Effect of Phenological Stage and Rooting Enhancers on Physiological Parameters in Stem Cuttings in the Process of Rhizogenesis of Rosa × alba ‘Maiden’s Blush’
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
- M1—flower buds closed (1 June 2012) (BBCH 54 404);
- M2—all flowers in an inflorescence open (9 June 2012) (BBCH 69 625);
- M3—immediately after petal shedding (17 June 2012) (BBCH 69 6N9);
- M4—7–14 days after petal fall (28 June 2012) (BBCH 71 702).
2.2. Evaluation of Rooted Cuttings
2.3. Protein Extraction and Analyse
2.4. Chlorophyll and Carotenoid Analyses
2.5. Statistical Analysis
3. Results
3.1. The Rooting Enhancers Affect Variously the Changes in Leaf Tissues and the Quality and Quantity of Rooted Cuttings Harvested in Four Phenology Stages
3.2. The Changes of Qualitative and Quantitative Parameters of Leaves Affect the Rooting Percentage
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moroz, E.K. Korniesoobstwiennyje rozy w Nacionalnom Parkie ‘Sofiewka’; Nacionalna Akademia Nauk Ukrainy; Centralny Botanicznyj Sad: Umań, Ukraine, 2006. [Google Scholar]
- Gustavsson, L.-Å. Rosen Leksikon; Rosinante: Copenhagen, Denmark, 1999; p. 138. [Google Scholar]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Plant Propagation, Principles and Practices, 7th ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 2011. [Google Scholar]
- Pihlajaniemi, H.; Siurainen, M.; Rautio, P.; Laine, K.; Peteri, S.L.; Huttunen, S. Field evaluation of phenology and success of hardy, micropropagated old shrub roses in northern Finland. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2005, 55, 275–286. [Google Scholar]
- Monder, M.J.; Pacholczak, A. Rhizogenesis and concentration of carbohydrates in cuttings harvested at different phenological stages of once-blooming rose shrubs and treated with rooting stimulants. Biol. Agric. Hortic. 2020, 36, 53–70. [Google Scholar] [CrossRef]
- Hoşafçi, H.; Arslan, N.; Sarihan, E.O. Propagation of dog rose (Rosa canina L.) plants by softwood cuttings. Acta Hortic. 2005, 690, 139–142. [Google Scholar] [CrossRef]
- Monder, M.J.; Niedzielski, M.; Woliński, K. Effect of rooting preparations on protein, chlorophyll and carotenoid content in leaves of Rosa gallica ‘Duchesse d`Angoulême’ cuttings. Dendrobiology 2014, 72, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Monder, M.J.; Niedzielski, M.; Woliński, K.; Pacholczak, A. The impact of seasonal changes in plant tissue on rhizogenesis of stem cuttings of the once flowering roses. Not. Bot. Horti. Agrobo. 2016, 4, 92–99. [Google Scholar] [CrossRef]
- Costa, J.M. The Role of the Leaf in Growth Dynamics and Rooting of Leafy Stem Cuttings of Rose. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2002. [Google Scholar]
- Costa, J.M.; Challa, H. The effect of the original leaf area on growth of softwood cuttings and planting material of rose. Sci. Hortic. 2002, 95, 111–121. [Google Scholar] [CrossRef]
- Okoro, O.O.; Grace, J. The physiology of rooting Populus cuttings. I. Carbohydrates and photosynthesis. Physiol. Plant. 1976, 36, 133–138. [Google Scholar] [CrossRef]
- Afitlhile, M.M. Constituent Processes of Leaf Senescence in Hordeum vulgare cv. Dyan. Master’s Thesis, Rhodes University, Grahamstown, South Africa, 1993. [Google Scholar]
- Gitelson, A.; Merzlyak, M.N. Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum L. and Acer platanoides L. leaves. Spectral features and relation to chlorophyll estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Etienne, P.; Laïné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F.; et al. Brassica napus growth is promoted by Ascophyllum nodosum L. Le. Jol. Seaweed extract, microarray analysis and physiological characterization of N, C, and S metabolism. J. Plant Growth Regul. 2013, 31, 32–52. [Google Scholar] [CrossRef]
