N2O and CO2 Emissions from Bare Soil: Effect of Fertilizer Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Experiment
2.2. N2O–N and CO2–C Emissions
2.3. Soil Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Soil N2O–N Fluxes
3.2. Soil CO2–C Fluxes
3.3. Cumulative Greenhouse Gas (GHG) Emissions from the Soil
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NIL | non-fertilized treatment |
| TD | top dressing |
| DP | deep placement |
References
- Nkebiwe, M.; Weinmann, M.; Bar-Tal, A.; Müller, T. Fertilizer placement to improve crop nutrient acquisition and yield: A review and meta-analysis. Field Crop Res. 2016, 196, 389–401. [Google Scholar] [CrossRef]
- Signor, D.; Cerri, C.E.P.; Conant, R. N2O emissions due to nitrogen fertilizer applications in two regions of sugarcane cultivation in Brazil. Environ. Res. Lett. 2013, 8, 9. [Google Scholar] [CrossRef]
- Liu, X.; Mosier, A.R.; Halvorson, A.D.; Zhang, F.S. The impact of nitrogen placement and tillage on NO, N2O, CH4 and CO2 fluxes from a clay loam soil. Plant Soil 2006, 280, 177–188. [Google Scholar] [CrossRef]
- Gaihre, Y.K.; Singh, U.; Huda, A.; Islam, S.M.M.; Islam, M.R.; Biswas, J.C.; DeWald, J. Nitrogen use efficiency, crop productivity and environmental impacts of urea deep placement in lowland rice fields. In Proceedings of the 2016 International Nitrogen Initiative Conference, “Solution to Improve Nitrogen use Efficiency for the World”, Melbourne, Australia, 4–8 December 2016; Volume 1, pp. 1–4. [Google Scholar]
- Chatterjee, D.; Mohanty, S.; Guru, P.K.; Swain, C.K.; Tripathi, R.; Shahid, M.; Kumar, U.; Kumar, A.; Bhattacharyya, P.; Gautam, P.; et al. Comparative assessment of urea briquette applicators on greenhouse gas emission, N loss and soil enzymatic activities in tropical lowland rice. Agric. Ecosyst. Environ. 2018, 252, 178–190. [Google Scholar] [CrossRef]
- Chapuis-Lardy, L.; Wrage, N.; Metay, A.; Bernoux, M. Soils, a sink for N2O? A review. Glob. Chang. Biol. 2006, 13, 1–17. [Google Scholar] [CrossRef]
- Rutkowska, B.; Szulc, W.; Szara, E.; Skowrońska, M.; Jadczyszyn, T. Soil N2O emissions under conventional and reduced tillage methods and maize cultivation. Plant Soil Environ. 2017, 63, 242–347. [Google Scholar]
- Adviento-Borbe, M.A.A.; Linquist, B. Assessing fertilizer N placement on CH4 and N2O emissions in irrigated rice systems. Geoderma 2016, 266, 40–45. [Google Scholar] [CrossRef]
- Linquist, B.A.; Adviento-Borbe, M.A.; Pittelkow, C.M.; van Kessel, C.; van Groenigen, K.J. Fertilizer management practices and greenhouse gas emissions from rice systems: A quantitative review and analysis. Field Crops Res. 2012, 135, 10–21. [Google Scholar] [CrossRef]
- Liu, T.Q.; Li, S.H.; Guo, L.G.; Cao, C.G.; Li, C.F.; Zhai, Z.B.; Zhou, J.Y.; Mei, Y.M.; Ke, H.J. Advantages of nitrogen fertilizer deep placement in greenhouse gas emissions and net ecosystem economic benefits from no-tillage paddy fields. J. Clean. Prod. 2020, 263, 121322. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Phil. Trans. R. Soc. B 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia oxidation pathways and nitrifier denitrification are significant source of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Martín, L.; Vallejo, A.; Dick, J.; Skiba, U.M. The influence of soluble carbon and fertilizer nitrogen on nitric oxide and nitrous oxide emissions from two contrasting agricultural soils. Soil Biol. Biochem. 2008, 40, 142–151. [Google Scholar] [CrossRef]
- Sosulski, T.; Szara, E.; Stępień, W.; Szymańska, M. Nitrous oxide emissions from the soil under different fertilization systems on a long-term experiment. Plant Soil Environ. 2014, 60, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Sosulski, T.; Szymańska, M.; Szara, E. CO2 Emissions from soil under fodder maize cultivation. Agronomy 2020, 10, 1087. [Google Scholar] [CrossRef]
- Sosulski, T.; Szara, E.; Stępień, W.; Szymańska, M.; Borowska-Komenda, M. Carbon and nitrogen leaching in long-term experiments and DOC/N-NO3- ratio in drainage water as an indicato of denitrification potential in different fertilization and crop rotation systems. Fresenius Environ. Bull. 2016, 25, 2813–2824. [Google Scholar]
- Doroszewski, A.; Pudełko, R.; Żyłowska, K.; Jadczyszyn, J.; Koza, P.; Wróblewska, E.; Nowak, A. Communication Report Regarding the Incidences of Drought Conditions in Poland. 2019. Available online: http://www.susza.iung.pulawy.pl/en/komentarz/2019,08/ (accessed on 8 August 2020).
