for 72 h at 37 °C. The number of S. hermonthica seeds that had germinated after this time was counted under a microscope.
2.3.2. Pot Experiment
Seeds from the maize lines were planted in pots, measuring 10 cm in diameter and 9.5 cm in height, the pots were filled with washed river sand. The plants were maintained in two stands per pot with five replicates per variety in a screen house. On the 14th day after planting root exudates were collected from each pot. This was done by depriving the plants of water for 24 h and then passing 100 mL of water through the sand per pot. Sterile Petri dishes were lined with a glass microfiber filter paper, and approximately 100 Striga seeds were transferred to the surface of each filter paper, followed by 10 mL root exudate. The Petri dishes were wrapped with aluminum foil, incubated at 37 °C in an incubator, and examined after eleven days for seeds with elongated radicles
2.4. Root Architecture Evaluation
Both lines were grown in test tubes as described in Section 2.3.1. At fifty-three days after planting, the plants were removed from the test tubes, and the roots were examined. The number and the lengths of all the major roots (crown, seminal and primary roots) of each plant were taken. The lengths were measured with a measuring tape (a major root was defined as any root that originates from the base of the plant). Furthermore, the two maize lines were planted in pots, measuring 38 cm in diameter and 45 cm in height, in a screen house, in replicates of five infested and five un-infested pots. At 28 days after planting, all the soil was carefully washed off the plant roots, and the lengths of all the major roots on each plant were taken using a measuring tape. The total major root length was determined by summing the lengths of the individual roots.
2.5. Rhizotron Studies
Two surface-sterilized maize seeds of either of the two maize lines (5057 and TZISTR1108) were placed in each individual table-top rhizotron (constructed as described in Supplementary File S1), on the black cotton fabric at a depth of approximately 5 cm. Upon germinating, they were thinned to one plant per rhizotron. The maize lines were infested by using a brush to carefully spread 2 g (about 10,000 seeds) of wet S. hermonthica seeds all over the rhizotron surface and especially on the exposed roots of the maize plants. Each rhizotron was fed with 125 mL of nutrient solution (40% Long Ashton solution prepared as described in Section 2.2) two times every day to provide a total volume of 250 mL/day of nutrient solution. All the treatments for each line were done in five replicates.
2.5.1. Determination of the Number of Attached S. hermonthica Plants and Maize Plant Responses to Infestation
This study was carried out in rhizotrons described in Supplementary File S1. The two maize lines used in this study were put in three groups of five plants each. At 20 days after planting, the first group was infested with untreated S. hermonthica seeds, in contrast the second group was infested with GR24 (a synthetic germination stimulant prepared as described in Supplementary File S2) pre-germinated S. hermonthica seeds. The third group was not infested and was used as a control. The number of attached S. hermonthica plants on the roots was observed and counted at three to four-day intervals that started when the attached S. hermonthica plants were first observed. Similar to what was described by [24], the growing S. hermonthica plants were categorized into five developmental stages. Namely, L1 = plants with 2–4 partially horizontal opened leaves, L2 = plants with 6–8 partially horizontal opened leaves, L3 = plants with 10–12 partially horizontal opened leaves, L4 = plants with 13 partially horizontal opened leaves and above, and Lx = discolored, dying or dead plants (Figure 1). The number of S. hermonthica plants in each of these developmental stages was identified and counted along with the total number of attached plants. Counting was done until the susceptible maize plants started dying at 46 days post-infection (DPI) for the plants infested with untreated S. hermonthica and 53 days post-infection for the plants infested with pre-germinated S. hermonthica. The plant height (distance from the base of the stem to the base of the tassel) of both un-infested and infested maize plants seeds was measured at 66 days after planting for all the different groups of plants.
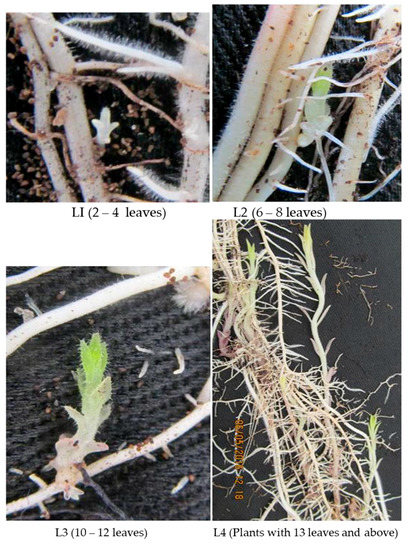
Figure 1.
Growth stages of attached Striga plants.
2.5.2. Evaluation of Physiological Changes Due to S. hermonthica Infestation
Two groups of five replicates each of both lines sown in rhizotrons described in Supplementary File S1. At twelve days after sowing one group of each line was infested with S. hermonthica seeds as described above. A chlorophyll meter SPAD502 plus (Konica Minolta Inc., Tokyo, Japan) chlorophyll meter and an SC-1 leaf porometer (Decagon Devices, Pullman, WA, USA) were used to measure the chlorophyll content and the stomatal conductance, respectively, of all the plants at fifty-six days after sowing. The SPAD readings were taken from the second and third uppermost leaves at a position two thirds from the leaf tip. The average of both measurements from each pant was taken as the value for that plant. Stomatal conductance was taken from the abaxial surface of the youngest fully open leaf of each plant.
2.5.3. Determination of the Growth Rate of Attached S. hermonthica Plants
The two maize lines (5057 and TZISTR1108) were sown in rhizotrons described in Supplementary File S1 and infested with Striga hermonthica seeds, as described in Section 2.5. Upon germination and attachment of the S. hermonthica plants, the number of partially opened leaves on two selected S. hermonthica plants (one on a major root and one on a lateral root of each maize plant) per plant were counted at 3–4 day intervals.
2.6. Field Evaluation of the Two Maize Lines (5057 and TZISTR1108)
The two maize lines were evaluated in two locations (Abuja and Mokwa) in Nigeria for two years, with two replicates in each location. In each location, each experimental unit was evaluated in a one-row plot, 4 m long, spaced 0.75 m apart with 0.25 m between hills. Two maize seeds were sown per hill and thinned to one plant per hill two weeks after planting. Before seed sowing and artificial infestation, ethene (ethylene) gas was injected into the soil to stimulate the suicidal germination of existing Striga seeds in the field. For the infested plots, the seeds were sown in a 6 cm deep hole that had been infested with approximately 3000 germinable S. hermonthica seeds. The Striga-infested and non-infested plots were arranged in such a way that Striga-infested blocks were placed back-to-back in strips with non-infested blocks across the field. Observations were recorded on yield, plant height, ear aspect, emerged Striga count and Striga damage ratings. Ear aspect was based on a scale of 1 to 5, where 1 = clean, uniform, large, and well-filled ears and 5 = ears with only undesirable features. Emerged Striga counts were taken at 10 (STRCO1) and 12 (STRCO2) weeks after planting (WAP) in Striga infested plots. Striga damage rating was taken at 10 (STRRAT1) and 12 (STRRAT2) weeks after planting (WAP) on S. hermonthica infested plots. Striga damage was scored per plot on a scale of 1–9, where 1 = no damage, indicating healthy plant growth and high resistance, and 9 = complete collapse or death of the maize plant.
2.7. Statistical Analysis
The Mann–Whitney U test and the Kruskal–Wallis H test was performed on the germination inducing ability experiments on R Software [25]. After which Dunn’s Multiple Comparison Test was performed on experiments with more than two groups also on R Software [25]. One-way analysis of variance (ANOVA) and a post hoc Tukey’s test were performed on the root architecture evaluation and rhizotron studies data with R software [25].
In the growth rate experiment, simple regression was used to fit a growth curve (the leaf count values were square root transformed (as indicated in Equation (1), and Analysis of Covariance was performed to compare the rate of growth of the parasite on the two maize lines using R Software [25].
where, y = predicted value of the dependent variable (leaf count), αi = intercept, β = regression coefficient—how much we expect y to change as x increases, x = independent variable (days post infestation), ε = error of the estimate.
For the field experiments analyses of variance combined across the two year-location combinations were computed for the three traits measured under Striga infested and non-infested conditions based on mixed-model analysis with the restricted maximum likelihood procedure in SAS version 9.4. (SAS institute inc., Cary nc, USA). The following model was adopted:
where μ is the overall mean, Gi is the effect of ith genotype, Ej is the effect of jth environment, GEij is the effect of the interaction of the ith genotype and the jth environment, R(E)jk is the effect of the kth replication within the jth environment, Ɛijk is the residual effect.
In these analyses, the maize lines were considered as fixed effects and the adjusted means and standard errors were estimated. The environments, replication (environment), block (replication x environment) were considered as random effects.