- Wojdyła, A. Effectiveness of Atonik SL in the control of powdery mildew, black spot and rust. Folia Hortic. 2004, 16, 175–181. [Google Scholar]
- Whapham, C.A.; Blunden, G.; Jenkins, T.; Hankins, S.D. Significance of betaines in the increased chlorophyll content of plants treated with seaweed extract. J. Appl. Phycol. 1993, 5, 231–234. [Google Scholar] [CrossRef]
- Volfová, A.; Chvojka, L.; Friedrich, A. The effect of kinetin and auxin on the chloroplast structure and chlorophyll content in wheat coleoptiles. Biol. Plantarum. 1978, 20, 440–445. [Google Scholar] [CrossRef]
- Czerpak, R.; Dobrzyń, P.; Krotke, A.; Kicińska, E. The effect of auxins and salicylic acid on chlorophyll and carotenoid contents in Wolffia Arrhiza (L.) Wimm. (Lemnaceae) growing on media of various trophicities. Pol. J. Environ. Stud. 2002, 11, 231–235. [Google Scholar]
- Ranwala, A.; Miller, W.B. Effects of gibberellin treatments on flower and leaf quality of cut hybrid lilies. Acta Hortic. 2002, 570, 205–210. [Google Scholar] [CrossRef]
- Skutnik, E.; Rabiza-Świder, J. Longevity of cut shoots of Molucella laevis L. as affected by flower preservatives and growth regulators. Folia Hortic. 2004, 16, 167–173. [Google Scholar]
- Official Journal of the European Union. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides. Off. J. Eur. Union 2009, 309, 71–86. [Google Scholar]
- Vasconcelos, A.C.F.; Chaves, L.H.G. Biostimulants and their role in improving plant growth under abiotic stresses. In Biostimulants in Plant Science; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Khan, W.; Rayrath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Thorsen, M.K.; Woodward, M.; McKenzie, B.M. Kelp (Laminaria digitata) increases germination and affects rooting and plant vigour in crops and native plants from an arable grassland in the Outer Hebrides, Scotland. J. Coast. Conserv. 2010, 14, 239–247. [Google Scholar] [CrossRef]
- Couée, I.; Hummel, I.; Sulfon, C.; Gouesbet, G.; Amrani, A. Involvement of polyamines in root development. Plant Cell Tiss Org. 2004, 76, 1–10. [Google Scholar] [CrossRef]
- Pacholczak, A.; Szydło, W.; Jacygrad, E.; Federowicz, M. Effect of auxins and the biostimulator Algaminoplant on rhizogenesis in stem cuttings of two dogwood cultivars (Cornus alba ‘Aurea’ and ‘Elegantissima’). Acta Sci. Pol. Hortorum Cultus 2012, 11, 93–103. [Google Scholar]
- Pacholczak, A.; Szydło, W.; Petelewicz, P.; Szulczyk, K. The effect if Algaminoplant on rhizogenesis in stem cuttings of Physocarpus opulifolius ‘Dart’s Gold’ and ‘Red Baron’. Acta Sci. Pol. Hortorum Cultus 2013, 12, 105–116. [Google Scholar]
- Monder, M.J.; Kozakiewicz, P.; Jankowska, A. Anatomical structure changes in stem cuttings of rambler roses induced with plant origin preparations. Sci. Hortic. 2019, 255, 242–254. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, 309, 1–50. [Google Scholar]
- United States Department of Agriculture. National Organic Program. 2017. Available online: http://www.ams.usda.gov/AMSv1.0/NOP2014 (accessed on 21 January 2017).
- Organic Materials Review Institute. OMRI Products List, Web Edition. Available online: http://www.omri.org/sites/default/files/opl_pdf/complete_company.pdf (accessed on 17 January 2017).
- Council Directive no. 91/414/EEC of concerning the placing of plant protection products on the market. Off. J. Eur. Union 1991, 230, 1–32.
- Canna Continental. Bio Rhizotonic. Available online: http://www.biocanna-organics.ca/BioRhizotonic/ (accessed on 19 January 2017).
- BioBizz Worldwide, B.V. Root Juice™. Available online: http://www.biobizz.com/products/#root%c2%b7juice (accessed on 19 January 2017).
