The Effect of Potassium and Micronutrient Foliar Fertilisation on the Content and Accumulation of Microelements, Yield and Quality Parameters of Potato Tubers
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- Foliar fertilisation with potassium and micronutrients increased the accumulation of zinc, copper and manganese in tubers of the Hermes variety, irrespective of the treatment analysed. In the Zorba variety, the increase in the nutrient accumulation in tubers due to foliar sprays varied between treatments and depending on the individual element. Compared to the control, the increase in the accumulation of manganese and iron was observed for all the treatments, while copper increased under the treatments with SOP + Micro and zinc—only under the Micro treatment.
- Compared to the control, the highest increase in the accumulation of nutrients in tubers was found under the regime of double foliar spray with micronutrients.
- From among the nutrients examined, it was zinc accumulation that was found to largely determine the tuber yield in the French fries variety, while no significant relationships were noted for the Hermes variety.
- The accumulated micronutrients determined the contents of protein and starch within the range 34–96% in tubers of both varieties.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fageria, N.K.; Baligar, V.C.; Li, Y.C. The role of nutrient efficient plants in improving crop yields in the twenty first Century. J. Plant Nutr. 2008, 31, 1121–1157. [Google Scholar] [CrossRef]
- Bailey, R.L.; West, K.P., Jr.; Balack, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metabol. 2015, 66, 22–33. [Google Scholar] [CrossRef] [PubMed]
- FAO; IFAD; WFP. The State of Food Insecurity in the World 2015. Meeting the 2015 International Hunger Targets: Talking Stock of Uneven Progress. Available online: http://www.fao.org/3/a-i4646e.pdf (accessed on 18 August 2020).
- Haverkort, A.J.; Struik, P.C. Yield levels of potato crops: (Recent achievements and future prospects). Field Crop. Res. 2015, 182, 75–86. [Google Scholar] [CrossRef]
- World Health Organization. The World Health Report 2002-Reducing Risks, Promoting Healthy Life. Available online: https://www.who.int/whr/2002/en/ (accessed on 18 August 2020).
- Robson, A.D.; Pitman, M.G. Interactions between nutrients in higher plants. In Inorganic Plant Nutrition; Laeuchli, A., Bieleski, R.L., Eds.; Springer: Berlin, Germany, 1983; pp. 147–180. [Google Scholar]