3. Results
3.1. Evaluation of the Germination-Inducing Activity of Root Exudates
3.1.1. Test Tube Experiments
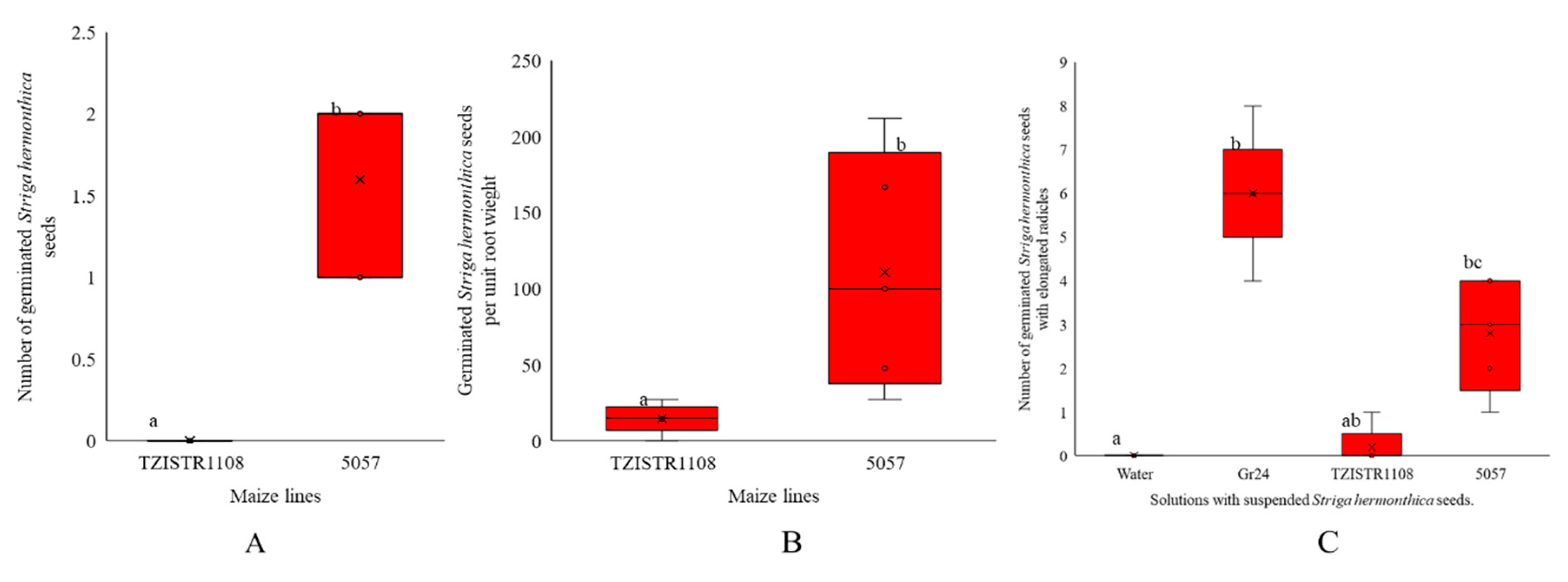
The differences in the number of S. hermonthica germinated by the exudates collected from test tubes and the number per unit of root weight between the susceptible and resistant lines were statistically significant. A Mann–Whitney test indicated that the number of S. hermonthica seeds germinated by 5057 (median = 2) was greater than the number germinated by TZISTR1108 (median 0), W = 25, p-value = 0.007. There was also a significant difference in the number of S. hermonthica seeds germinated per unit weight 5057 (median = 100), TZISTR1108 (median = 14). W = 24.5, p-value = 0.02. (Figure 2A,B).
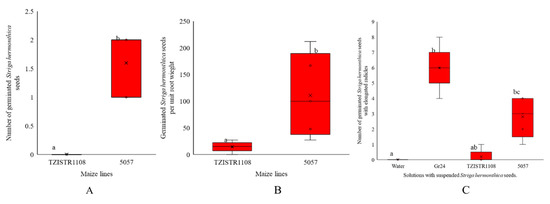
Figure 2.
Maize root exudate S. hermonthica germination ability. Boxplots of (A) number of Striga hermonthica seeds germinated by the root exudates of the two lines after 72 h. (B). number of Striga hermonthica seeds germinated per unit root weight of both lines. (C) number of Striga hermonthica seeds with elongated radicles on suspension in the root exudates of the two varieties GR24 and water for 11 days. The letters in the figure show significant differences at α = 0.05.
3.1.2. Pot Experiments
There were significant differences in the number of germinated S. hermonthica seeds with elongated radicles (Supplementary Figure S1) (H (3) = 17.348, p-value = 0.0006). Water, Gr24, TZISTR1108 and 5057 had mean ranks of 0, 6.0, 0.2 and 2.8 respectively Dunn test did not show a significant difference between TZISTR1108 and 5057 (Figure 2C).
3.2. Root Architecture Evaluation
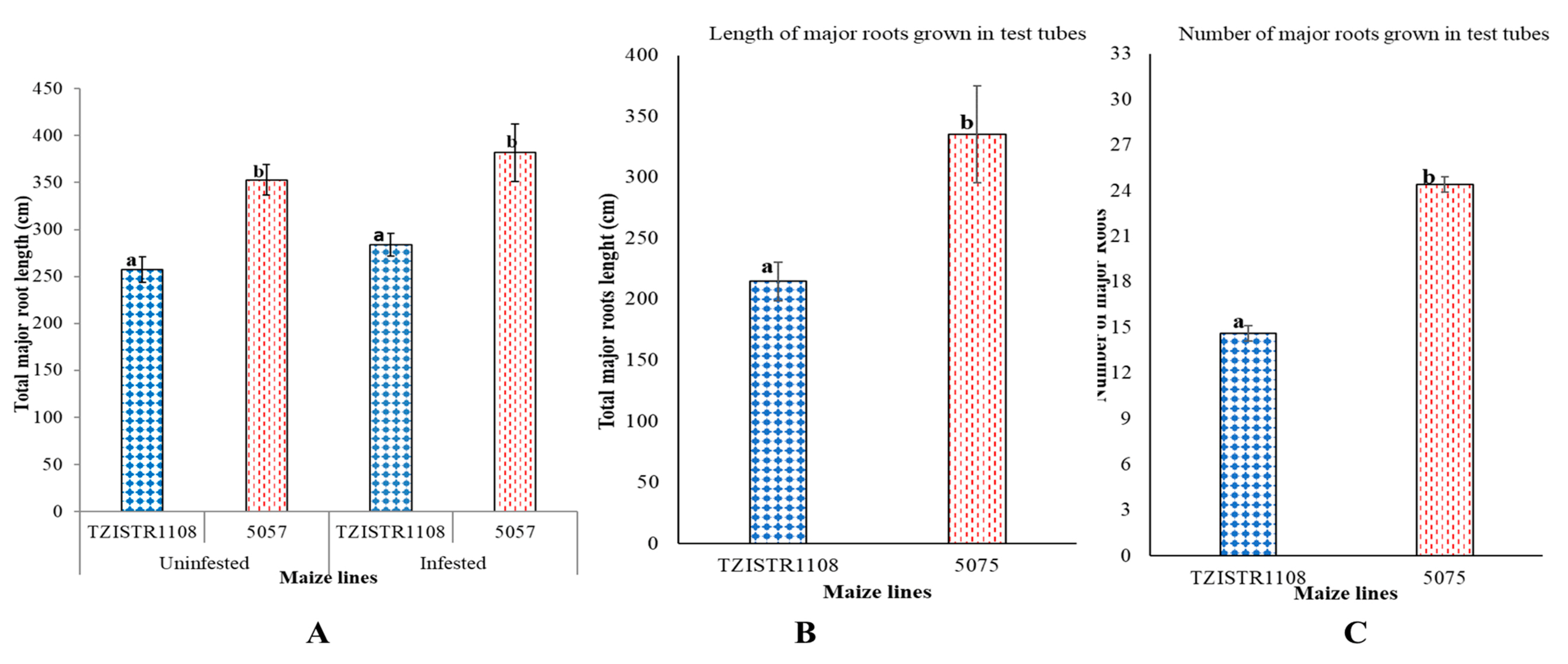
Total root length. In the pot experiment, TZISTR1108 had significantly shorter total major root lengths (257 ± 13.5 cm) than 5057 (352.9 ± 16 cm) before infestation. Upon infestation, the total major root length of TZISTR1108 (284 ± 12.2 cm) was also significantly shorter than that of 5057 (382 ± 30 cm). There was no significant difference in total root length of infested 5057, and un-infested 5057, as well as between infested TZISTR1108 and un-infested TZISTR1108 (Figure 3A). When the plants were grown in test tubes, similar results were observed. Line 5057 had more major roots than TZISTR1108, 24.4 ± 2.6 and 14.6 ± 0.5. respectively (Figure 3B). The total major root length of the 5057 plants was also significantly higher than that of TZISTR1108 (Figure 3C and Supplementary Figure S2). TZISTR1108 had an average major root length of 14.7 cm, while 5057 had an average major root length of 13.7 cm.