- General Hydroponics Europe. Bio Roots. Homepage. Available online: http://gb.eurohydro.com/bio_roots.html/ (accessed on 19 January 2017).
- Morais, T.B.; Swarowsky, A.; Rodrigues, S.N.; Quadros, D.; Christofari, L.Z.; Posser, T.; Pivetta, M. Efeito dos bioestimulantes seed+® e crop+® no índice de clorofila total da soja sob estresse hídrico. Agric. Foco. 2018, 1, 166–171. [Google Scholar] [CrossRef]
- Bulgari, R.; Trivellini, A.; Ferrante, A. Effects of two doses of organic extract based biostimulant on greenhouse lettuce grown under increasing NaCl concentrations. Front. Plant Sci. 2019, 9, 1870. [Google Scholar] [CrossRef] [PubMed]
- El-Baky, H.H.A.; Hussein, M.M.; El-Baroty, G.S. Algal extracts improve antioxidant defense abilities and salt tolerance of wheat plant irrigated with sea water. Afr. J. Biochem. Res. 2008, 2, 151–164. [Google Scholar]
- Blunden, G.; Jenkins, T.; Liu, Y. Enhanced leaf chlorophyll levels in plants treated with seaweed extract. J. App. Phycol. 1997, 8, 535–543. [Google Scholar] [CrossRef]
- Meier, U.; Bleiholder, H.; Brumme, H.; Bruns, E.; Mehring, B.; Proll, T.; Wiegand, J. Phenological growth stages of roses (Rosa sp.): Codification and description according to the BBCH scale. Ann. Appl. Biol. 2008, 154, 231–238. [Google Scholar] [CrossRef]
- Moberg, U.; Winter, A.; Hyden, N.; Ek, K. Analysis of Monoclonal Antibodies during Production and Purification. A Review of Electrophoretic Methods. LKB INSTRUMENTS. Available online: https://www.lkb.com.au/ (accessed on 28 October 2020).
- Ghosh, S.; Gepstein, S.; Heikkila, J.J.; Dumbroff, E.B. Use of scaning densitometer or an ELISA Plate Reader for measurement of nanogram amounts of protein in crude extracts from biological tissues. Anal. Biochem. 1988, 169, 227–233. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzyme in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Wójcik, A.R.; Laudański, Z. Planowanie i Wnioskowanie Statystyczne w Doświadczalnictwie; Wydawnictwo Naukowe PWN: Warsaw, Poland, 1989. [Google Scholar]
- Szydło, W. Intensyfikacja Procesu Rozmnażania Krzewów Ozdobnych Przez Sadzonki Pędowe. Ph.D. Thesis, University of Life Science, Warsaw, Poland, 1999. [Google Scholar]
- Mola, I.; Cozzolini, E.; Ottaiano, L.; Giordano, M.; Rouphael, G.; Mori, M. Effect of vegetal- and seaweed extract-based biostimulants on agronomical and leaf quality traits of plastic tunnel-grown baby lettuce under four regimes of nitrogen fertilization. Agronomy 2019, 9, 571. [Google Scholar] [CrossRef] [Green Version]