- Zhai, L.; Liu, H.; Zhang, J.; Huang, J.; Wang, B. Long-term application of organic manure and mineral fertilizer on N2O and CO2 emissions in a red soil from cultivated maize-wheat rotation in China. Agric. Sci. China 2011, 10, 1748–1757. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J. Effects of soil moisture, temperature, and nitrogen fertilization on soil respiration and nitrous oxide emission during maize growth period in northeast China. Acta Agric. Scand. B 2009, 59, 97–106. [Google Scholar] [CrossRef]
- Dai, Z.; Mengjie Yu, M.; Chen, H.; Zhao, H.; Huang, Y.; Su, W.; Xia, F.; Chang, S.X.; Brookes, P.C.; Dahlgren, R.A.; et al. Elevated temperature shifts soil N cycling from microbial immobilization to enhanced mineralization, nitrification and denitrification across global terrestrial ecosystems. Glob. Chang. Biol. 2020, 26, 5267–5276. [Google Scholar] [CrossRef]
- Pareja-Sánchez, E.; Cantero-Martinez, C.; Alvaro-Fuentes, J.; Plaza-Bonilla, D. Tillage and nitrogen fertilization in irrigated maize: Key practices to reduce soil CO2 and CH4 emission. Soil Tillage Res. 2019, 191, 29–36. [Google Scholar] [CrossRef]
- Adviento-Borbe, M.A.A.; Haddix, M.L.; Binder, D.L.; Walters, D.T.; Dobermann, A. Soil greenhouse gas fluxes and global warming potential in four high-yielding maize systems. Glob. Chang. Biol. 2007, 13, 1972–1988. [Google Scholar] [CrossRef]
- Amos, B.; Arkebauer, T.J.; Doran, J.W. Soil surface fluxes of greenhouse gases in an irrigated maize-based agroecosystem. Soil Sci. Soc. Am. J. 2005, 69, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Kopiński, J. Diversity of nitrogen management in Polish agriculture. Pol. J. Agron. 2018, 32, 2–16. (In Polish) [Google Scholar]
- Burton, D.L.; Zebarth, B.J.; Gillam, K.M.; MacLeod, J.A. Effect of split application of fertilizer nitrogen on N2O emissions from potatoes. Can. J. Soil Sci. 2008, 88, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Bosco, S.; Volpi, I.; Antichi, D.; Ragaglini, G.; Frasconi, C. Greenhouse Gas Emissions from Soil Cultivated with Vegetables in Crop Rotation under Integrated, Organic and Organic Conservation Management in a Mediterranean Environment. Agronomy 2019, 9, 446. [Google Scholar] [CrossRef] [Green Version]