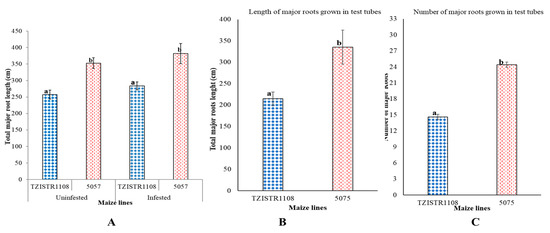
Figure 3.
Root architecture evaluation. (A). Mean total length of major roots (infested and un-infested) on both maize lines at 28 days after planting in pots. (B). Mean number of major roots at 53 days after planting in test tubes. (C). Mean total length of major roots at 53 days after planting in test tubes. The different letters (a and b) show significant differences among groups (α = 0.05). The bars show standard error.
3.3. Rhizotron Studies (Post-Attachment Resistance Evaluation)
3.3.1. Evaluation of the Number of Attached S. hermonthica Plants
The number of attached S. hermonthica plants on 5057 was consistently higher than that on TZISTR1108, regardless of whether the S. hermonthica seeds were treated with GR24 or not, and this difference became significant as the experiment progressed. In both cases, the experiment was terminated after two susceptible maize plants (5075) died before the next observation period.
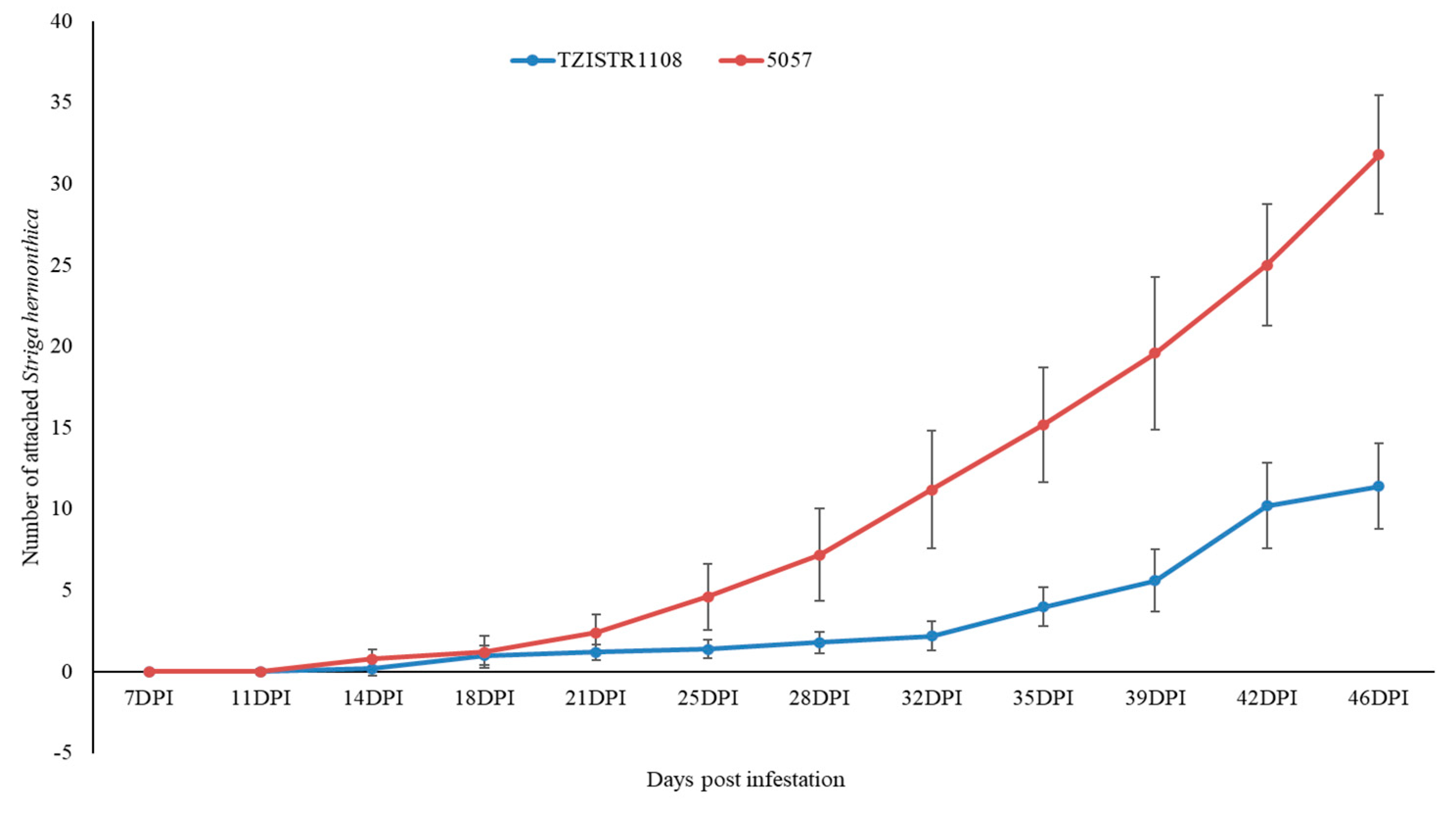
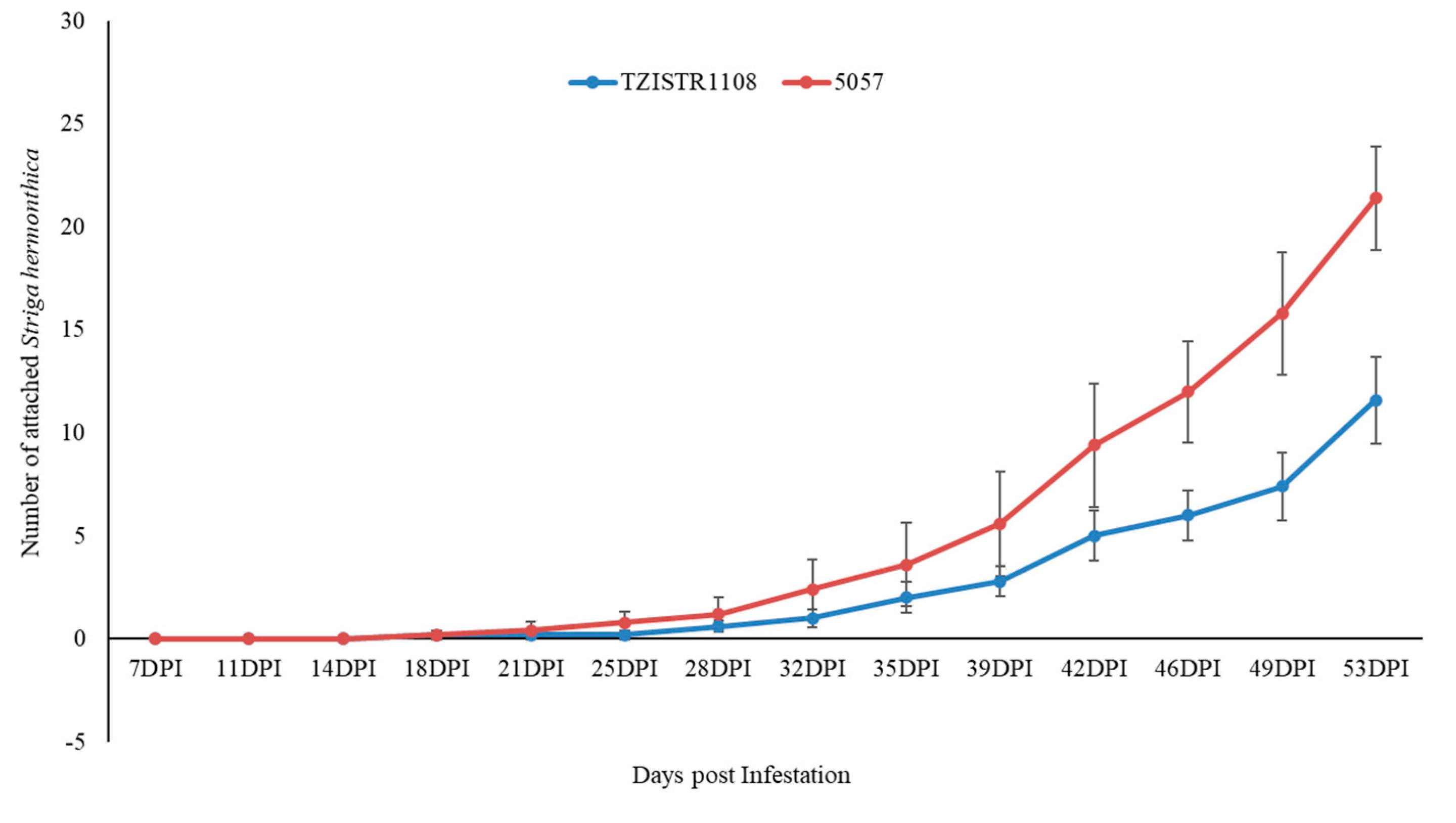
In group A, at the last recorded time point (46 dpi), TZISTR1108 had 11.8 ± 2.2 attached S. hermonthica plants while 5057 had 31.8 ± 3.67 (Figure 4 and Supplementary Figure S3). The plants in group B (infested with GR24 pre-germinated S. hermonthica seeds) also showed a statistically significant difference in the number of S. hermonthica plants attached to TZISTR1108 and 5057, with 11.6 ± 2.1 attached S. hermonthica plants on TZISTR1108 and 21.4 ± 2.5 attahced plants on 5057 (Figure 5 and Supplementary Figure S4).
