Enteric Methane Emissions of Dairy Cattle Considering Breed Composition, Pasture Management, Housing Conditions and Feeding Characteristics along a Rural-Urban Gradient in a Rising Megacity
Abstract
1. Introduction
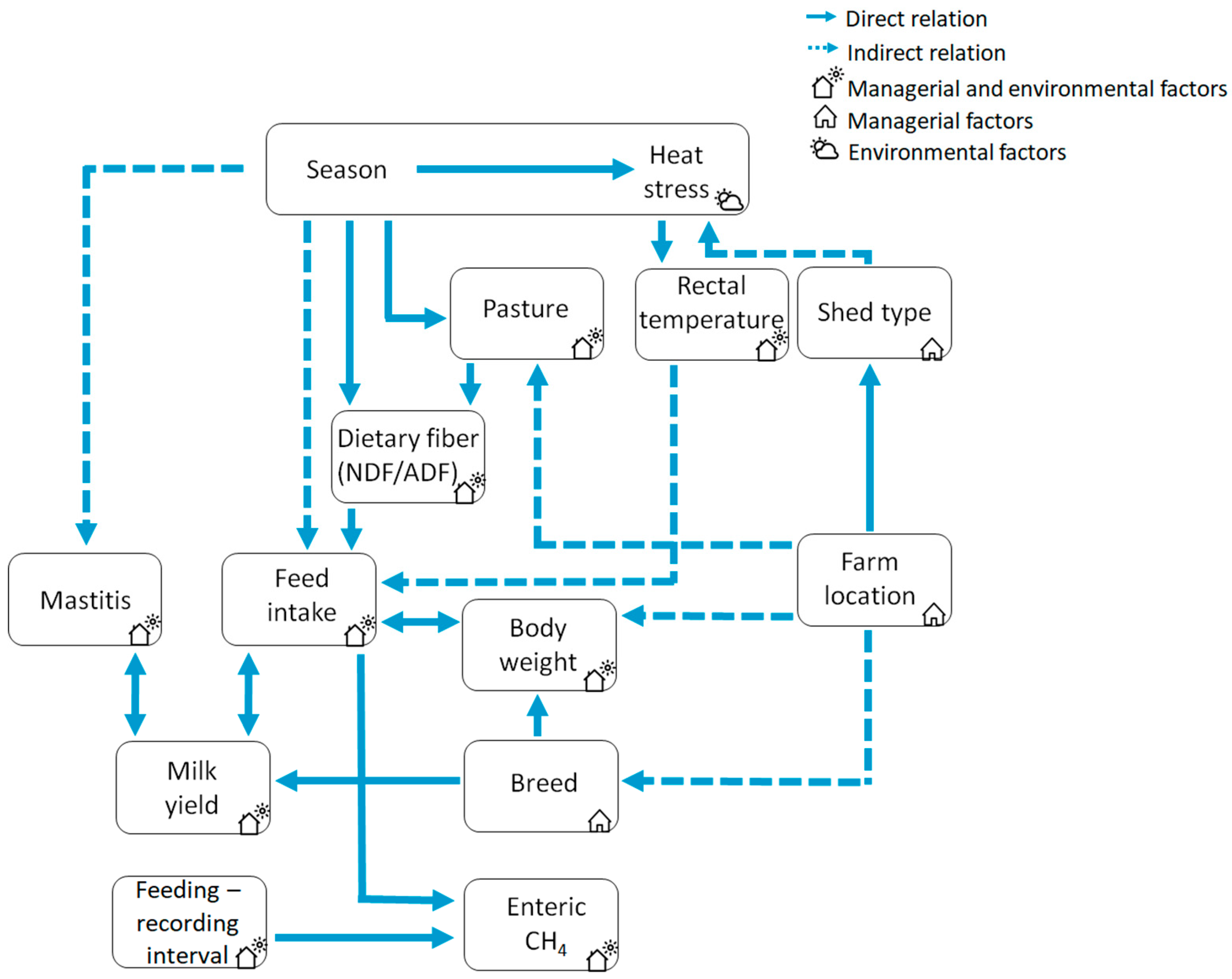
2. Materials and Methods
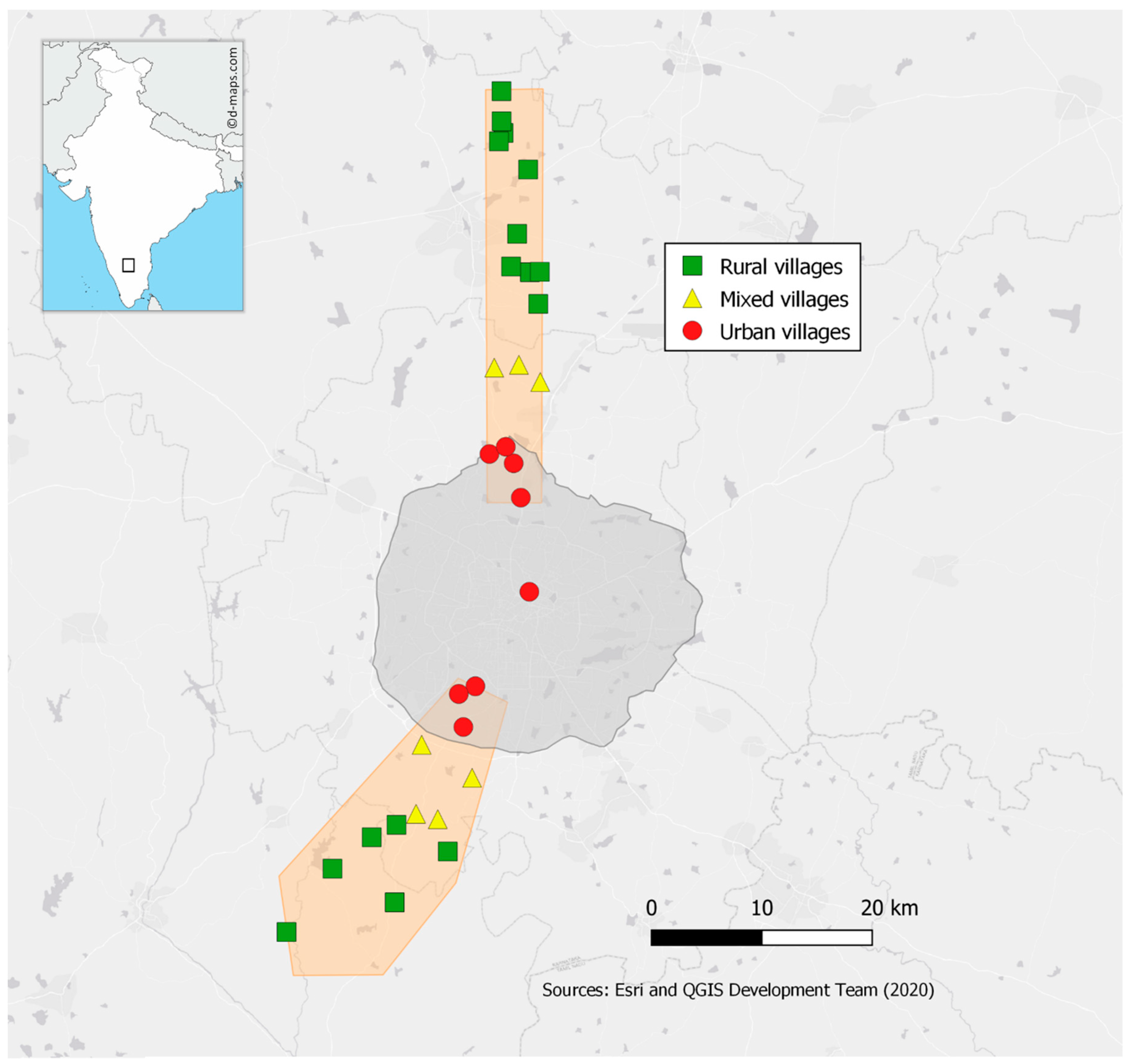
2.1. Farm Selection and Description
2.2. Cattle Production and Conformation Traits
2.3. Methane Spot Measurements
2.4. Nutrition Monitoring Subsample