- Ierna, A.; Pellegrino, A.; Malvuccio, A. Effects of micronutrient fertilization on the overall quality of raw and minimally processed potatoes. Postharvest Biol. Technol. 2017, 134, 38–44. [Google Scholar] [CrossRef]
- Sinclair, A.H.; Edwards, A.C. Micronutrient Deficiency Problems in Agricultural Crops in Europe. In Micronutrient Deficiencies in Global Crop. Production; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 225–244. [Google Scholar]
- Lipński, W. Evaluation of the Polish soils abundance in microelements. Zesz Nauk. Wroc. Univ. Life Sci. 2016, 621, 49–58. [Google Scholar]
- Lobell, D.B.K.G.; Casman, K.G.; Field, C.N. Crop yield gabs: Their, magnitudes, and causses. Annu. Rev. Envir Res. 2009, 34, 179–204. [Google Scholar] [CrossRef]
- Ryan, J.; Sommer, R. Soil fertility and crop nutrition research at an international centre in the Mediterranean region: Achievements and future perspective. Arch. Agron. Soil Sci. 2012, 58, 41–54. [Google Scholar] [CrossRef]
- Zhang, K.; Greenwood, D.J.; White, P.J.; Burns, I.G. A dynamic model for the combined effects of N, P and K fertilizers on yield and mineral composition; description and experimental test. Plant Soil 2007, 298, 81–98. [Google Scholar] [CrossRef]
- Roberts, T.L. Improving nutrient use efficiency. Turk. J. Agric. For. 2008, 32, 177–182. [Google Scholar]
- Öborn, I.; Andrist-Randel, Y.; Askekaard, M.; Grant, C.A.; Watson, C.A.; Edwards, A.C. Critical aspects of potassium management in agricultural systems. Soil Use Manag. 2005, 21, 102–112. [Google Scholar] [CrossRef]
- Cakmak, I.; Torun, B.; Ernoglu, B.; Ozturk, L.; Marschner, H.; Kalayci, M.; Ekiz, H. Morfological and physiological differences in cereals in response to zinc deficiency. Ephytica 1998, 100, 349–357. [Google Scholar] [CrossRef]
- Oury, F.X.; Leenhardt, F.; Rémésy, C.; Chanliaud, E.; Duperrier, B.; Balfourier, F.; Charmet, G. Genetic variability and stability of grain magnesium, zinc and iron concentrations in bread wheat. Eur. J. Agron. 2006, 25, 177–185. [Google Scholar] [CrossRef]
- Baghour, M.; Moreno, D.A.; Hernández, J.; Castilla, N.; Romero, L. Influence of root temperature on uptake and accumulation of Ni and Co in potato. J. Plant Physiol. 2002, 159, 1113–1122. [Google Scholar] [CrossRef]