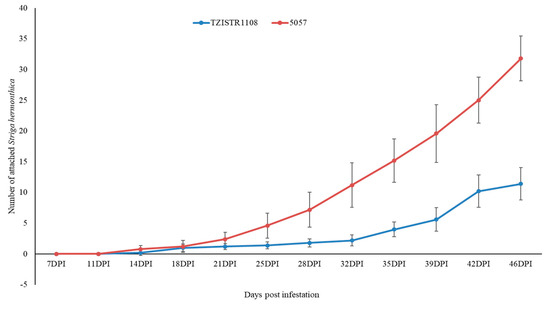
Figure 4.
Mean number of attached Striga hermonthica plants on resistant (TZISTR1108) and susceptible (5057) maize lines in group A (No GR24 treatment) as the experiment progressed. Error bars show Standard error.
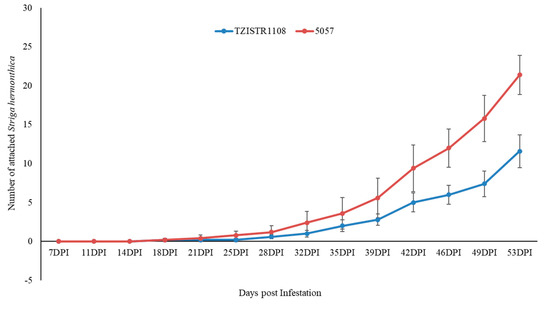
Figure 5.
Mean number of attached Striga hermonthica on resistant (TZISTR1108) and susceptible (5057) maize lines in group B (GR24 treatment) as the experiment progressed. Error bars show standard error.
3.3.2. Physiological Changes Due to S. hermonthica Infestation
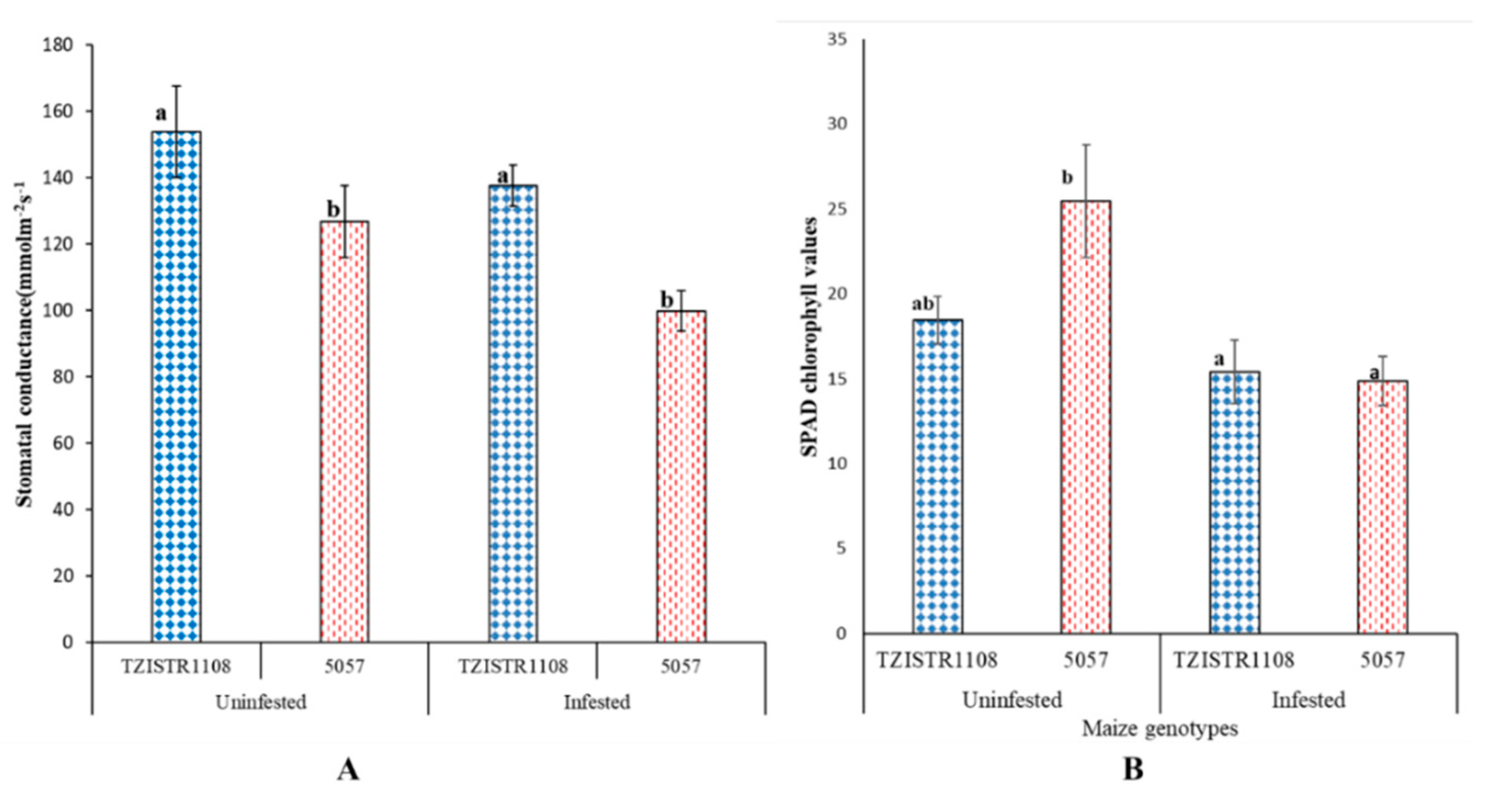
The chlorophyll content and stomatal conductance in the infested plants was less than in the un-infested plants. The difference in chlorophyll content between the infested and un-infested plants was significant in the 5057 line but was not in the TZISTR1108. An 11% decrease was observed in the stomatal conductance of the infested TZISTR1108 when compared to the un-infested plants. While 5057 showed a 22% decrease in stomatal conductance between the infested and un-infested plants (Figure 6A). This decrease in stomatal conductance was however not significant. There was a 43% decrease in chlorophyll content between the infested and un-infested 5057 plants. While the infested TZISTR1108 plants showed a 21% decrease in chlorophyll content when compared to the un-infested TZISTR1108 (Figure 6B).
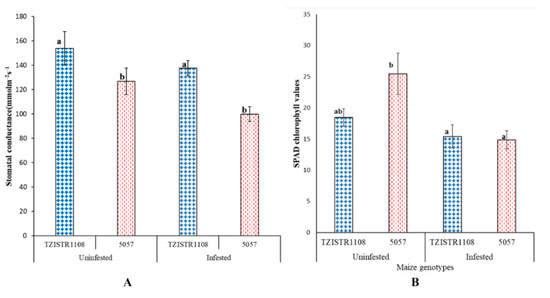
Figure 6.
Physiological parameters upon infestation and without infestation. (A). Average stomatal conductance of both lines with and without infestation. (B). Average chlorophyll content of both lines with and without infestation. The different letters (a and b) show significant differences among groups at α = 0.05. The bars show standard error.
