Application of Artificial Neural Networks to Analyze the Concentration of Ferulic Acid, Deoxynivalenol, and Nivalenol in Winter Wheat Grain
Abstract
:1. Introduction
2. Materials and Methods
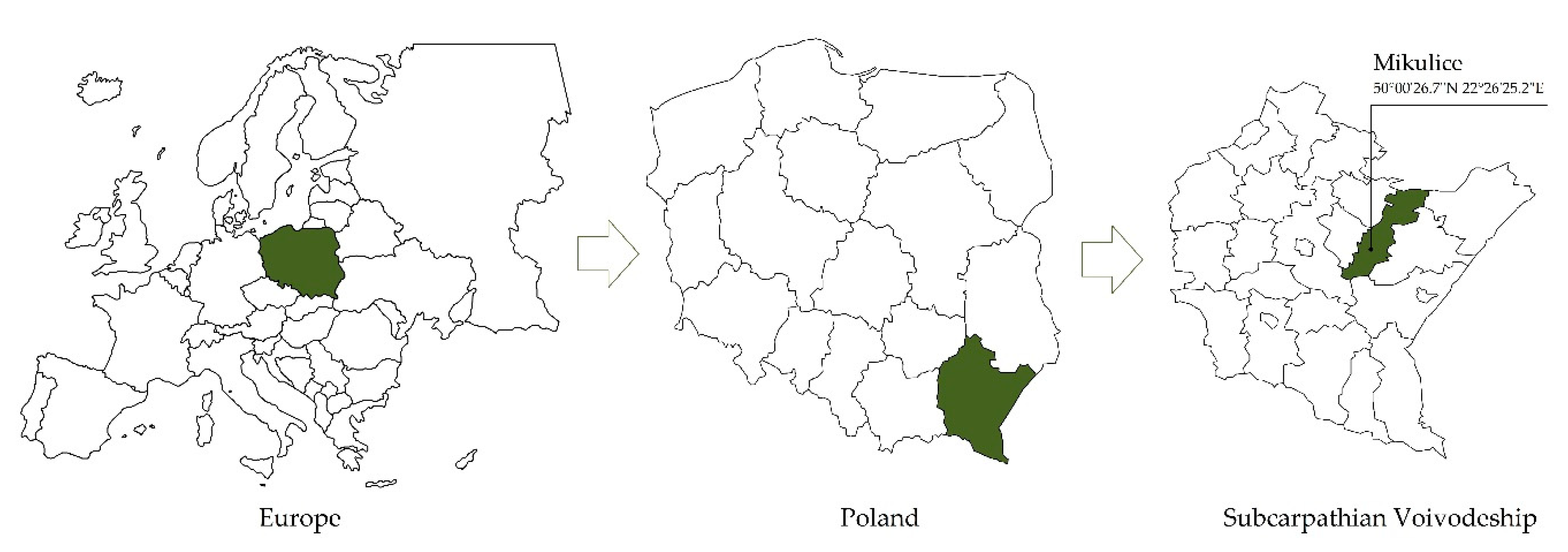
2.1. Site Location
- -
- full chemical crop protection (CH)—fungicides: Duet Ultra 497 SC (epoxyconazole, thiophanate methyl) at a concentration of 0.6 l·ha−1, and Capalo 337.5 SE (fenpropimorph, epoxyconazole, and metrafenone) at 2 l·ha−1;
- -
- no chemical crop protection, natural infestation (K);
- -
- no chemical crop protection, artificial inoculation with fungi from the genus Fusarium (I).
2.2. Determination of Ferulic Acid
2.3. Determination of Trichothecenes
2.4. The Method of Constructing Neural Models
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernandez, O.; Béthencourt, L.; Quero, A.; Sangwan, R.S.; Clément, C. Trehalose and plant stress responses: Friend or foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.A.; Lipps, P.E.; Hershman, D.E.; McMullen, M.P.; Draper, M.A.; Madden, L.V. A Quantitative Review of Tebuconazole Effect on Fusarium Head Blight and Deoxynivalenol Content in Wheat. Phytopathology 2007, 97, 211–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, B.A.; Linde, C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 2002, 40, 349–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, S.; Xu, F.; Tang, M.; Zhou, Y.; Li, G.; An, X.; Lin, F.; Xu, H.; Jia, H.; Zhang, L.; et al. Precise mapping Fhb5, a major QTL conditioning resistance to Fusarium infection in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2011, 123, 1055–1063. [Google Scholar] [CrossRef]
- Xue, S.; Li, G.; Jia, H.; Xu, F.; Lin, F.; Tang, M.; Wang, Y.; An, X.; Xu, H.; Zhang, L.; et al. Fine mapping Fhb4, a major QTL conditioning resistance to Fusarium infection in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2010, 121, 147–156. [Google Scholar] [CrossRef]
- Dhokane, D.; Karre, S.; Kushalappa, A.C.; McCartney, C. Integrated Metabolo-Transcriptomics Reveals Fusarium Head Blight Candidate Resistance Genes in Wheat QTL-Fhb2. PLoS ONE 2016, 11, e0155851. [Google Scholar]
- Giancaspro, A.; Giove, S.L.; Zito, D.; Blanco, A.; Gadaleta, A. Mapping QTLs for Fusarium Head Blight Resistance in an Interspecific Wheat Population. Front. Plant Sci. 2016, 7, 1381. [Google Scholar] [CrossRef]
- Arruda, M.P.; Brown, P.; Brown-Guedira, G.; Krill, A.M.; Thurber, C.; Merrill, K.R.; Foresman, B.J.; Kolb, F.L. Genome-Wide Association Mapping of Fusarium Head Blight Resistance in Wheat using Genotyping-by-Sequencing. Plant Genome 2016, 9. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Ban, T.; Anderson, J.A. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: A review. Plant Breed. 2009, 128, 1–26. [Google Scholar]
- Riley, R.T.; An, N.H.; Showker, J.L.; Yoo, H.S.; Norred, W.P.; Chamberlain, W.J.; Wang, E.; Merrill, A.H.; Motelin, G.; Beasley, V.R.; et al. Alteration of Tissue and Serum Sphinganine to Sphingosine Ratio: An Early Biomarker of Exposure to Fumonisin-Containing Feeds in Pigs. Toxicol. Appl. Pharmacol. 1993, 118, 105–112. [Google Scholar] [CrossRef]