- Myhre, G.D.; Shindell, F.-M.; Bréon, W.; Collins, J.; Fuglestvedt, J.; Huang, D.; Koch, J.-F.; Lamarque, D.; Lee, B.; Mendoza, T.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F.D., Qin, G.-K., Plattner, M., Tignor, S.K., Allen, J., Boschung, A., Nauels, Y., Xia, V., Bex, P., Midgley, M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 659–740. [Google Scholar]
- Zsolnay, A. Dissolved Humus in Soil Waters. In Humic Substances in Terrestrial Ecosystems; Piccolo, A., Ed.; Elsevier: Amsterdam, The Netherlands, 1996; pp. 171–223. [Google Scholar]
- McVicar, K.; Kellman, L. Growing season nitrous oxide fluxes across a 125+ year harvested red spruce forest chronosequence. Biochemistry 2014, 120, 225–238. [Google Scholar] [CrossRef]
- McSwiney, C.P.; Robertson, G.P. Nonlinear response of N2O flux to incremental fertilizer addition in a continuous maize (Zea mays L.) cropping system. Glob. Chang. Biol. 2005, 11, 1712–1719. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E. Effects of nitrogen fertilizer application on greenhouse gas emissions and economics of corn production. Environ. Sci. Technol. 2008, 42, 6028–6033. [Google Scholar] [CrossRef]
- Hofstra, N.; Bouwman, A.F. Denitrification in agricultural soils: Summarizing published data and estimating global annual rates. Nutr. Cycl. Agroecosyst. 2005, 72, 267–278. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Emissions of N2O and NO from fertilized fields: Summary of available measurement data. Glob. Biogeochem. Cycles 2002, 16, 1–13. [Google Scholar] [CrossRef]
- Van Groenigen, J.W.; Velthhof, G.L.; Oenema, O.; Van Groeningen, K.J.; Van Kessel, C. Towards an agronomic assessment of N2O emissions: A case study for arable crops. Eur. J. Soil Sci. 2010, 61, 903–913. [Google Scholar] [CrossRef]
- Meng, L.; Ding, W.; Cai, Z. Long-term application of organic manure and nitrogen fertilizer on N2O emissions, soil quality and crop production in a sandy loam soil. Soil Biol. Biochem. 2005, 37, 2037–2045. [Google Scholar] [CrossRef]