3.3.3. Effect of Parasitization on Plant Height
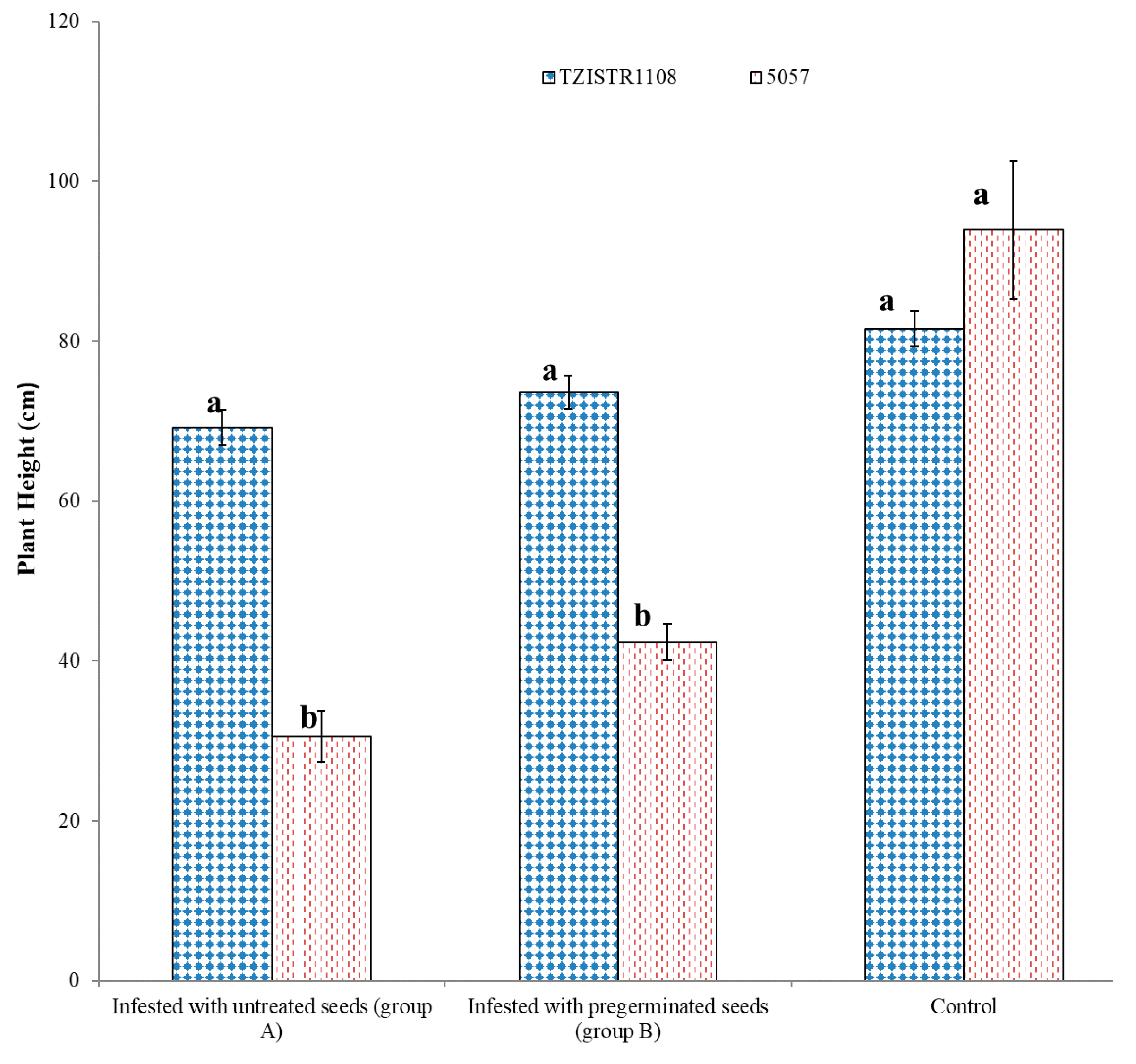
Upon infestation by S. hermonthica, TZISTR1108 had significantly taller shoots than the 5057 regardless of the treatment of the S. hermonthica seeds. Upon infestation with untreated seeds TZISTR1108 and 5057 had heights of 69.20 ± 2.15 cm and 30.60 ± 3.1 cm, respectively. When pre-germinated S. hermonthica seeds were used to infest both maize lines, TZISTR1108 plants had an average height of 73.60 ± 2.1 cm, and the 5057 plants had an average height of 42.4 ± 2.2 cm. Both maize lines did not differ significantly in height without S. hermonthica infestation. (TZISTR1108 = 81.50 ± 2.1 cm and 5057 = 93.96 ± 8.6 cm). There was no significant difference among the heights of the infested TZISTR1108 line with pre-germinated S. hermonthica seeds, the infested TZISTR1108 line with untreated S. hermonthica seeds, and the un-infested TZISTR1108. There was no significant difference in height between the 5057 plants infested with treated and untreated S. hermonthica seeds. (Figure 7 and Figure 8).

Figure 7.
Infested and un-infested 5057 and TZISTR1108 plants growing in rhizotron at 66 days after planting.
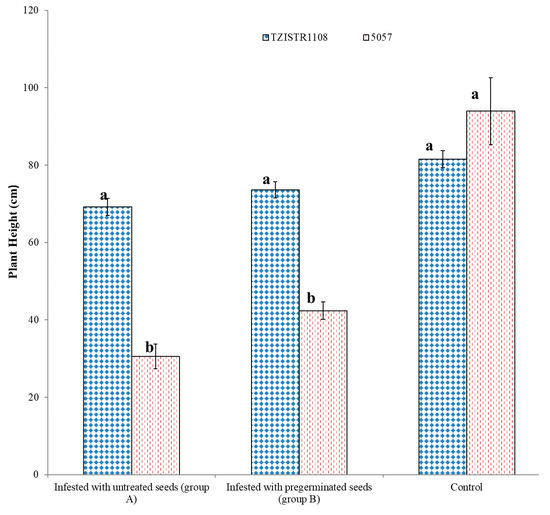
Figure 8.
Mean plant height (cm) of infested (with seeds that were not pregerminated and with pregerminated seeds) and un-infested maize plants of both lines grown on rhizotrons. The different letters (a and b) show significant differences among groups at α = 0.05. The bars show standard error.
3.3.4. Growth Rate of Attached S. hermonthica Plants
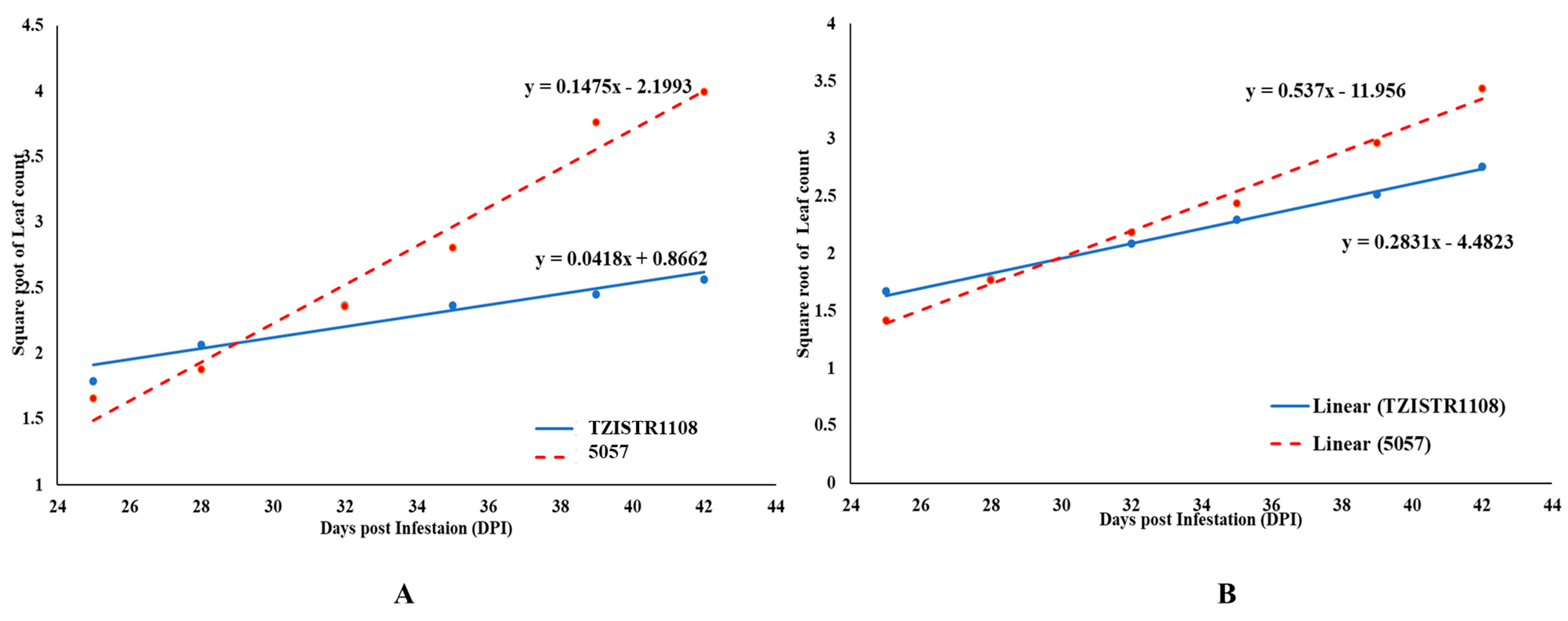
It was also observed that the growth (as indicated by the emergence rate of leaves) of S. hermonthica plants that were attached to the TZISTR1108 was slower when compared to that of plants attached to the susceptible line. This slower rate of growth was significant both for Striga plants attached to lateral roots and plants attached to major roots (Figure 9A,B).