- Miyasaka, S.C.; Grunes, D.L. Root zone temperature and calcium effects on phosphorus, sulfur, and micronutrients in winter wheat forage. Argon J. 1997, 89, 743–748. [Google Scholar] [CrossRef]
- Bowen, G.D. Soil temperature, root growth, and plant function. In Plant Roots: The Hidden Half; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1991; pp. 309–330. [Google Scholar]
- Rao, C.R.M.; Sahuquillo, A.; Lopez Sanchez, J.F. A review of the different methods applied in environmental geochemistry for single and sequential extraction of trace elements in soils and related materials. Water Air Soil Pollut. 2008, 189, 291–333. [Google Scholar] [CrossRef]
- Rengel, Z. Availability of Mn, Zn and Fe in the rhizosphere. J. Soil Sci. Plant Nutr. 2015, 15, 397–409. [Google Scholar] [CrossRef]
- Agrawal, R.; Kumar, B.; Priyanka, K.; Narayan, C.; Shukla, K.; Sarkar Anshumali, J. Micronutrient fractionation in coal mine-affected agricultural soils, India. Bull. Environ. Contam. Toxicol. 2016, 96, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Voortman, R.; Bindraban, P.S. Beyond N and P toward a Land Resource Ecology Perspective and Impactful Fertilizer Interventions in Sub-Saharan Africa; VFRC Report 2015/1; Virtual Fertilizer Research Center: Washington, DC, USA, 2015; p. 49. [Google Scholar]
- Bagci, S.A.; Ekiz, H.; Yilmaz, A.; Cakmak, I. Effects of zinc deficiency and drought on grain yield of field-grown wheat cultivars in Central Anatolia. J. Agron. Crop. Sci. 2007, 193, 198–206. [Google Scholar] [CrossRef]
- Costa, L.D.; Vedove, G.D.; Giantquinto, G.; Giovanardi, R.; Peressotti, A. Yield, water use efficiency and nitrogen uptake in potato: Influence of drought stress. Potato Res. 1997, 40, 19–34. [Google Scholar]
- Yuan, B.Z.; Nishiyama, S.; Kang, Y. Effects of different irrigation regimes on the growth and yield of drip-irrigated potato. Agric. Water Manag. 2003, 63, 153–167. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, L.; Xin, Z. Effect of foliar application of zinc, selenium, and iron fertilizers on nutrients concentration and yield of rice grain in China. J. Agric. Food Chem. 2008, 56, 2079–2084. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J.L. Effects of foliar application of a byproduct of the two-step olive oil mill process on rice yield. Eur. J. Agron. 2004, 21, 31–40. [Google Scholar] [CrossRef]