- Jungkunst, H.F.; Freibauer, A.; Neufeldt, H.; Bareth, G. Nitrous oxide emissions from agricultural land use in German—A synthesis of available annual field data. J. Plant Nutr. Soil Sci. 2006, 169, 341–351. [Google Scholar] [CrossRef]
- Rudaz, A.O.; Wälti, E.; Kyburz, G.; Lehmann, P.; Fuhrer, J. Temporal variation in N2O and N2 fluxes from a permanent pasture in Switzerland in relation to management, soil water content and soil temperature. Agric. Ecosyst. Environ. 1999, 73, 83–91. [Google Scholar] [CrossRef]
- Kaiser, E.-A.; Kohrs, K.; Kücke, M.; Schnug, E.; Heinemeyer, O.; Munch, J.C. Nitrous oxide release from arable soil: Importance of N-fertilization, crops and temporal variation. Soil Biol. Biochem. 1998, 30, 1553–1563. [Google Scholar] [CrossRef]
- Kaiser, E.A.; Heinemeyer, O. Temporal changes in N2O losses from two arable soils. Plant Soil. 1996, 181, 57–63. [Google Scholar] [CrossRef]
- Sosulski, T.; Szara, E.; Stępień, W.; Rutkowska, B. The influence of mineral fertilization and legumes cultivation on the N2O soil emissions. Plant Soil Environ. 2015, 61, 529–536. [Google Scholar] [CrossRef]
- Morkved, P.T.; Dorsch, P.; Bakken, L.R. The N2O product ratio of nitrification and its dependence on long-term changes in soil pH. Soil Biol. Biochem. 2007, 39, 2048–2057. [Google Scholar] [CrossRef]
- Van Den Heuvel, R.N.; Bakker, S.E.; Jetten, M.S.M.; Hefting, M.M. Decreased N2O reduction by low soil pH causes high N2O emissions in a riparian ecosystem. Geobiology 2011, 9, 294–300. [Google Scholar] [CrossRef]
- Kesik, M.; Blagodatsky, S.; Papen, H.; Butterbach-Bahl, K. Effect of pH, temperature and substrate on N2O, NO and CO2 production by Alcaligenes faecalis p. J. Appl. Microbiol. 2006, 101, 655–667. [Google Scholar] [CrossRef]
- Sosulski, T.; Szara, E.; Stępień, W.; Szymańska, M. Impact of liming management on N2O emissions from arable soils in three long-term fertilization experiments in Central Poland. Fresenius Environ. Bull. 2016, 25, 6111–6119. [Google Scholar]