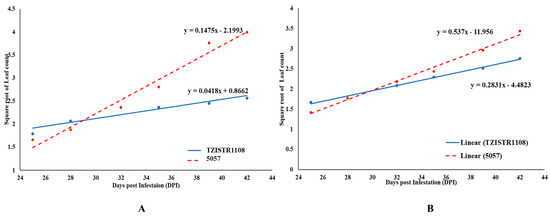
Figure 9.
Regression lines of mean leaf count of Striga hermonthica plants on lateral roots (A) and major roots (B) of the two maize lines. The points represent the mean of the Square root of the number of leaves fully or partially opened leaves at days post infestation on selected S. hermonthica plants attached to a lateral root on 5057 and TZSTRI108 replicated 5 times.
3.4. Field Evaluation
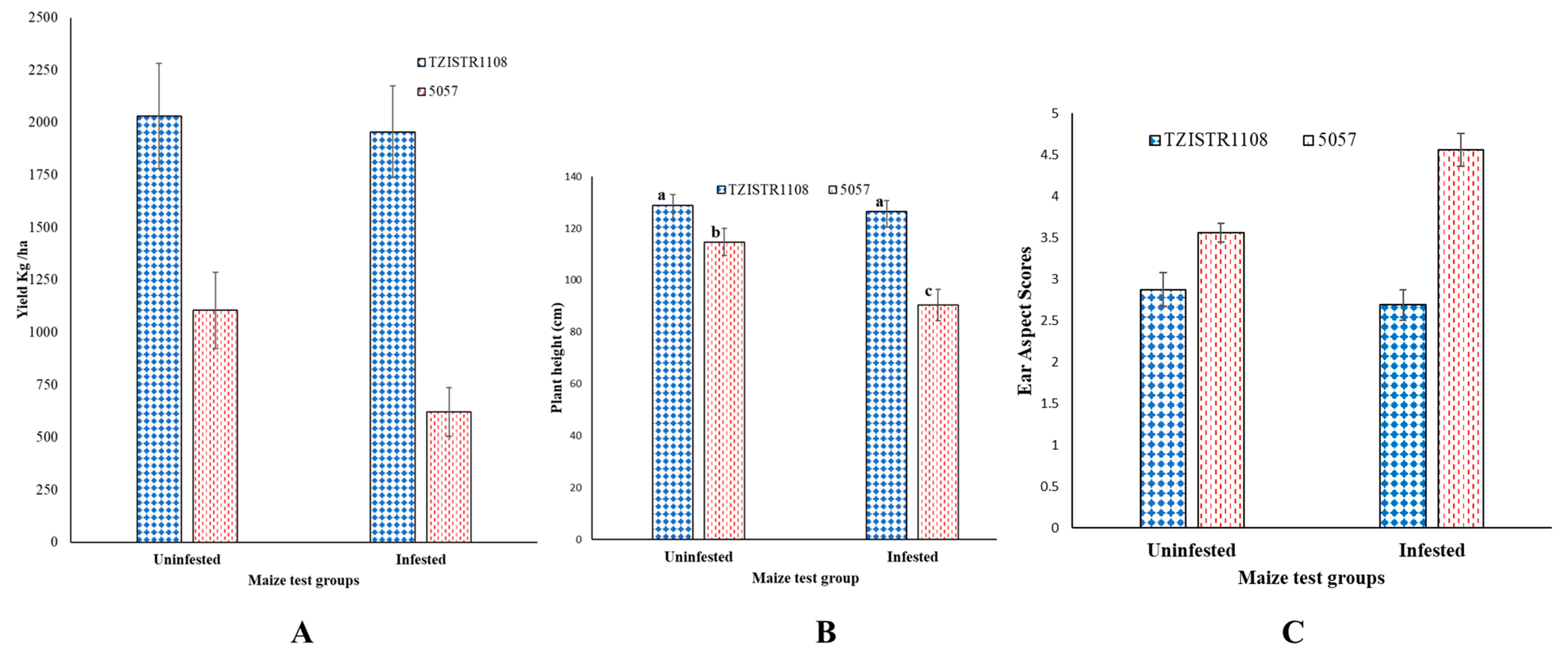
Field studies also revealed that the two lines show significant differences in S. hermonthica related traits. Upon infestation, TZISTR1108 did not show a significant decrease in yield (un-infested = 2030.04 ± 251.07 kg/ha, infested = 1955.36 ± 219.17 kg/ha) compared to the un-infested state, while 5057 showed a significant (43.7%) decrease in yield (un-infested = 1104.11 ± 180.78 kg/ha, infested = 619.9 ± 116.4 kg/ha, Figure 10A). A significant difference (21.13%) was also observed in the heights of both the infested and un-infested 5057 plants (un-infested = 114.75 ± 5.32 cm, infested = 90.54 ± 6.05 cm), this difference in height was not significant in TZISTR1108 (un-infested = 128.88 ± 4.14 cm, infested = 126.63 2 ± 4.14 cm) (Figure 10B). The ear aspects scores of the groups of plants (TZISTR1108: un-infested = 2.88 ± 0.21, infested = 2.69 ± 0.19; 5057: un-infested = 3.56 ± 0.11, infested = 4.56 ± 0.2) revealed a significant deterioration (28%) in the ear aspect of 5057 upon infestation, but no significant effect on TZISTR1108 (Figure 10C).
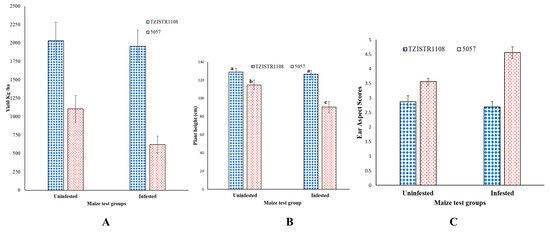
Figure 10.
Field estimates of grain yield and two associated traits upon infestation and without infestation. (A) Mean grain yield of both lines. (B) Mean plant height of both lines. (C) Mean ear aspect scores of both lines. The different letters (a, b and c) show significant differences among the groups (α = 0.05). The bars show standard error.
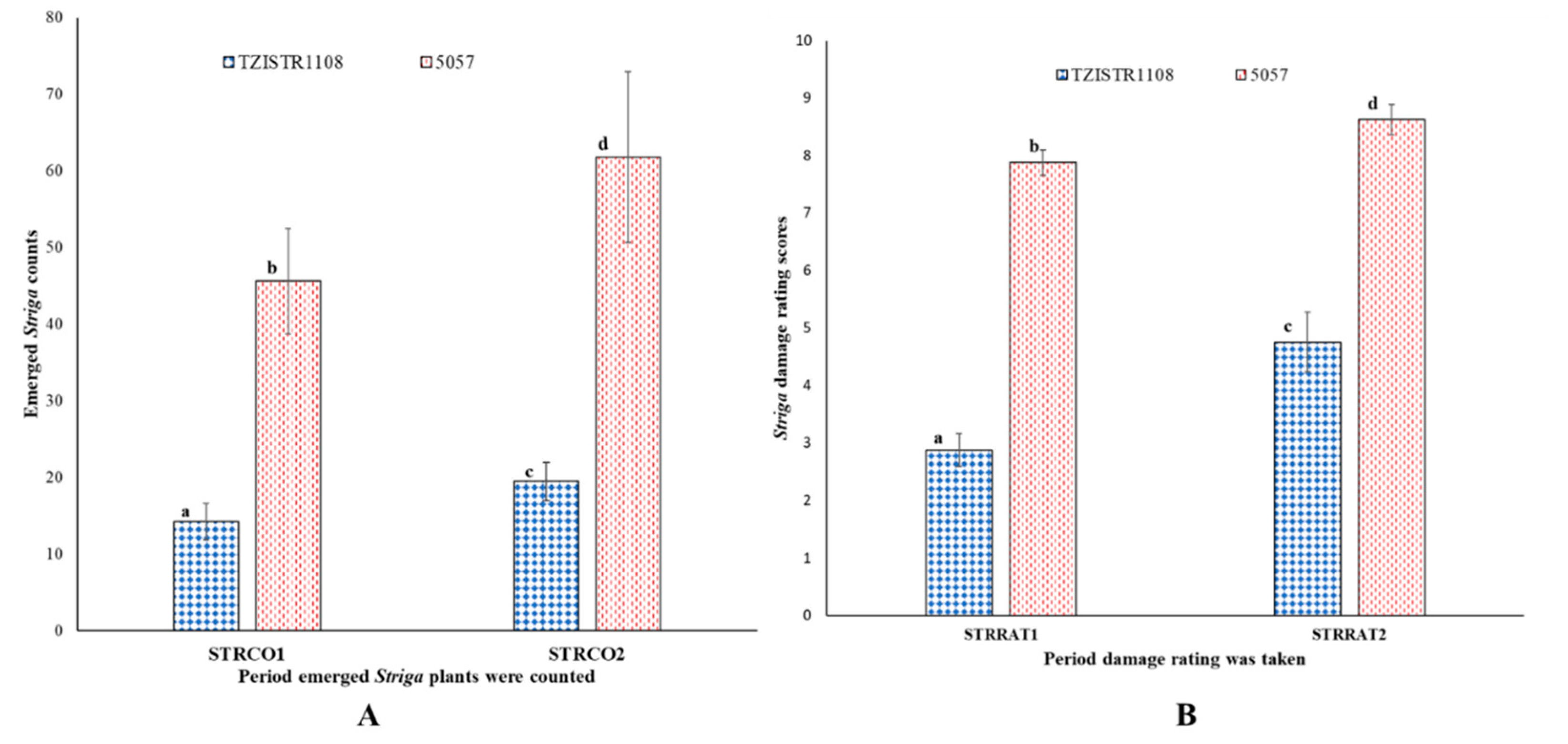
There were significantly more emerged S. hermonthica on the 5057 plants than the TZISTR1108 plants, 232% more at 10 WAP (5057 = 45.6 ± 6.9, TZISTR1108 = 14.3 ± 2.4) and 209% more at 12 WAP (5057 = 61.8 ± 11.18, TZISTR1108 = 19.5 ± 2.5, Figure 11A). Upon infestation 5057 showed a higher amount of the deleterious effect of infestation as measured by Striga damage ratings compared to TZISTR1108. There was 173.9% more damage on 5057 at 10 WAP (STRRAT1; TZISTR1108 = 2.87 ± 0.22, 5057 = 7.88 ± 0.29) and 81.9% more damage on 5057 at 12 WAP (STRRAT2; TZISTR1108 = 4.75 ± 0.26, 5057 = 8.63 ± 0.52, Figure 11B).