- Abbas, M.K.; Ali, A.S. Effects of foliar application of NPK on some growth characters of two cultivars of roselle (Hibiscus sabdariffa L.). Am. J. Plant Physiol. 2011, 6, 220–227. [Google Scholar] [CrossRef][Green Version]
- Osman, E.A.M.; EL-Masry, A.A.; Khatab, K.A. Effect of nitrogen fertilizer sources and foliar spray of humic and/or fulvic acids on yield and quality of rice plants. Adv. Appl. Sci. Res. 2013, 4, 174–183. [Google Scholar]
- Gaj, R.; Borowski-Beszta, J. Effects of foliar fertilization with potassium and micronutrients on potato yield and quality. Eur. J. Hortic. Sci. 2020. in print. [Google Scholar]
- IGAC. Métodos Analíticos de Laboratorios de Suelo Y Tejidos; Instituto Geográfico Agustín Codazzi: Bogotá, Colombia, 2006. [Google Scholar]
- Jones, J.B.; Case, V.W. Samplig, Handling, Analyzing Plant Tissue Samples. Soil Test. Plant Anal. 1990, 3, 389–427. [Google Scholar]
- Van Gelder, W.N.J. Conversion factor from nitrogen to protein for potato tuber protein. Potato Res. 1981, 24, 423–425. [Google Scholar] [CrossRef]
- Stanisławska-Glubiak, E.; Strączyński, S.; Sienkiewicz-Cholewa, U. Effect of diversified level of yields on the micronutrient content in wheat grain. Zesz. Probl. Post. Nauk. Rol. 1996, 434, 77–81. [Google Scholar]
- Abdoli, M.; Esfandiari, E.; Mousavi, S.B.; Sadeghzadeh, B. Effects of foliar application of zinc sulfate at different phenological stages on yield formation and grain zinc content of bread wheat (cv. Kohdasht). Azarian J. Agric. 2014, 1, 11–17. [Google Scholar]
- Bergmann, W. Colour Atlas: Nutritional Disorders of Plants; Gustav Fisher: New York, NY, USA, 1992; pp. 204–239. [Google Scholar]
- Fageria, N.K.; Baligar, V.C.; Jones, C.A. Growth and Mineral Nutrition of Field Crops, 3rd ed.; Taylor & Francis Group: Oxford, UK, 2011; Available online: http://www.crcpress.com (accessed on 18 August 2015).
- Petrie, S.E.; Jackson, T.L. Effects of nitrogen-fertilization on manganese concentration and yield of barley and oats. Soil Sci. Soc. Am. J. 1984, 48, 319–322. [Google Scholar] [CrossRef]
- Husted, S.; Thomsen, M.U.; Mattsson, M.; Schjoerring, J. Influence of nitrogen and sulphur form on manganese acquisition by barley (Hordeum vulgare). Plant Soil 2005, 268, 309–317. [Google Scholar] [CrossRef]
- Zhang, R.; Guo, Y.X.; Nan, C.Q. Study of trace elements of wheat grain in different fertilizer treatments. Acta Bot. Boreal. Occident. Sin. 2004, 24, 125–129. [Google Scholar]