- Russow, R.; Sich, I.; Neue, H.U. The formation of the trace gases NO and N2O in soils by the coupled process of nitrification and denitrification: Results of kinetic 15N tracer investigations. Chemosphere Glob. Chang. Sci. 2000, 2, 359–366. [Google Scholar] [CrossRef]
- Vermoesen, A.; de Groot, C.J.; Nollet, L.; Boeckx, P.; van Cleemput, O. Effect of ammonium and nitrate application on the NO and N2O emission out of different soils. Plant Soil 1996, 181, 153–162. [Google Scholar] [CrossRef]
- Baggs, E.M.; Smales, C.L.; Bateman, E.J. Changing pH shifts the microbial source as well as the magnitude of N2O emission from soil. Biol. Fertil. Soils 2010, 46, 793–805. [Google Scholar] [CrossRef]
- Van Kessel, C.; Venterea, R.; Six, J.; Adviento-Borbe, M.A.; Linquist, B.; van Groenigen, K.J. Climate, duration, and N placement determine N2O emissions in reduced tillage systems: A meta-analysis. Glob. Chang. Biol. 2013, 19, 33–44. [Google Scholar] [CrossRef]
- Millar, N.; Doll, J.E.; Robertson, G.P. Management of nitrogen fertilizer to reduce nitrous oxide (N2O) emissions from field crops. In Climate Change and Agriculture Fact Sheet Series—MSU Extension Bulletin, E3152; Michigan State University: East Lansing, MI, USA, November 2014; Volume 5. [Google Scholar]
- Hosen, Y.; Paisancharoen, K.; Tsuruta, H. Effects of deep application of urea on NO, N2O emissions from an Andidol. Nutr. Cycl. Agroecosyst. 2002, 63, 197–206. [Google Scholar] [CrossRef]
- Bateman, E.J.; Baggs, E.M. Contribution of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Wang, L.F.; Cai, Z.C.; Yang, L.F.; Meng, L. Effects of disturbance and glucose addition on nitrous oxide and carbon dioxide emissions from a paddy soil. Soil Tillage Res. 2005, 82, 185–194. [Google Scholar] [CrossRef]
- Rutkowska, B.; Szulc, W.; Sosulski, T.; Skowrońska, M.; Szczepaniak, J. Impact of reduced tillage on emission from soil under maize cultivation. Soil Tillage Res. 2018, 180, 21–28. [Google Scholar] [CrossRef]