2.5. Statistical Models
3. Results
3.1. Basic Environmental Factors on CH4 Concentrations
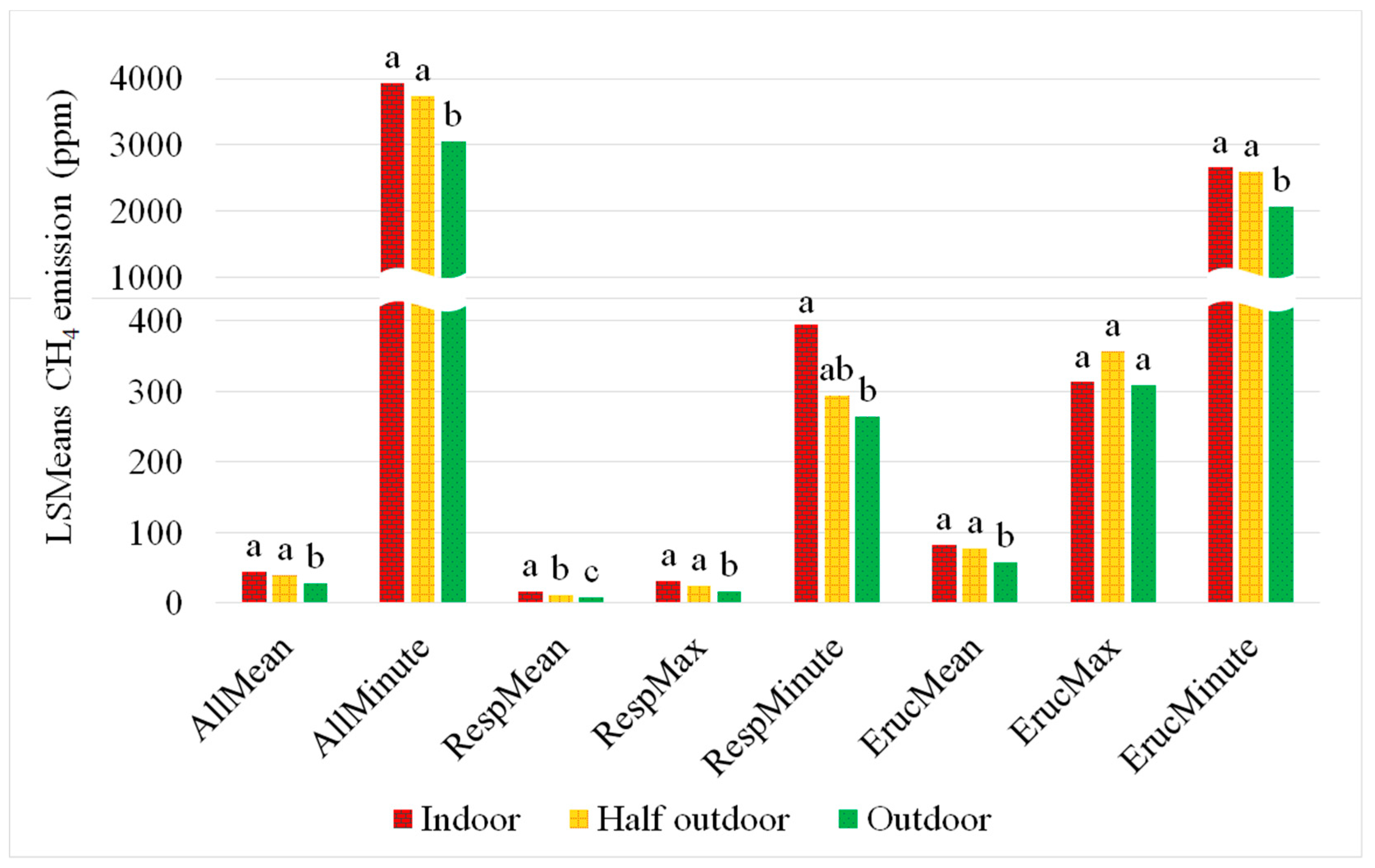
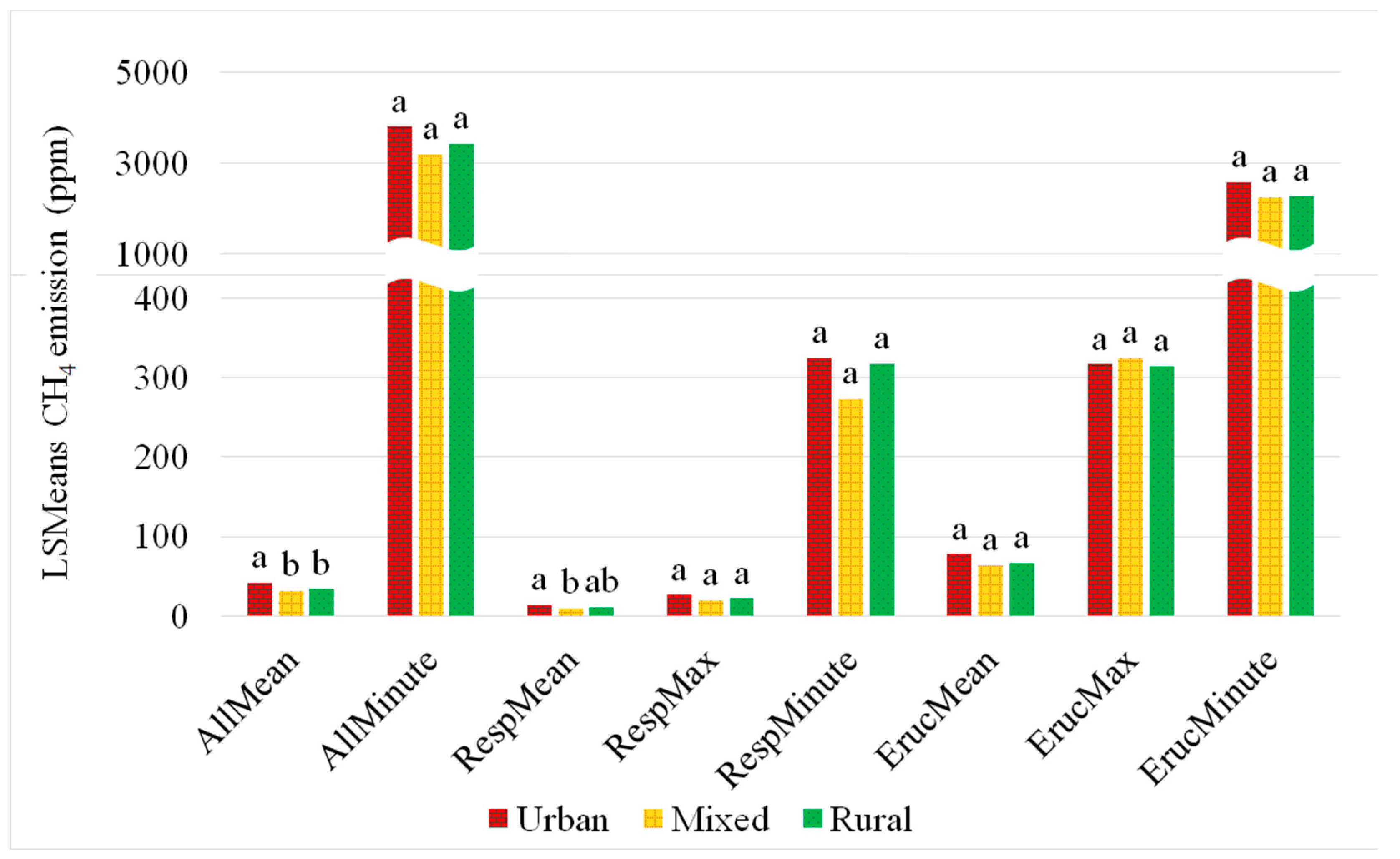
3.2. Impact of Measurement Location on CH4 Concentrations
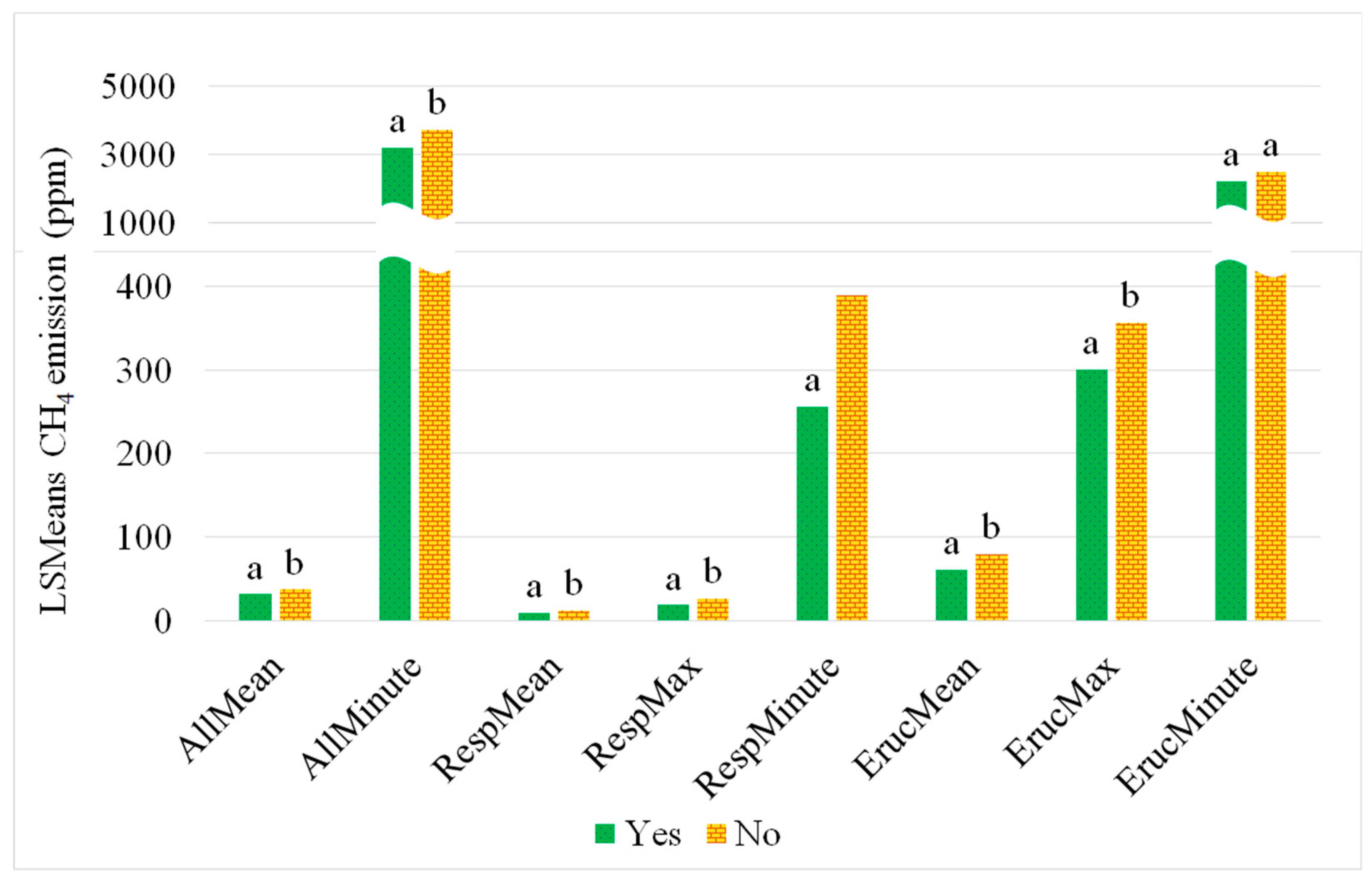
3.3. Impact of Access to Pasture on CH4 Concentrations
3.4. Impact of Fibre Intake on CH4 Concentrations
3.5. Impact of SSI Cluster on CH4 Concentrations
4. Discussion
4.1. Methane Emission Traits
4.2. Body Weight and Milk Yield
4.3. Time Interval from Feeding to CH4 Recording
4.4. Breed
4.5. Heat Stress and Measurement Location
4.6. Pasture Access and Fibre Intake
4.7. Rural-Urban Gradient
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Live Animals & Livestock Primary Dataset. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data/QA (accessed on 13 February 2020).
- International Farm Comparison Network Dairy Report 2011. For a Better Understanding of Milk Production World-Wide. IFCN—Dairy Research Center, Kiel, Germany. Available online: https://ifcndairy.org/wp-content/uploads/2017/07/Dairy-Report-2011.pdf (accessed on 11 February 2020).
- International Farm Comparison Network Dairy Report 2019. For a Better Understanding of the Dairy World. IFCN—Dairy Research Center, Kiel, Germany. Available online: https://ifcndairy.org/wp-content/uploads/2019/10/Dairy-Report_2019_extraction_for-marketing.pdf (accessed on 11 February 2020).