- René, P.J.J.R.; Heinen, M.; Chistian, O.; Dimkpa, C.O.; Bindraban, P.S. Effects of Nutrient Antagonism and Synergism on Yield and Fertilizer Use Efficiency. Commun. Soil Sci. Plan. 2017, 48, 1895–1920. [Google Scholar]
- Gunes, A.; Alpaslan, M.; Inal, A. Critical nutrient concentrations and antagonistic and synergistic relationships among the nutrients of NFT-grown young tomato plants. Commun. Soil Sci. Plan. 1998, 21, 2035–2047. [Google Scholar] [CrossRef]
- Aciksoz, S.B.; Ozturk, L.; Yazici, A.; Cakmak, I. Inclusion of urea in a 59FeEDTA solution stimulated leaf penetration and translocation of 59Fe within wheat plants. Physiol. Plant. 2014, 151, 348–357. [Google Scholar] [CrossRef]
- Yassen, A.; Abou El-Nour, E.A.A.; Shedeed, S. Response of wheat to foliar spray with urea and micronutrients. Am. J. Sci. 2010, 6, 14–22. [Google Scholar]
- Garcia, R.L.; Hanway, J.J. Foliar fertilization of soybeans during the seed-filling period. J. Agron. 1976, 68, 653–657. [Google Scholar] [CrossRef]
- Gómez, M.I.; Magnitskiy, S.; Rodriguez, L.E. Nitrogen, phosphorus and potassium accumulation and partitioning by the potato group Andigenum in Colombia. Nut. Cycl. Agroecosyst. 2019, 113, 349–363. [Google Scholar] [CrossRef]
- Kimball, B.A.; Morris, C.F.; Pinter, J.P.J.; Wall, G.W.; Hunsaker, D.J.; Damsen, F.J.; Lamorte, R.L.; Leavitt, S.W.; Tompson, T.L.; Mathias, A.D.; et al. Elevated CO2, drought and soil nitrogen effects on wheat grain quality. New Phytol. 2001, 150, 295–303. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, Y.; Chen, X.; Sun, Q.; Zhang, F.; Römhed, V.; Zou, C. Influence of long-term nitrogen fertilization on micronutrient density in grain of winter wheat (Triticum aestivum L.). J. Cereal Sci. 2010, 51, 165–170. [Google Scholar] [CrossRef]
- Ross, F.; Matteo, J.; Cerrudo, A. Maize prolificacy: A source of reproductive plasticity that contributes to yield stability when plant population varies in drought-prone environments. Field Crops Res. 2020, 247, 107699. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil. Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Joudu, J. The chemical composition of potato tuber. In Potato Cultivating; Joudu, J., Ed.; Estonian University of Life Sciences: Tartu, Estonia, 2003; pp. 59–62. [Google Scholar]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and human health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef]