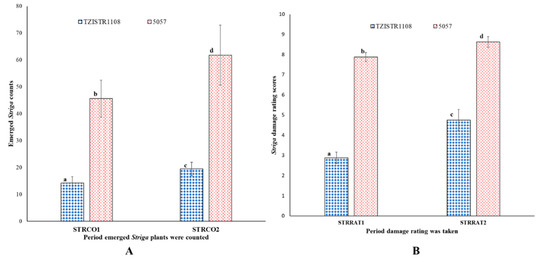
Figure 11.
Field estimates of Striga resistance related traits. (A) Mean values for number of emerged Striga hermonthica plants on both lines at 10 weeks after planting (STRCO1) and 12 weeks after planting (STRCO2). (B) Mean Striga damage ratings of both lines at 10 weeks after planting (STRRAT1) and 12 weeks after planting (STRRAT2). The different letters (a, b, c and d) show significant differences among the groups (α = 0.05). The bars show standard error.
4. Discussion
This study investigated the response of two maize lines to S. hermonthica parasitism. Our results revealed that the resistant line TZISTR1108 showed lower germination and radicle elongation of Striga hermonthica seeds when compared with the susceptible variety. This is consistent with observations by [26] on another Zea diploperennis-derived maize line on Striga asiatica seeds. The observed reduction in germination stimulation ability may be due to reduced production or type of germination stimulants present in the root exudate of the two lines. Gobena et al. [27] provided evidence that a mutation at the LGS1 gene locus changes the dominant strigolactone in root exudates from a highly active Striga germination stimulant, 5-deoxystrigol, to orobanchol. Ejeta et al. [28] reported that low-stimulant sorghum varieties showed Striga resistance in field tests, indicating that a reduced germination ability leads to some amount of resistance to S. hermonthica. In addition, it was observed that TZISTR1108 had a smaller number of major roots and a shorter total major root length than 5057. In contrast, its average root length was longer than 5057’s. These apparent differences in root architecture between the two maize lines may account for an additional mechanism of avoidance in the resistant inbred line. This is because S. hermonthica is a root parasite, and a plant with relatively fewer roots has a lower likelihood of Striga-host root contact. Cherif-Ari et al. [29] showed that low root length density might be one mechanism that is adopted by certain sorghum varieties to avoid Striga parasitism. Van Delft et al. [30] observed a vertical stratification in the distribution of Striga hermonthica seeds in the soil profile, where very few seeds were found below a depth of 20 cm. This stratification would favour plants with fewer roots, especially if these roots are relatively longer and extend into the ground strata with fewer Striga seeds.
In the present study, attached S. hermonthica plants on the resistant maize line had a slower growth rate compared to the ones attached to the susceptible line. This result is consistent with what was observed by [24], and this finding may indicate that TZISTR1108 has some intrinsically induced physiological/biochemical defense response to parasitism by Striga. Amusan et al. [24] reported the accumulation or deposition of an unidentified substance at the haustoria-host interface in TZISTR1108, as well as poor invasion of the host tissues by the haustoria of the parasite; This will likely lead to poor nutrient absorption by the parasite from the host and thus the observed retardation in growth. The observed growth retardation might also be a result of some host-induced compounds that are toxic to the parasite. The slower growth rate, as well as the weaker S. hermonthica germinating ability of TZISTR1108 recorded, may be responsible for the lower number of emerged plants on the TZISTR1108 in the field.
Striga hermonthica infestation causes a plethora of deleterious effects in its host. These effects are expected to be reduced in the resistant plant when compared to those in the susceptible plant. Our results showed that the susceptible line supported a larger number of Striga plants than the resistant line, both on the field and in the screen house. This result agrees with the field test results of [22]. The higher the number of attached plants, the more deleterious the effects of the parasite, where the host is not tolerant. The ability to reduce attachment is, therefore, an important trait in the fight for the survival of the host. We also observed that the infested resistant maize plants were significantly taller than the infested susceptible plants. Since both maize lines did not differ significantly in height without S. hermonthica, this result implies that the susceptible plant was significantly affected by infestation. There was no significant difference in heights of both maize lines between treatment (pre-germinated or not) of Striga seeds. This finding indicates that the deleterious effect of Striga on host plant height does not depend only on the number of attached plants but also on some inherent biochemical and physiological properties of the host or the parasite.
Leaf stomatal conductance is essential for CO2 acquisition [31]. Striga hermonthica infestation led to a decrease in the stomatal conductance of the 5057 plants. Stomatal closure of the host may benefit the parasite, as the stomatal conductance of a parasitic angiosperm is generally higher than that of its host, and thus water and nutrients will be diverted to the parasite. Reduced stomatal conductance, however, leads to a reduction in host photosynthesis [7]. Chlorophyll largely determines the photosynthetic capacity of a plant and hence its growth [32]. Furthermore photosynthesis, a vital part of plant metabolism, is sensitive to different kinds of abiotic and biotic stresses it can be used for screening of plant material for tolerance or sensitivity [33]. The infected TZISTR1108 significantly mitigated the drop in its chlorophyll SPAD value upon infestation, indicating that the plant was responding well despite the Striga infestation.
5. Conclusions
The findings of this study show that TZISTR1108 resists infestation by the parasite through multiple mechanisms. TZISTR1108 actively resists infestation by limiting attachment of the parasite. If the parasite attaches, it slows the parasite’s development and limits its adverse effects to levels comparable to those in the un-infested state. The susceptible plants (5057), however, show the deleterious effects of Striga infestation. The mechanisms of resistance observed in TZISTR1108 require further genetic and genomic investigations to determine their molecular controls. Furthermore, it will be essential to study the effect of Striga parasitism on other biochemical and physiological parameters. The results of this study will facilitate the development of improved selection methods for Striga resistance in maize. This is because maize lines with different resistance mechanisms can be crossbred to produce plant lines with enhanced durable tolerance and resistance to S. hermonthica.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0472/10/10/485/s1, Table S1: Chemical composition of 40% Long Ashton Solution (Hewitt, 1966), amended to provide 1 mM NH4NO3. File S1: Assembly of and introduction of plant material into a rhizotron. File S2: Preparation of 1 mg/L GR24 solution. File S3: R scripts used for statistical analysis. A = Script for Mann-Whitney u test, B = Script for Kruskal wallis and post hoc Dunn tests C. Script for ANCOVA. Figure S1: Striga hermonthica seeds treated with exudates from 5057 and TZISTR1108. A. Seeds treated with exudates from TZISTR1108, B = Seeds treated with exudates from 5057. (The red circles show germinating Striga hermonthica seeds) C. Germinated S. hermonthica seeds with elongated radicles. Figure S2: Scanned pictures of the roots of both genotypes. (A, C) are 5057 plants, while (B, D) are TZISTR1108 plants. The 5057 plants are seen to have more major roots that are slightly shorter than in the TZISTR1108 plants.