- World Bank Urban Population Growth (Annual %)—India. Available online: https://data.worldbank.org/indicator/SP.URB.TOTL.IN.ZS?end=2018&locations=IN&start=1960&view=chart (accessed on 13 February 2020).
- Prasad, C.S.; Anandan, S.; Gowda, N.K.S.; Schlecht, E.; Buerkert, A. Managing nutrient flows in Indian urban and peri-urban livestock systems. Nutr. Cycling Agroecosyst. 2019, 115, 159–172. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; ISBN 978-92-5-107920-1. [Google Scholar]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited review: Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K. Trends and projected estimates of GHG emissions from Indian livestock in comparisons with GHG emissions from world and developing countries. Asian-Australas. J. Anim. Sci. 2014, 27, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Goopy, J.P.; Chang, C.; Tomkins, N. A Comparison of Methodologies for Measuring Methane Emissions from Ruminants. In Methods for Measuring Greenhouse Gas Balances and Evaluating Mitigation Options in Smallholder Agriculture; Rosenstock, T.S., Rufino, M.C., Butterbach-Bahl, K., Wollenberg, L., Richards, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 97–117. ISBN 978-3-319-29794-1. [Google Scholar]
- Grainger, C.; Clarke, T.; McGinn, S.M.; Auldist, M.J.; Beauchemin, K.A.; Hannah, M.C.; Waghorn, G.C.; Clark, H.; Eckard, R.J. Methane emissions from dairy cows measured using the sulfur hexafluoride (SF6) tracer and chamber techniques. J. Dairy Sci. 2007, 90, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Garnsworthy, P.C.; Difford, G.F.; Bell, M.J.; Bayat, A.R.; Huhtanen, P.; Kuhla, B.; Lassen, J.; Peiren, N.; Pszczola, M.; Sorg, D.; et al. Comparison of methods to measure methane for use in genetic evaluation of dairy cattle. Animals (Basel) 2019, 9, 837. [Google Scholar] [CrossRef]
- Chagunda, M.G.G.; Ross, D.; Roberts, D.J. On the use of a laser methane detector in dairy cows. Comput. Electron. Agric. 2009, 68, 157–160. [Google Scholar] [CrossRef]
- Chagunda, M.G.G.; Yan, T. Do methane measurements from a laser detector and an indirect open-circuit respiration calorimetric chamber agree sufficiently closely? Anim. Feed. Sci. Technol. 2011, 165, 8–14. [Google Scholar] [CrossRef]
- Reintke, J.; Brügemann, K.; Yin, T.; Engel, P.; Wagner, H.; Wehrend, A.; König, S. Assessment of methane emission traits in ewes using a laser methane detector: Genetic parameters and impact on lamb weaning performance. Arch. Anim. Breed. 2020, 63, 113–123. [Google Scholar] [CrossRef]
- Ricci, P.; Chagunda, M.G.G.; Rooke, J.M.; Houdijk, J.G.; Duthie, C.-A.; Hyslop, J.; Roehe, R.; Waterhouse, A. Evaluation of the laser methane detector to estimate methane emissions from ewes and steers. J. Anim. Sci. 2014, 92, 5239–5250. [Google Scholar] [CrossRef]
- Sorg, D.; Mühlbach, S.; Rosner, F.; Kuhla, B.; Derno, M.; Meese, S.; Schwarm, A.; Kreuzer, M.; Swalve, H. The agreement between two next-generation laser methane detectors and respiration chamber facilities in recording methane concentrations in the spent air produced by dairy cows. Comput. Electron. Agric. 2017, 143, 262–272. [Google Scholar] [CrossRef]
- Pickering, N.K.; Chagunda, M.G.G.; Banos, G.; Mrode, R.; McEwan, J.C.; Wall, E. Genetic parameters for predicted methane production and laser methane detector measurements. J. Anim. Sci. 2015, 93, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ricci, P.; Rooke, J.A.; Nevison, I.; Waterhouse, A. Methane emissions from beef and dairy cattle: Quantifying the effect of physiological stage and diet characteristics. J. Anim. Sci. 2013, 91, 5379–5389. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Pinent, T.; Brügemann, K.; Simianer, H.; König, S. Simulation, prediction, and genetic analyses of daily methane emissions in dairy cattle. J. Dairy Sci. 2015, 98, 5748–5762. [Google Scholar] [CrossRef] [PubMed]
- Hammond, K.J.; Muetzel, S.; Waghorn, G.C.; Pinares-Patino, C.S.; Burke, J.L.; Hoskin, S.O. The variation in methane emissions from sheep and cattle is not explained by the chemical composition of ryegrass. Proc. N. Z. Soc. Anim. Prod. 2009, 69, 174–178. [Google Scholar]