- Włodarczyk, T.; Brzezińska, M.; Borkowska, A.; Kotowska, U.; Nosalewicz, M.; Szarlip, P.; Pazur, M. Carbon dioxide fluxes from arable soils under conventional and reduced tillage methods. Acta Agrophys. 2007, 150, 29–41. (In Polish) [Google Scholar]
- Brzezińska, M.; Włodarczyk, T.; Witkowska-Walczak, B.; Sławinski, C.; Borkowska, A.; Kotowska, U.; Nosalewicz, M.; Szarlip, P. Influence of soil moisture and temperature on the concentration and CO2 emissions from arable soils under conventional and reduced tillage methods. Acta Agrophys. 2007, 150, 41–53. (In Polish) [Google Scholar]
- Rochette, P.; Flanagan, L.B.; Gregorich, E.G. Separating soil respiration into plant and soil components using analyses of the natural abundance of carbon-13. Biology and Biochemistry. Soil Sci. Soc. Am. J. 1999, 63, 1207–1213. [Google Scholar] [CrossRef]
- Ding, W.; Cai, Y.; Cai, Z.; Yagi, K.; Zheng, X. Soil Respiration under Maize Crops: Effects of Water, Temperature, and Nitrogen Fertilization. Soil Biol. Biochem. 2007, 71, 944–951. [Google Scholar] [CrossRef]
- DeForest, J.L.; Zak, D.R.; Pregitzer, K.S.; Burton, A.J. Atmospheric nitrate decomposition, microbial community composition, and enzyme activity in Northern Hardwood Forests. Soil Sci. Soc. Am. J. 2004, 68, 132–138. [Google Scholar] [CrossRef] [Green Version]
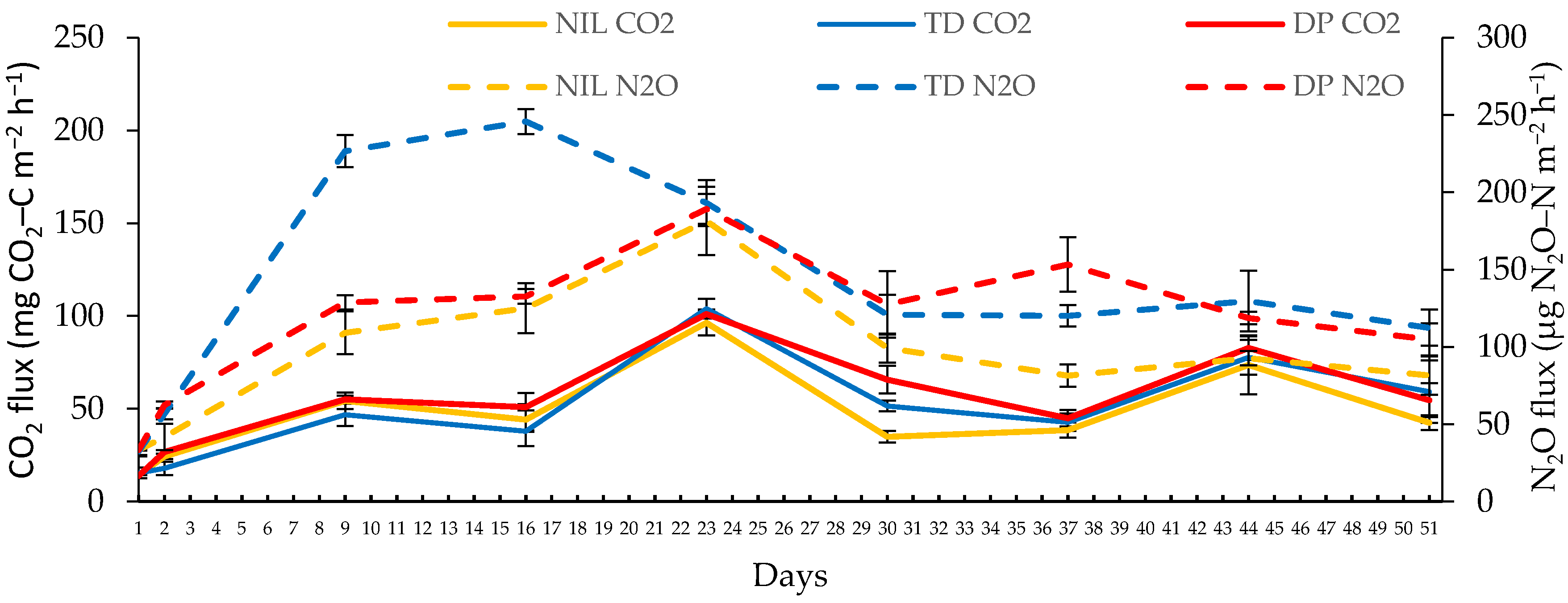
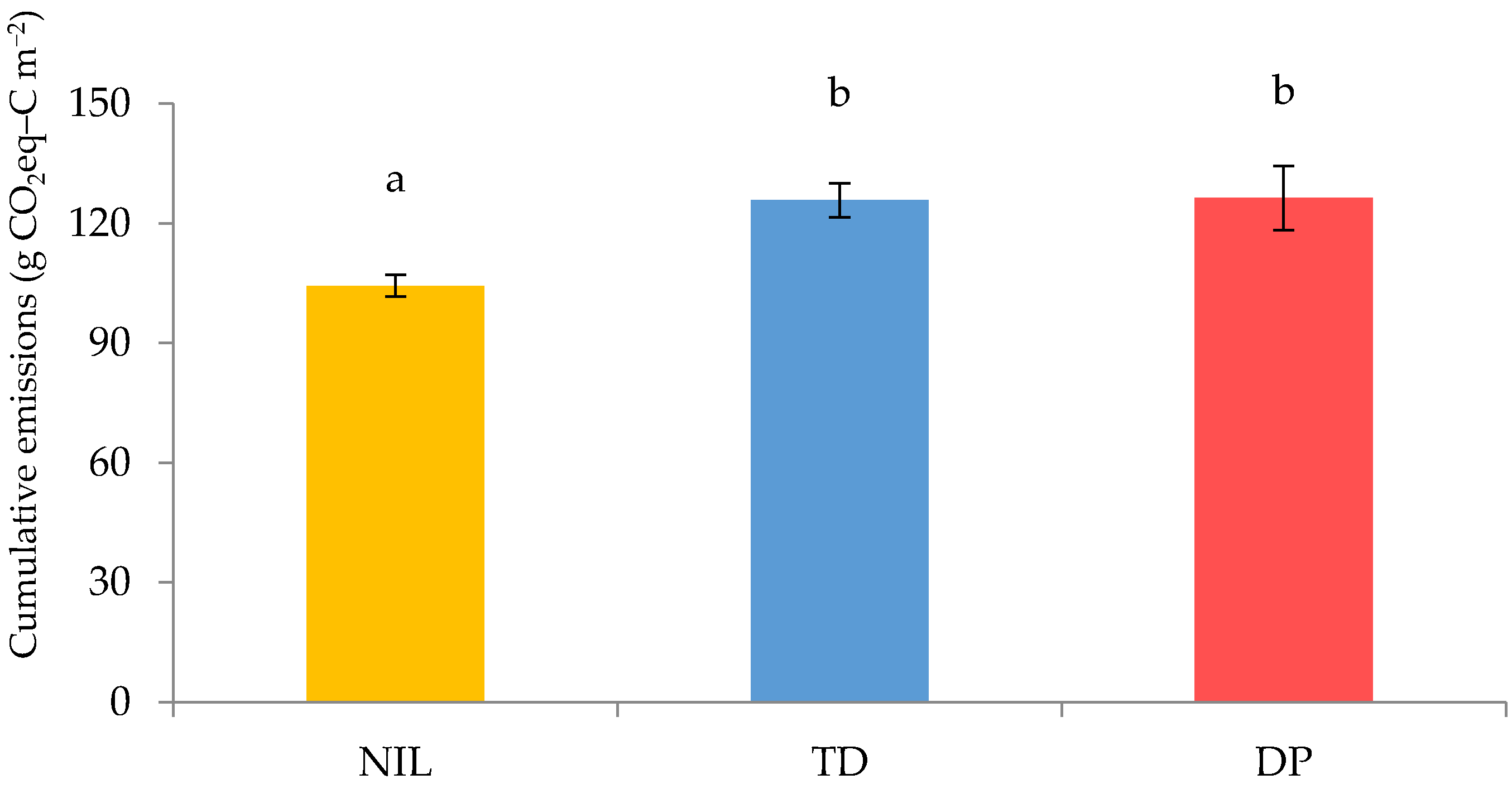
| Treatment | N2O–N Emissions | |||
|---|---|---|---|---|
| Daily | Cumulative From: | |||
| Soil | NH4NO3 | |||
| µg N m−2 h−1 | mg N m−2 | |||
| NIL | mean | 94.0 a ± 5.6 | 133.5 a ± 6.7 | - |
| median | 93.3 | 137.4 | - | |
| min–max | 33.0–206.7 | 125.7–137.4 | - | |
| TD | mean | 137.5 b ± 3.3 | 190.8 b ± 5.6 | 57.3 a ± 7.3 |
| median | 125.9 | 188.8 | 59.8 | |
| min–max | 30.2–254.8 | 186.4–197.2 | 49.0–63.1 | |
| DP | mean | 116.8 c ± 4.7 | 169.4 b ± 20.2 | 35.9 b ± 26.7 |
| median | 123.5 | 164.7 | 27.3 | |
| min–max | 29.5–200.2 | 151.9–191.5 | 14.5–65.8 | |
| Treatment | Soil Content | ||||
|---|---|---|---|---|---|
| NO3−–N | DOC | ||||
| 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | ||
| mg N kg−1 | mg C kg−1 | ||||