- Pacholczak, A.; Nowakowska, K.; Mika, N.; Borkowska, M. The effect of the biostimulator Goteo on the rooting of ninebark stem cuttings. Folia Hortic. 2016, 28, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Smart, C.M. Gene expression during leaf senescence. New Phytol. 1994, 126, 419–448. [Google Scholar] [CrossRef]
- Watanabe, M.; Balazadeh, S.; Tohge, T.; Erban, A.; Giavalisco, P.; Kopka, J.; Mueller-Roeber, B.; Fernie, A.; Hoefgen, R. Comprehensive dissection of spatiotemporal metabolic shifts in primary, secondary, and lipid metabolism during developmental senescence in Arabidopsis. Plant Physiol. 2013, 162, 1290–1310. [Google Scholar] [CrossRef] [Green Version]










| Preparation | Characteristic | Certificate |
|---|---|---|
| Bio Rhizotonic (Canna Continental, 2017) | seaweed-based, 100% organic and contains N-P-K 0.6-0.2-0.6, vitamins such as B1 and B2, and other biologically active substances | Organic Materials Review Institute (OMRI) |
| Root JuiceTM (BioBizz Worldwide B.V. 2017) | combination of humic acid and seaweed extracts, containing N-P-K 2-6-6 | National Organic Program (NOP); Control Union Certified EU; Good Soil Quality Mark; Point Vert; Organic Materials Review Institute (OMRI), Clean Green Certified |
| Bio Roots (GHE, 2017) | amino acids and oligosaccharins, fruit oil up to 1%; humic acids 1%; pectinate 1%; sodium alginate 3%; seaweed species 10%, organic matter 84% | European regulation EC No 834/2007 on organic agriculture (Certificaat Bio Roots 2014). |
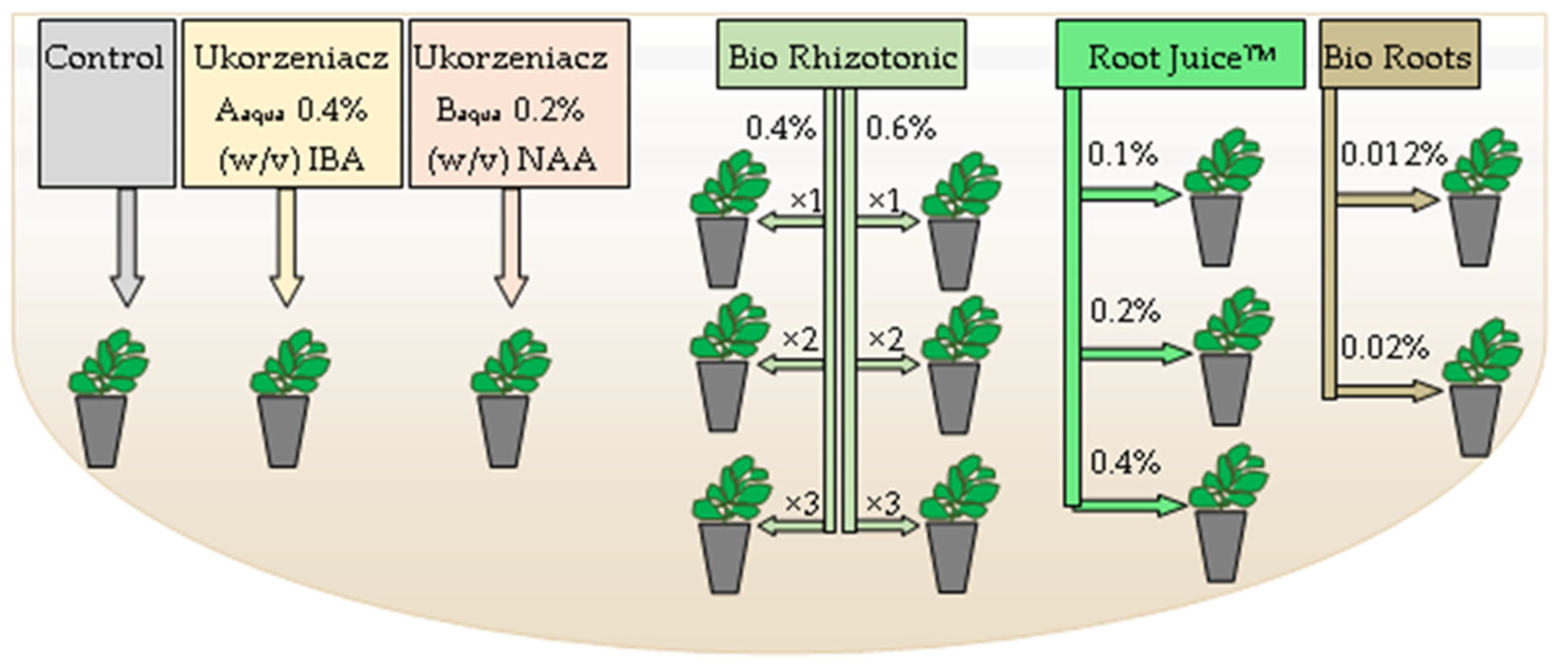
| No. of Treatment | Treatment of Cuttings | |
|---|---|---|
| 1 | Control | |
| 2 | Ukorzeniacz Aaqua 0.4% IBA | |
| 3 | Ukorzeniacz Baqua 0.2% NAA | |
| 4 | 0.4% Bio Rhizotonic | watering (10 mL) after cutting |
| 5 | 0.6% Bio Rhizotonic | |
| 6 | 0.4% Bio Rhizotonic | watering (10 mL) after cutting and 10 days later |
| 7 | 0.6% Bio Rhizotonic | |
| 8 | 0.4% Bio Rhizotonic | watering (10 mL) after cuttings, 10 and 20 days later |
| 9 | 0.6% Bio Rhizotonic | |
| 10 | 0.1% RootJuice | watering (10 mL) after cuttings |
| 11 | 0.2% RootJuice | |
| 12 | 0.4% RootJuice | |
| 13 | 0.012% Bio Roots | |
| 14 | 0.02% Bio Roots | |