- Chagunda, M.G.G. Opportunities and challenges in the use of the Laser Methane Detector to monitor enteric methane emissions from ruminants. Animal 2013, 7, 394–400. [Google Scholar] [CrossRef]
- O’Brien, D.; Capper, J.L.; Garnsworthy, P.C.; Grainger, C.; Shalloo, L. A case study of the carbon footprint of milk from high-performing confinement and grass-based dairy farms. J. Dairy Sci. 2014, 97, 1835–1851. [Google Scholar] [CrossRef]
- Rhoads, M.L.; Rhoads, R.P.; VanBaale, M.J.; Collier, R.J.; Sanders, S.R.; Weber, W.J.; Crooker, B.A.; Baumgard, L.H. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef]
- Broaddus, B. Impact of Season and Heat Stress on Somatic Cell Counts. Master Thesis, University of Kentucky, Kentucky, KY, USA, 2001. [Google Scholar]
- Garnsworthy, P.C.; Craigon, J.; Hernandez-Medrano, J.H.; Saunders, N. Variation among individual dairy cows in methane measurements made on farm during milking. J. Dairy Sci. 2012, 95, 3181–3189. [Google Scholar] [CrossRef]
- Yan, T.; Mayne, C.S.; Gordon, F.G.; Porter, M.G.; Agnew, R.E.; Patterson, D.C.; Ferris, C.P.; Kilpatrick, D.J. Mitigation of enteric methane emissions through improving efficiency of energy utilization and productivity in lactating dairy cows. J. Dairy Sci. 2010, 93, 2630–2638. [Google Scholar] [CrossRef]
- Pinto, A.; Yin, T.; Reichenbach, M.; Bhatta, R.; Schlecht, E.; König, S. Phenotypic Dairy Cattle Trait Expressions in Dependency of Social-Ecological Characteristics along Rural–Urban Gradients. Sustainability 2020, 12, 9021. [Google Scholar] [CrossRef]
- Martin-Collado, D.; Soini, K.; Mäki-Tanila, A.; Toro, M.A.; Díaz, C. Defining farmer typology to analyze the current state and development prospects of livestock breeds: The Avileña-Negra Ibérica beef cattle breed as a case study. Livest. Sci. 2014, 169, 137–145. [Google Scholar] [CrossRef]
- Kraas, F. Megacities and global change: Key priorities. Geogr. J. 2007, 173, 79–82. [Google Scholar] [CrossRef]
- Groupe SCE India Revised of Structure Plan—2031 Bangalore Metropolitan Region. Available online: http://opencity.in/documents/bmrda-revised-structure-plan-2031-draft-report (accessed on 17 August 2020).
- Hoffmann, E.M.; Jose, M.; Nölke, N.; Möckel, T. Construction and use of a simple index of urbanisation in the rural–urban interface of Bangalore, India. Sustainability 2017, 9, 2146. [Google Scholar] [CrossRef]
- Reichenbach, M. Dairy Production in an Urbanizing Environment. A System Approach in Bengaluru, India. Ph.D. Thesis, University of Kassel, Witzenhausen, Germany, 2020. [Google Scholar]
- National Research Council (NRC). A Guide to Environmental Research on Animals; National Academy of Sciences: Washington, DC, USA, 1971. [Google Scholar]
- Ferguson, J.D.; Galligan, D.T.; Thomsen, N. Principal descriptors of body condition score in Holstein cows. J. Dairy Sci. 1994, 77, 2695–2703. [Google Scholar] [CrossRef]
- Grund, J.-N. Biometric Characteristics of Dairy Cattle in Karnataka. Master’s Thesis, Georg-August Universität Göttingen, Göttingen, Germany, 2018. [Google Scholar]
- Tokyo Gas Engineering Solutions Corporation. Laser Methane Mini SA3C32A Operation Manual; Tokyo Gas Engineering Solutions Corporation: Tokio, Japan, 2015. [Google Scholar]
- Blaxter, K.L.; Joyce, J.P. The accuracy and ease with which measurements of respiratory metabolism can be made with tracheostomised sheep. Br. J. Nutr. 1963, 17, 523–537. [Google Scholar] [CrossRef]
- Murray, R.M.; Bryant, A.M.; Leng, R.A. Rates of production of methane in the rumen and large intestine of sheep. Br. J. Nutr. 1976, 36, 1–14. [Google Scholar] [CrossRef]
- Benaglia, T.; Chauveau, D.; Hunter, D.R.; Young, D.S. Mixtools: An R package for analyzing mixture models. J. Stat. Softw. 2009, 32, 1–29. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated marginal means, aka least-squares means.2018. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 11 February 2020).