- Haynes, K.G.; Yenchi, G.C.; Clough, M.E.; Henninger, M.R.; Sterrett, S.B. Genetic variation for potato tuber micronutrient content and implications for biofortification of potatoes to reduce micronutrient malnutrition. Am. J. Potato Res. 2012, 89, 192–198. [Google Scholar] [CrossRef]
- Mitrus, J.; Stankiewicz, C.; Steé, E.; Kamecki, M.; Starczewski, J. The influence of selected cultivation on the content of total protein and amino acids in the potato tubers. Plant Soil Environ. 2003, 49, 131–134. [Google Scholar] [CrossRef]
- Fontes, P.C.R.; Braun, H.; Busato, C.; Cecon, P.R. Economic optimum nitrogen fertilization rates and nitrogen fertilization rate effects on tuber characteristics of potato cultivars. Potato Res. 2010, 53, 167–179. [Google Scholar] [CrossRef]
- Gómez, M.I.; Magnitaskiy, S.; Rodrigez, L.E. Critical dilution curves for nitrogen, phosphorus, and potassium in potato Grup Andigenum. Agron. J. 2019, 111, 419–427. [Google Scholar] [CrossRef]
- Liu, H.E.; Wang, Q.Y.; Rengel, Z.; Zhao, P. Zinc fertilization alters flour protein composition of winter wheat genotypes varying in gluten content. Plant Soil Environ. 2015, 61, 195–200. [Google Scholar]
- Peck, A.W.; McDonald, G.K.; Graham, R.D. Zinc nutrition influences the protein composition of flour in bread wheat (Triticum aestivum L.). J. Cereal Sci. 2008, 47, 266–274. [Google Scholar] [CrossRef]
- Graham, R.D.; Rengel, Z. Genotypic variation in zinc uptake and utilization by plants. In Zinc in Soils and Plants; Robson, A.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 107–118. [Google Scholar]

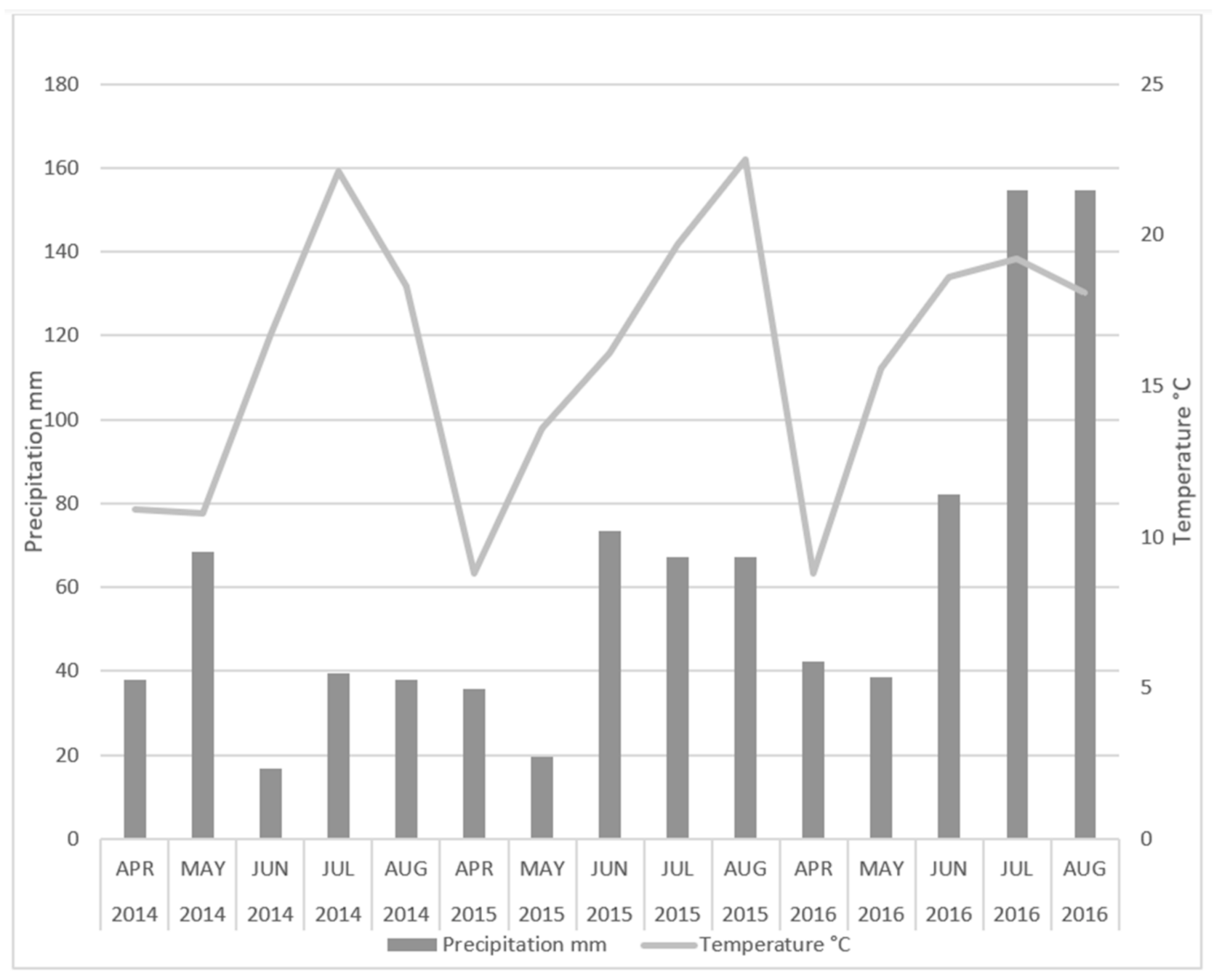

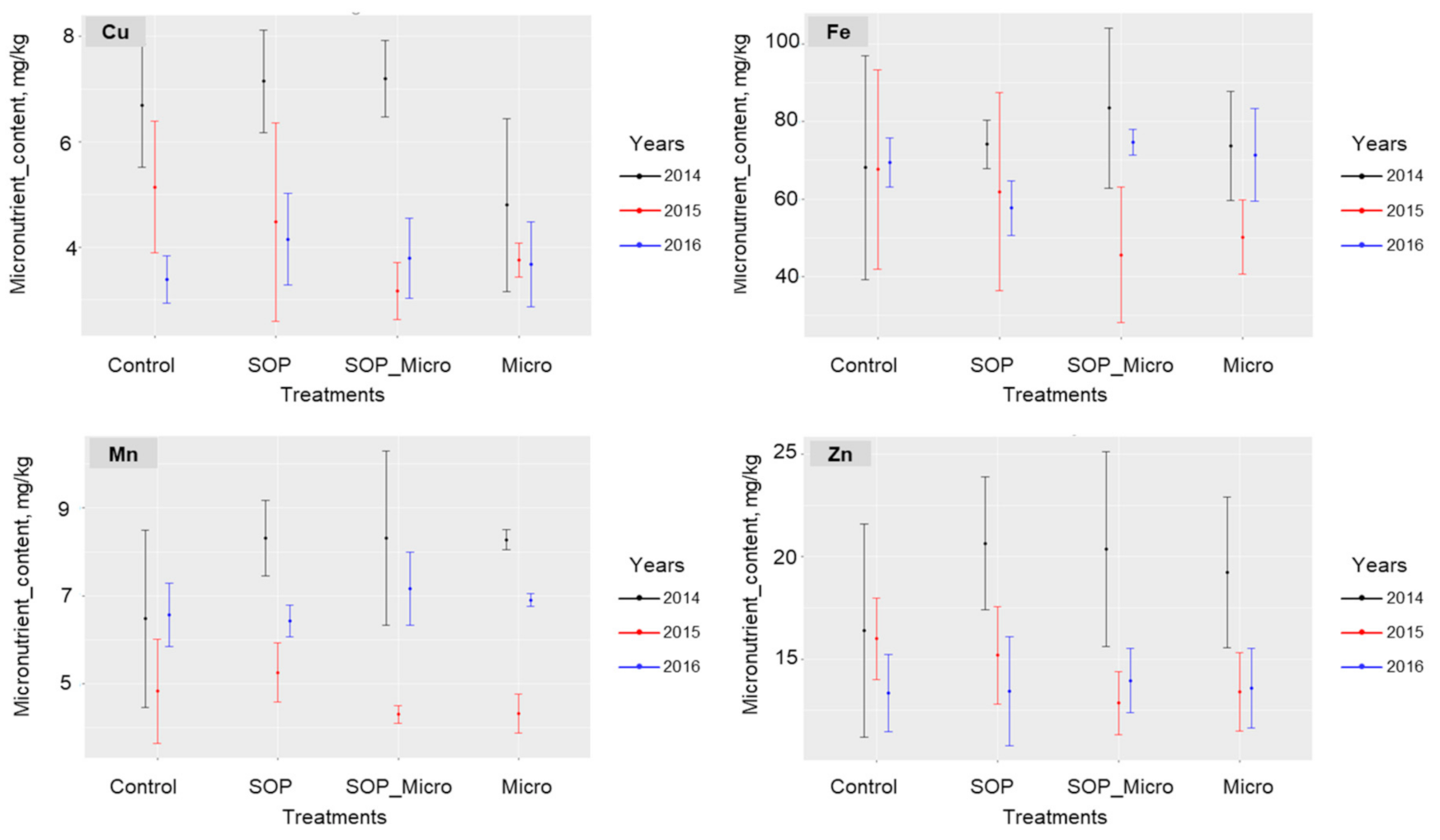


| Varieties | Parameters | Treatments | Zn | Cu | Mn | Fe |
|---|---|---|---|---|---|---|
| Zorba | Yield | Control | −0.292 | 0.142 | −0.451 | 0.129 |
| SOP | 0.636 * | 0.632 * | −0.183 | 0.674 * | ||
| SOP + Micro | −0.212 | 0.013 | −0.752 ** | 0.069 | ||
| Micro | 0.573 | 0.520 | 0.109 | 0.638 * | ||
| Protein content | Control | 0.142 | 0.557 | 0.672 * | −0.421 | |
| SOP | 0.295 | 0.149 | 0.413 | −0.034 | ||
| SOP + Micro | 0.536 * | 0.304 | 0.703 * | 0.247 | ||
| Micro | −0.210 | −0.145 | 0.898 *** | 0.141 | ||
| Hermes | Yield | Control | −0.633 * | −0.687 * | −0.128 | −0.163 |