| Variable | Rooting Percentage | Percentage of Cuttings with Retained Stock Plant Leaf | Percentage of Cuttings Created a New Shoot | New Shoot Length | Total Leaf Area | Protein | Chloro-phyll a | Chloro-phyll b | Carote-Noids |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1 | 1.00 | ||||||||
| 2 | 0.38 *** | 1.00 | |||||||
| 3 | 0.05 | −0.20 * | 1.00 | ||||||
| 4 | 0.22 * | 0.26 ** | −0.23 * | 1.00 | |||||
| 5 | 0.24 ** | 0.25 ** | −0.06 | 0.19 * | 1.00 | ||||
| 6 | 0.23 * | 0.29 ** | −0.12 * | 0.21 * | 0.07 | 1.00 | |||
| 7 | 0.14 * | 0.06 | −0.09 | 0.15 * | 0.05 | 0.28 | 1.00 | ||
| 8 | 0.15 * | 0.07 | −0.02 | 0.14 * | 0.07 | 0.26 | 0.95 ***** | 1.00 | |
| 9 | 0.13 * | 0.06 | −0.05 | 0.15 * | 0.05 | 0.29 | 0.95 ***** | 0.97 ***** | 1.00 |
| Variable | Rooting Percen-Tage | Percentage of Cuttings with Retained Stock Plant Leaf | Percentage of Cuttings Created a New Shoot | New Shoot Length | Total Leaf Area | Protein | Chloro-phyll a | Chloro-phyll b | Carote-Noids |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1 | 1.00 | ||||||||
| 2 | 0.17 | 1.00 | |||||||
| 3 | 0.09 | 0.15 | 1.00 | ||||||
| 4 | 0.11 | 0.01 | −0.29 ** | 1.00 | |||||
| 5 | −0.05 | 0.04 | −0.01 | 0.01 | 1.00 | ||||
| 6 | −0.25 ** | 0.02 | −0.07 | −0.05 | −0.19 * | 1.00 | |||
| 7 | −0.09 | −0.10 | −0.29 ** | 0.08 | −0.10 | 0.06 | 1.00 | ||
| 8 | −0.07 | −0.08 | −0.26 ** | 0.07 | −0.08 | 0.11 | 0.95 **** | 1.00 | |
| 9 | -0.08 | −0.12 | −0.28 ** | 0.09 | −0.09 | 0.14 | 0.96 **** | 0.97 **** | 1.00 |
| Variable | Rooting Percen-Tage | Percentage of Cuttings with Retained Stock Plant Leaf | Percentage of Cuttings Created a New Shoot | New Shoot Length | Total Leaf Area | Protein | Chloro-phyll a | Chloro-phyll b | Carote-Noids |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1 | 1.00 | ||||||||
| 2 | 0.23 * | 1.00 | |||||||
| 3 | 0.14 | 0.07 | 1.00 | ||||||
| 4 | −0.02 | 0.12 | 0.20 * | 1.00 | |||||
| 5 | 0.33 ** | 0.09 | 0.27 ** | 0.03 | 1.00 | ||||
| 6 | −0.02 | 0.12 | 0.08 | −0.01 | −0.20 | 1.00 | |||
| 7 | 0.00 | 0.09 | 0.02 | −0.04 | 0.07 | 0.13 | 1.00 | ||
| 8 | 0.00 | 0.15 | 0.05 | −0.02 | 0.09 | 0.10 | 0.96 ***** | 1.00 | |
| 9 | −0.01 | 0.16 | 0.05 | −0.01 | 0.06 | 0.14 | 0.94 ***** | 0.98 ***** | 1.00 |
| Variable | Rooting Percen-Tage | Percentage of Cuttings with Retained Stock Plant Leaf | Percentage of Cuttings Created a New Shoot | New Shoot Length | TOTAL leaf Area | Protein | CHLORO-phyll a | chloro-phyll b | Carote-Noids |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1 | 1.00 | ||||||||