- Garnsworthy, P.C.; Craigon, J.; Hernandez-Medrano, J.H.; Saunders, N. On-farm methane measurements during milking correlate with total methane production by individual dairy cows. J. Dairy Sci. 2012, 95, 3166–3180. [Google Scholar] [CrossRef]
- Pszczola, M.; Rzewuska, K.; Mucha, S.; Strabel, T. Heritability of methane emissions from dairy cows over a lactation measured on commercial farms. J. Anim. Sci. 2017, 95, 4813–4819. [Google Scholar] [CrossRef] [PubMed]
- Lassen, J.; Løvendahl, P. Heritability estimates for enteric methane emissions from Holstein cattle measured using noninvasive methods. J. Dairy Sci. 2016, 99, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- van Engelen, S.; Bovenhuis, H.; van der Tol, P.P.J.; Visker, M.H.P.W. Genetic background of methane emission by Dutch Holstein Friesian cows measured with infrared sensors in automatic milking systems. J. Anim. Sci. 2018, 101, 2226–2234. [Google Scholar] [CrossRef] [PubMed]
- Ngwabie, N.M.; Jeppsson, K.-H.; Nimmermark, S.; Swensson, C.; Gustafsson, G. Multi-location measurements of greenhouse gases and emission rates of methane and ammonia from a naturally-ventilated barn for dairy cows. Biosyst. Eng. 2009, 103, 68–77. [Google Scholar] [CrossRef]
- Berry, D.P.; Buckley, F.; Dillon, P. Body condition score and live-weight effects on milk production in Irish Holstein-Friesian dairy cows. Animal. 2007, 1, 1351–1359. [Google Scholar] [CrossRef]
- Chagunda, M.G.G.; Römer, D.A.M.; Roberts, D.J. Effect of genotype and feeding regime on enteric methane, non-milk nitrogen and performance of dairy cows during the winter feeding period. Livest. Sci. 2009, 122, 323–332. [Google Scholar] [CrossRef]
- Kirchgessner, M.; Windisch, W.; Mueller, H.L.; Kreuzer, M. Release of methane and of carbon dioxide by dairy cattle. Agribiol. Res. 1991, 44, 91–102. [Google Scholar]
- Basarab, J.A.; Beauchemin, K.A.; Baron, V.S.; Ominski, K.H.; Guan, L.L.; Miller, S.P.; Crowley, J.J. Reducing GHG emissions through genetic improvement for feed efficiency: Effects on economically important traits and enteric methane production. Animal 2013, 7, 303–315. [Google Scholar] [CrossRef]
- Swamy, M.; Bhattacharya, S. Budgeting anthropogenic greenhouse gas emission from Indian livestock using country-specific emission coefficients. Curr. Sci. 2006, 91, 1340–1353. [Google Scholar]
- Ngwabie, N.M.; Jeppsson, K.-H.; Gustafsson, G.; Nimmermark, S. Effects of animal activity and air temperature on methane and ammonia emissions from a naturally ventilated building for dairy cows. Atmos. Environ. 2011, 45, 6760–6768. [Google Scholar] [CrossRef]
- Alford, A.R.; Hegarty, R.S.; Parnell, P.F.; Cacho, O.J.; Herd, R.M.; Griffith, G.R. The impact of breeding to reduce residual feed intake on enteric methane emissions from the Australian beef industry. Aust. J. Exp. Agric. 2006, 46, 813. [Google Scholar] [CrossRef]
- Hammond, K.J.; Jones, A.K.; Humphries, D.J.; Crompton, L.A.; Reynolds, C.K. Effects of diet forage source and neutral detergent fiber content on milk production of dairy cattle and methane emissions determined using GreenFeed and respiration chamber techniques. J. Dairy Sci. 2016, 99, 7904–7917. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.; Lee, C.-C.; Meinen, R.; Montes, F.; Ott, T.; Firkins, J.; Rotz, C.A.; Dell, C.; Adesogan, A.; Yang, W.; et al. Mitigation of Greenhouse Gas Emissions in Livestock Production—A Review of Technical Options for Non-CO2 Emissions; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; Volume 177, ISBN 978-92-5-107658-3. [Google Scholar]
- Hristov, A.N.; Oh, J.; Firkins, J.L.; Dijkstra, J.; Kebreab, E.; Waghorn, G.; Makkar, H.P.S.; Adesogan, A.T.; Yang, W.; Lee, C.; et al. SPECIAL TOPICS—Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J. Anim. Sci. 2013, 91, 5045–5069. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.J.; Waghorn, G.C. Dairy industry perspectives of methane emissions and production from cattle fed pasture or total mixed rations in New Zealand. Proc. N. Z. Soc. Anim. Prod. 2002, 62, 213–218. [Google Scholar]
- Ali, A.I.M.; Wassie, S.E.; Korir, D.; Merbold, L.; Goopy, J.P.; Butterbach-Bahl, K.; Dickhoefer, U.; Schlecht, E. Supplementing tropical cattle for improved nutrient utilization and reduced enteric methane emissions. Animals 2019, 9, 210. [Google Scholar] [CrossRef]
- Mertens, D.R. Regulation of forage intake. In Forage Quality, Evaluation, and Utilization; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1994; pp. 450–493. ISBN 978-0-89118-579-6. [Google Scholar]
- Standing Committee of Agriculture (SCA). Feeding Standards for Australian Livestock Ruminants. Ruminants Subcommittee; Corbett, J.L., Ed.; CSIRO: Melbourne, Australia, 1990; p. 266. [Google Scholar]