| SOP | −0.393 | −0.240 | 0.032 | 0.0150 | ||
| SOP + Micro | −0.196 | −0.160 | 0.061 | 0.054 | ||
| Micro | −0.229 | −0.223 | −0.403 | −0.393 | ||
| Protein content | Control | 0.552 | 0.471 | −0.264 | −0.001 | |
| SOP | 0.727 ** | 0.675 * | 0.421 | 0.442 | ||
| SOP + Micro | 0.274 | 0.191 | −0.045 | −0.077 | ||
| Micro | 0.480 | 0.466 | −0.126 | −0.139 |
| Varieties | Treatments | Parameters | Regression Models | R2 |
|---|---|---|---|---|
| Zorba | SOP | Yield | (1) y = 0.6428Fe + 3.36 ± 11.71 * | 0.4056 |
| SOP + Micro | Yield | (2) y = 0.5227Fe-2.8978Mn + 39.43 ± 6.25 ** | 0.7721 | |
| Micro | Yield | (3) y = 0.3382Fe + 25.24 ± 6.63 * | 0.4554 | |
| Control | Protein | (4) y = −0.6792Fe + 15.48Mn + 27.05 ± 7.27 ** | 0.7497 | |
| SOP + Micro | Protein | (5) y = 2.5528Mn + 62.36 ± 10.82 * | 0.4954 | |
| Micro | Protein | (6) y = 10.3804Mn + 13.97 ± 6.98 *** | 0.8078 | |
| SOP | Starch | (7) y = 0.0543Fe + 8.79 ± 0.89 ** | 0.5425 | |
| SOP + Micro | Starch | (8) y = −0.1545Mn + 14.33 ± 0.61 ** | 0.5322 | |
| Micro | Starch | (9) y = 0.0583Fe + 8.29 ± 0.96* | 0.4550 | |
| Hermes | Control | Yield | (10) y = −3.7827Cu + 71.67 ± 7.06 * | 0.4733 |
| Control | Protein | (11) y = 5.2410Zn-9.1092Mn + 79.01 ± 9.68 ** | 0.7465 | |
| SOP | Protein | (12) y = 3.1316Zn + 48.42 ± 12.78 ** | 0.5234 | |
| Control | Starch | (13) y = −0.6500Cu + 17.85 ± 1.08 ** | 0.6022 | |
| SOP | Starch | (14) y = −0.4730Cu + 17.89 ± 1.01 * | 0.4530 | |
| SOP + Micro | Starch | (15) y = −0.2613Mn + 16.78 ± 0.5851 * | 0.3801 |
| Variety | Treatments | Zn | Cu | Mn | Fe |
|---|---|---|---|---|---|
| Zorba | Control | 0.664 * | 0.598 * | 0.811 ** | 0.771 ** |
| SOP | 0.961 *** | 0.872 *** | 0.377 | 0.891 *** | |
| SOP + Micro | 0.841 *** | 0.632 * | −0.113 | 0.853 *** | |
| Micro | 0.960 *** | 0.830 *** | 0.880 *** | 0.923 *** | |
| Hermes | Control | 0.083 | −0.413 | 0.499 | 0.436 |
| SOP | 0.184 | 0.142 | 0.644 * | 0.521 | |
| SOP + Micro | 0.608 * | 0.383 | 0.201 | 0.183 | |
| Micro | 0.295 | −0.076 | −0.268 | −0.274 |
| Varieties | Treatments | Parameters | Regression Models | R2 |
|---|---|---|---|---|
| Zorba | Control | Yield | (1) y = +0.0267 × UpFe + 0.4559 × UpMn + 10.16 ± 3.89 *** | 0.7998 |
| SOP | Yield | (2) y = +0.2655 × UpZn-0.0635 × UpMn + 20.51 ± 1.89 *** | 0.9602 | |
| SOP + Micro | Yield | (3) y = +0.1324 × UpZn + 0.0398 × UpFe-0.2171 × UpMn + 24.20 ± 3.90 *** | 0.9212 | |