Author Contributions
M.G., A.M. and E.O.F. Conceptualization, supervision, resources, and writing—reviewing and editing. A.S.: Investigation and writing—Original draft, reviewing and editing. N.N.U.: Investigation, methodology and writing—Original draft, reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the CGIAR Research Program on Maize (MAIZE) and the Bill & Melinda Gates Foundation and USAID funded project Stress Tolerant Maize for Africa (STMA) (grant number OPP1134248).
Acknowledgments
The authors sincerely thank Yinka Ilesanmi, Deborah Babalola, and Queen Obi for their help with setting up and running the various experiments. We also acknowledge Tayo Ojo and Paul Ibrahim for supplying the maize and Striga hermonthica seeds and for helping with the screen house and field experiments. We are also especially grateful for the comments 3 anonymous reviewers.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bozkurt, M.L.; Muth, P.; Parzies, H.K.; Haussmann, B.I.G. Genetic diversity of East and West African Striga hermonthica populations and virulence effects on a contrasting set of sorghum cultivars. Weed Res. 2014, 55, 71–81. [Google Scholar] [CrossRef]
- Parker, C.; Riches, C.R. Parasitic Weeds of the World: Biology and Control; CAB International: Wallingford, CT, USA, 1993. [Google Scholar]
- Hearne, S.J. Control—The Striga conundrum. Pest Manag. Sci. 2009, 65, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Press, M.C. How do the parasitic weeds Striga and Orobanche influence host carbon relations? Asp. Appl. Biol. 1995, 42, 63–70. [Google Scholar]
- Press, C.; Scholes, J.D.; Watling, J.R. Parasitic plants: Physiological and ecological interactions with their hosts. In Physiological Plant Ecology; Press, M.C., Scholes, J.D., Barker, M.G., Eds.; Blackwell Science: Oxford, UK, 1999; pp. 175–197. [Google Scholar]
- Frost, D.L.; Gurney, A.L.; Press, M.C.; Scholes, J.D. Striga hermonthica reduces photosynthesis in sorghum: The importance of stomatal limitations and a potential role for ABA? Plant Cell Environ. 1997, 20, 483–492. [Google Scholar] [CrossRef]
- Taylor, A.; Martin, J.; Seel, W.E. Physiology of the parasitic association between maize and witchweed (Striga hermonthica): Is ABA involved? J. Exp. Bot. 1996, 47, 1057–1065. [Google Scholar] [CrossRef]
- Parker, C. The Parasitic Weeds of the Orobanchaceae. In Parasitic Orobanchaceae. Parasitic Mechanisms and Control Strategies; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: New York, NY, USA, 2013; pp. 313–343. [Google Scholar]
- Graves, J.D.; Press, M.C.; Stewart, G.R. A carbon balance model of the sorghum-Striga hermonthica host-parasite association. Plant Cell Environ. 1989, 12, 101–107. [Google Scholar] [CrossRef]
- Eplee, R.E.; Norris, R. Control of Parasitic Weeds. Parasitic Plants; Press, M.C., Graves, J.D., Eds.; Chapman and Hall: London, UK, 1995; pp. 256–278. [Google Scholar]
- Kim, S.-K.; Adetimirin, V.O. Responses of Tolerant and Susceptible Maize Varieties to Timing and Rate of Nitrogen under Striga hermonthica Infestation. Agron. J. 1997, 89, 38–44. [Google Scholar] [CrossRef]
- Egley, G.H.; Eplee, R.E.; Norris, R.S. Discovery and development of ethylene as a witchweed seed germination stimulant. In Witchweed Research and Control in the U.S.; Sand, P.F., Eplee, R.E., Westbrooks, R.G., Eds.; WSSA: Champain, IL, USA, 1990; pp. 56–67. [Google Scholar]
- De Groote, H.; Wangare, L.; Kanampiu, F.; Odendo, M.; Diallo, A.; Karaya, H.; Friesen, D. The potential of a herbicide resistant maize technology for Striga control in Africa. Agric. Syst. 2008, 97, 83–94. [Google Scholar] [CrossRef]
- Parker, C. Protection of crops against parasitic weeds. Crop. Prot. 1991, 10, 6–22. [Google Scholar] [CrossRef]
- Kling, J.G.; Fajemisin, J.M.; Badu-Apraku, B.; Diallo, A.; Menkir, A.; Melake-Berhan, A. Striga resistance breeding in maize. In Breeding for Striga Resistance in Cereals; Haussmann, B.I.G., Hess, D.E., Koyama, M.L., Grivet, L., Rattunde, H.F.W., Geiger, H.H., Eds.; Margraf Verlag. Haussmann: Weikersheim, Germany, 2000; pp. 103–118. [Google Scholar]
- Kim, S. Genetics of Maize Tolerance of Striga hermonthica. Crop. Sci. 1994, 34, 900–907. [Google Scholar] [CrossRef]
- Riches, C.R.; Parker, C. Parasitic plants as weeds. In Press Parasitic; Plants, M.C., Graves, J.D., Eds.; Chapman and Hall: London, UK, 1995; pp. 226–255. [Google Scholar]
- Timko, M.P.; Scholes, J.D. Host Reaction to Attack by Root Parasitic Plants. In Parasitic Orobanchaceae; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 115–141. [Google Scholar] [CrossRef]
- Rodenburg, J.; Riches, C.R.; Kayeke, J.M. Addressing current and future problems of parasitic weeds in rice. Crop. Prot. 2010, 29, 210–221. [Google Scholar] [CrossRef]
- Rich, P.J.; Ejeta, G. Towards effective resistance to Striga in African maize. Plant Signal. Behav. 2008, 3, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Menkir, A.; Makumbi, D.; Franco, J. Assessment of Reaction Patterns of Hybrids to Striga hermonthica (Del.) Benth. under Artificial Infestation in Kenya and Nigeria. Crop. Sci. 2012, 52, 2528–2537. [Google Scholar] [CrossRef]
- Menkir, A. Assessment of reactions of diverse maize inbred lines to Striga hermonthica (Del.) Benth. Plant Breed. 2006, 125, 131–139. [Google Scholar] [CrossRef]
- Menkir, A.; Kling, J.; Badu-Apraku, B.; Ibikunle, O. Registration of 26 Tropical Maize Germplasm Lines with Resistance to Striga hermonthica. Crop. Sci. 2006, 46, 1007–1009. [Google Scholar] [CrossRef]
- Amusan, I.O.; Rich, P.J.; Menkir, A.; Housley, T.; Ejeta, G. Resistance to Striga hermonthica in a maize inbred line derived from Zea diploperennis. New Phytol. 2008, 178, 157–166. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2018. Available online: https://www.r-project.org/ (accessed on 13 February 2012).
- Pierce, S.; Mbwaga, A.M.; Press, M.C.; Scholes, J.D. Xenognosin production and tolerance to Striga asiatica infection of high-yielding maize cultivars. Weed Res. 2003, 43, 139–145. [Google Scholar] [CrossRef]
- Gobena, D.; Shimels, M.; Rich, P.J.; Ruyter-Spira, C.; Bouwmeester, H.; Kanuganti, S.; Mengiste, T.; Ejeta, G. Mutation in Sorghum LOW GERMINATION STIMULANT 1 alters Strigolactones and Causes Striga resistance. Proc. Natl. Acad. Sci. USA 2017, 1149, 4471–4476. [Google Scholar] [CrossRef]
- Ejeta, G.; Gressel, J. Integrating New Technologies for Striga Control: Towards Ending the Witch-Hunt; World Scientific Pub. Co Pte Lt: Singapore, 2007; pp. 3–16. [Google Scholar]
- Cherif-Ari, O.; Housley, T.L.; Ejeta, G. Sorghum root length density and the potential for avoiding striga parasitism. Plant Soil 1990, 121, 67–72. [Google Scholar] [CrossRef]
- Van Delft, G.-J.; Graves, J.D.; Fitter, A.H.; Pruiksma, M.A. Spatial distribution and population dynamics of Striga hermonthica seeds in naturally infested farm soils. Plant Soil 1997, 195, 1–15. [Google Scholar] [CrossRef]
- Dodd, I.C. Hormonal Interactions and Stomatal Responses. J. Plant Growth Regul. 2003, 22, 32–46. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Franić, M.; Galić, V.; Lončarić, Z.; Šimić, D. Genotypic Variability of Photosynthetic Parameters in Maize Ear-Leaves at Different Cadmium Levels in Soil. Agronomy 2020, 10, 986. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).