- National Research Council (NRC). Nutrient Requirements of Dairy Cattle, 6th ed.; National Academy of Sciences: Washington, DC, USA, 1989; ISBN 0-309-03826-X. [Google Scholar]
- Moe, P.W.; Tyrrell, H.F. Methane Production in Dairy Cows. J. Dairy Sci. 1979, 62, 1583–1586. [Google Scholar] [CrossRef]
- Chagunda, M.G.G.; Ross, D.; Rooke, J.; Yan, T.; Douglas, J.-L.; Poret, L.; McEwan, N.R.; Teeranavattanakul, P.; Roberts, D.J. Measurement of enteric methane from ruminants using a hand-held laser methane detector. Acta Agric. Scand. A Anim. Sci. 2013, 63, 68–75. [Google Scholar] [CrossRef]


| SSI Cluster | No. of Measurements | Breed (%) | Past (%) | Loc (%) | MY | BW | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exotic | Crossbreed | Native | No | Yes | Indoor | Half-Outdoor | Outdoor | (L/day) | (kg) | ||
| Urban | 191 | 51.3 | 45.6 | 3.1 | 29.3 | 70.7 | 46.1 | 16.7 | 37.2 | 11.5 | 392.6 |
| Mixed | 250 | 62.0 | 31.2 | 6.8 | 34.4 | 65.6 | 32.0 | 11.2 | 56.8 | 10.5 | 382.8 |
| Rural | 394 | 68.3 | 31.5 | 0.2 | 62.7 | 37.3 | 22.6 | 29.7 | 47.7 | 10.4 | 379.1 |
| CH4 Trait | # Farm | # Cow | # Measurement | Methane Emission (ppm) | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Min. | Max. | ||||
| AllMean | 119 | 448 | 835 | 42.8 | 34.92 | 8.2 | 351.7 |
| AllMinute | 119 | 448 | 835 | 3969.0 | 2446.35 | 771.1 | 23,785.2 |
| RespMean | 119 | 448 | 835 | 16.0 | 17.21 | 1.0 | 160.3 |
| RespMax | 119 | 448 | 835 | 48.4 | 81.40 | 1.0 | 900.0 |
| RespMinute | 119 | 448 | 835 | 879.9 | 1249.82 | 0.5 | 12,873.9 |
| ErucMean | 119 | 448 | 835 | 108.1 | 148.28 | 9.7 | 1425.0 |
| ErucMax | 119 | 448 | 835 | 410.2 | 371.99 | 31.0 | 4152.0 |
| ErucMinute | 119 | 448 | 835 | 3089.0 | 2253.80 | 106.0 | 22,098.0 |
| CH4 Trait | # Farm | # Cow | # Measurement | Methane Emission (ppm) | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Min. | Max. | ||||
| AllMean | 27 | 78 | 146 | 37.2 | 25.00 | 8.2 | 119.2 |
| AllMinute | 27 | 78 | 146 | 3645.0 | 2110.67 | 905.2 | 11,353.7 |
| RespMean | 27 | 78 | 146 | 13.3 | 12.86 | 1.0 | 74.8 |
| RespMax | 27 | 78 | 146 | 48.6 | 89.58 | 1.0 | 721.0 |
| RespMinute | 27 | 78 | 146 | 891.0 | 1207.36 | 0.5 | 7550.2 |
| ErucMean | 27 | 78 | 146 | 114.2 | 174.42 | 10.1 | 1271.2 |
| ErucMax | 27 | 78 | 146 | 489.1 | 537.96 | 39.0 | 4152.0 |
| ErucMinute | 27 | 78 | 146 | 2754.1 | 1993.99 | 106.0 | 10,747.6 |
| CH4 Trait | Fixed Effects | |||||
|---|---|---|---|---|---|---|
| MY/BW 1 | THI 1 | FasT 1 | Breed 2 | |||
| Exotic | Crossbreed | Native | ||||
| AllMean | 0.03 * | −0.00 ns | −0.01 * | 33.19 a | 33.33 a | 35.38 a |
| AllMinute | 0.02 ns | −0.01 ns | −0.01 ns | 3328.17 a | 3351.99 a | 3550.51 a |
| RespMean | 0.03 ns | −0.01 ns | −0.00 ns | 10.09 a | 10.88 a | 10.81 a |
| RespMax | 0.03 ns | −0.00 ns | −0.02 ns | 19.80 a | 23.63 a | 21.48 a |
| RespMinute | −0.01 ns | 0.01 ns | 0.00 ns | 275.74 a | 344.81 a | 284.45 a |
| ErucMean | 0.02 ns | −0.03 ** | −0.03 ** | 61.50 a | 70.33 a | 70.31 a |
| ErucMax | −0.01 ns | −0.04 *** | −0.04 *** | 296.92 a | 305.91 a | 358.25 a |
| ErucMinute | 0.04 ns | 0.02 * | −0.00 ns | 2406.74 a | 2242.53 a | 2313.07 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, A.; Yin, T.; Reichenbach, M.; Bhatta, R.; Malik, P.K.; Schlecht, E.; König, S. Enteric Methane Emissions of Dairy Cattle Considering Breed Composition, Pasture Management, Housing Conditions and Feeding Characteristics along a Rural-Urban Gradient in a Rising Megacity. Agriculture 2020, 10, 628. https://doi.org/10.3390/agriculture10120628
Pinto A, Yin T, Reichenbach M, Bhatta R, Malik PK, Schlecht E, König S. Enteric Methane Emissions of Dairy Cattle Considering Breed Composition, Pasture Management, Housing Conditions and Feeding Characteristics along a Rural-Urban Gradient in a Rising Megacity. Agriculture. 2020; 10(12):628. https://doi.org/10.3390/agriculture10120628
Chicago/Turabian StylePinto, Ana, Tong Yin, Marion Reichenbach, Raghavendra Bhatta, Pradeep Kumar Malik, Eva Schlecht, and Sven König. 2020. "Enteric Methane Emissions of Dairy Cattle Considering Breed Composition, Pasture Management, Housing Conditions and Feeding Characteristics along a Rural-Urban Gradient in a Rising Megacity" Agriculture 10, no. 12: 628. https://doi.org/10.3390/agriculture10120628
APA StylePinto, A., Yin, T., Reichenbach, M., Bhatta, R., Malik, P. K., Schlecht, E., & König, S. (2020). Enteric Methane Emissions of Dairy Cattle Considering Breed Composition, Pasture Management, Housing Conditions and Feeding Characteristics along a Rural-Urban Gradient in a Rising Megacity. Agriculture, 10(12), 628. https://doi.org/10.3390/agriculture10120628