| mean | 6.84 a ± 1.6 | 6.27 a ± 1.6 | 9.12 ab ± 0.7 | 8.03 b ± 0.9 | |
| NIL | median | 5.93 | 5.59 | 8.99 | 8.04 |
| min–max | 5.90–8.69 | 5.13–5.59 | 8.52–9.86 | 7.15–8.90 | |
| mean | 163.13 b ± 22.5 | 14.55 a ± 1.9 | 9.54 ab ± 1.4 | 11.81 a ± 1.3 | |
| TD | median | 174.2 | 14.46 | 9.56 | 11.51 |
| min–max | 137.2–178.0 | 12.74–16.46 | 8.18–10.88 | 10.69–13.23 | |
| mean | 10.03 a ± 0.8 | 160.63 b ± 20.4 | 12.64 a ± 2.3 | 9.78 ab ± 0.9 | |
| DP | median | 9.76 | 167.2 | 13.61 | 10.30 |
| min–max | 9.45–10.89 | 127.8–176.9 | 10.04–14.26 | 8.73–10.32 | |
| Treatment | CO2–C Emissions | ||
|---|---|---|---|
| Daily | Cumulative | ||
| mg C m−2 h−1 | g C m−2 | ||
| NIL | mean | 47.0 a ± 25.0 | 64.0 a ± 4.0 |
| median | 40.7 | 64.7 | |
| min–max | 14.1–100.5 | 59.8–67.6 | |
| TD | mean | 50.4 ab ± 27.5 | 68.4 ab ± 2.6 |
| median | 46.4 | 68.0 | |
| min–max | 12.7–109.2 | 66.0–71.1 | |
| DP | mean | 55.1 b ± 25.8 | 75.4 b ± 2.6 |
| median | 52.9 | 75.1 | |
| min–max | 12.9–102.5 | 71.5–79.42 | |
| Parameter | N2O–N | NO3−–N | DOC |
|---|---|---|---|
| N2O–N | 1.00 | 0.55 * | 0.57 * |
| CO2–C | 0.24 | 0.34 | 0.44 * |
| NO3−–N | 0.55 * | 1.00 | 0.03 |
| DOC | 0.57 * | 0.03 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosulski, T.; Stępień, W.; Wąs, A.; Szymańska, M. N2O and CO2 Emissions from Bare Soil: Effect of Fertilizer Management. Agriculture 2020, 10, 602. https://doi.org/10.3390/agriculture10120602
Sosulski T, Stępień W, Wąs A, Szymańska M. N2O and CO2 Emissions from Bare Soil: Effect of Fertilizer Management. Agriculture. 2020; 10(12):602. https://doi.org/10.3390/agriculture10120602
Chicago/Turabian StyleSosulski, Tomasz, Wojciech Stępień, Adam Wąs, and Magdalena Szymańska. 2020. "N2O and CO2 Emissions from Bare Soil: Effect of Fertilizer Management" Agriculture 10, no. 12: 602. https://doi.org/10.3390/agriculture10120602
APA StyleSosulski, T., Stępień, W., Wąs, A., & Szymańska, M. (2020). N2O and CO2 Emissions from Bare Soil: Effect of Fertilizer Management. Agriculture, 10(12), 602. https://doi.org/10.3390/agriculture10120602