| Micro | Yield | (4) y = +0.2751 × UpZn + 13.78 ± 4.21 *** | 0.9231 | |
| Control | Protein | (5) y = −0.0715 × UpFe + 124.07 ± 9.38 ** | 0.5363 | |
| SOP + Micro | Protein | (6) y = −0.0433 × UpFe + 0.6257 × UpMn + 64.74 ± 10.23 * | 0.5938 | |
| Micro | Protein | (7) y = −0.4933 × UpZn + 0.9579 × UpMn + 87.80 ± 10.05 ** | 0.6415 | |
| SOP | Starch | (8) y = +0.0040 × UpFe + 9.98 ± 0.77 ** | 0.6595 | |
| SOP + Micro | Starch | (9) y = +0.0025 × UpFe + 11.69 ± 0.72 * | 0.3428 | |
| Micro | Starch | (10) y = +0.0375 × UpMn + 9.98 ± 0.71 *** | 0.6997 | |
| Hermes | Control | Yield | (11) y = −0.4779 × UpCu + 0.0278 × UpFe + 57.16 ± 6.76 * | 0.5648 |
| SOP | Yield | (12) y = +0.2319 × UpMn + 39.88 ± 5.63 * | 0.4153 | |
| SOP + Micro | Yield | (13) y = +0.1264 × UpZn + 34.11 ± 8.18 * | 0.3699 | |
| Micro | Yield | (14) y = −0.2449 × UpCu + 0.2559 × UpZn + 26.99 ± 5.75 ** | 0.6941 | |
| Control | Protein | (15) y = +0.4905 × UpZn-0.8333 × UpMn + 77.50 ± 9.90 ** | 0.7353 | |
| Control | Starch | (16) y = −0.0252 × UpMn + 16.91 ± 0.54 * | 0.4726 | |
| Micro | Starch | (17) y = −0.0266 × UpCu + 0.0261 × UpZn + 12.42 ± 0.72 * | 0.6105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaj, R.; Górski, D.; Majchrzak, L. The Effect of Potassium and Micronutrient Foliar Fertilisation on the Content and Accumulation of Microelements, Yield and Quality Parameters of Potato Tubers. Agriculture 2020, 10, 530. https://doi.org/10.3390/agriculture10110530
Gaj R, Górski D, Majchrzak L. The Effect of Potassium and Micronutrient Foliar Fertilisation on the Content and Accumulation of Microelements, Yield and Quality Parameters of Potato Tubers. Agriculture. 2020; 10(11):530. https://doi.org/10.3390/agriculture10110530
Chicago/Turabian StyleGaj, Renata, Dariusz Górski, and Leszek Majchrzak. 2020. "The Effect of Potassium and Micronutrient Foliar Fertilisation on the Content and Accumulation of Microelements, Yield and Quality Parameters of Potato Tubers" Agriculture 10, no. 11: 530. https://doi.org/10.3390/agriculture10110530
APA StyleGaj, R., Górski, D., & Majchrzak, L. (2020). The Effect of Potassium and Micronutrient Foliar Fertilisation on the Content and Accumulation of Microelements, Yield and Quality Parameters of Potato Tubers. Agriculture, 10(11), 530. https://doi.org/10.3390/agriculture10110530