| 2 | 0.32 ** | 1.00 | |||||||
| 3 | 0.14 | −0.21 * | 1.00 | ||||||
| 4 | 0.07 | 0.22 * | −0.47 *** | 1.00 | |||||
| 5 | 0.09 | 0.07 | −0.15 | 0.24 ** | 1.00 | ||||
| 6 | 0.00 | −0.19 * | 0.40 *** | −0.33 ** | −0.16 | 1.00 | |||
| 7 | 0.13 | −0.09 | 0.19 * | 0.06 | −0.08 | 0.35 *** | 1.00 | ||
| 8 | 0.10 | −0.06 | 0.22 * | 0.04 | 0.00 | 0.30 ** | 0.93 ***** | 1.00 | |
| 9 | 0.12 | −0.08 | 0.23 * | 0.00 | 0.00 | 0.34 *** | 0.93 ***** | 0.96 ***** | 1.00 |
| Variable | Rooting Percen-Tage | Percentage of Cuttings with Retained Stock Plant Leaf | Percentage of Cuttings Created a New Shoot | NEW Shoot Length | Total Leaf Area | Protein | Chloro-phyll a | Chloro-phyll b | Carote-Noids |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1 | 1.00 | ||||||||
| 2 | 0.06 | 1.00 | |||||||
| 3 | 0.11 | −0.15 | 1.00 | ||||||
| 4 | 0.12 | 0.19 * | 0.01 | 1.00 | |||||
| 5 | 0.10 | 0.18 | -0.01 | 0.14 | 1.00 | ||||
| 6 | −0.01 | −0.06 | −0.02 | 0.04 | −0.23 * | 1.00 | |||
| 7 | 0.16 | −0.13 | −0.07 | 0.02 | −0.14 | 0.25 ** | 1.00 | ||
| 8 | 0.16 | −0.13 | −0.09 | 0.03 | −0.17 | 0.29 ** | 0.96 ***** | 1.00 | |
| 9 | 0.15 | −0.13 | −0.11 | 0.05 | −0.21 * | 0.30 ** | 0.94 ***** | 0.98 ***** | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monder, M.J.; Niedzielski, M.; Woliński, K. Effect of Phenological Stage and Rooting Enhancers on Physiological Parameters in Stem Cuttings in the Process of Rhizogenesis of Rosa × alba ‘Maiden’s Blush’. Agriculture 2020, 10, 572. https://doi.org/10.3390/agriculture10110572
Monder MJ, Niedzielski M, Woliński K. Effect of Phenological Stage and Rooting Enhancers on Physiological Parameters in Stem Cuttings in the Process of Rhizogenesis of Rosa × alba ‘Maiden’s Blush’. Agriculture. 2020; 10(11):572. https://doi.org/10.3390/agriculture10110572
Chicago/Turabian StyleMonder, Marta Joanna, Maciej Niedzielski, and Konrad Woliński. 2020. "Effect of Phenological Stage and Rooting Enhancers on Physiological Parameters in Stem Cuttings in the Process of Rhizogenesis of Rosa × alba ‘Maiden’s Blush’" Agriculture 10, no. 11: 572. https://doi.org/10.3390/agriculture10110572
APA StyleMonder, M. J., Niedzielski, M., & Woliński, K. (2020). Effect of Phenological Stage and Rooting Enhancers on Physiological Parameters in Stem Cuttings in the Process of Rhizogenesis of Rosa × alba ‘Maiden’s Blush’. Agriculture, 10(11), 572. https://doi.org/10.3390/agriculture10110572