- Bal, G. Scab of Wheat: Prospects For Control. Plant Dis. 1994, 78, 760. [Google Scholar]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of Wheat and Barley: A Re-emerging Disease of Devastating Impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [PubMed] [Green Version]
- Gang, G.; Miedaner, T.; Schuhmacher, U.; Schollenberger, M.; Geiger, H.H. Deoxynivalenol and Nivalenol Production by Fusarium culmorum Isolates Differing in Aggressiveness Toward Winter Rye. Phytopathology 1998, 88, 879–884. [Google Scholar]
- D’Mello, J.P.F.; Placinta, C.M.; Macdonald, A.M.C. Fusarium mycotoxins: A review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Technol. 1999, 80, 183–205. [Google Scholar]
- Muthomi, J.W.; Schütze, A.; Dehne, H.-W.; Mutitu, E.W.; Oerke, E.-C. Characterization of Fusarium culmorum isolates by mycotoxin production and aggressiveness to winter wheat/Charakterisierung von Isolaten von Fusarium culmorum anhand der Mykotoxinbildung und Aggressivität an Winterweizen. Z. Pflanzenkrankh. Pflanzenschutz/J. Plant Dis. Prot. 2000, 107, 113–123. [Google Scholar]
- Peng, W.-X.; Marchal, J.L.M.; van der Poel, A.F.B. Strategies to prevent and reduce mycotoxins for compound feed manufacturing. Anim. Feed Sci. Technol. 2018, 237, 129–153. [Google Scholar]
- Martínez, G.; Regente, M.; Jacobi, S.; Del Rio, M.; Pinedo, M.; de la Canal, L. Chlorogenic acid is a fungicide active against phytopathogenic fungi. Pestic. Biochem. Physiol. 2017, 140, 30–35. [Google Scholar]
- Siranidou, E.; Kang, Z.; Buchenauer, H. Studies on Symptom Development, Phenolic Compounds and Morphological Defence Responses in Wheat Cultivars Differing in Resistance to Fusarium Head Blight. J. Phytopathol. 2002, 150, 200–208. [Google Scholar]
- Stuper-Szablewska, K.; Kurasiak-Popowska, D.; Nawracała, J.; Perkowski, J. Response of non-enzymatic antioxidative mechanisms to stress caused by infection with Fusarium fungi and chemical protection in different wheat genotypes. Chem. Ecol. 2017, 33, 949–962. [Google Scholar]
- Stuper-Szablewska, K.; Kurasiak-Popowska, D.; Nawracała, J.; Perkowski, J. Quantitative profile of phenolic acids and antioxidant activity of wheat grain exposed to stress. Eur. Food Res. Technol. 2019, 245, 1595–1603. [Google Scholar] [CrossRef] [Green Version]
- Stuper-Szablewska, K.; Kurasiak-Popowska, D.; Nawracała, J.; Perkowski, J. Study of metabolite profiles in winter wheat cultivars induced by Fusarium infection. Cereal Res. Commun. 2016, 44, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Goral, T.; Stuper-Szablewska, K.; Busko, M.; Boczkowska, M.; Walentyn-Goral, D.; Wisniewska, H.; Perkowski, J. Relationships between Genetic Diversity and Fusarium Toxin Profiles of Winter Wheat Cultivars. Plant Pathol. J. 2015, 31, 226–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuper-Szablewska, K.; Perkowski, J. Phenolic acids in cereal grain: Occurrence, biosynthesis, metabolism and role in living organisms. Crit. Rev. Food Sci. Nutr. 2019, 59, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Abdipour, M.; Ramazani, S.H.R.; Younessi-Hmazekhanlu, M.; Niazian, M. Modeling Oil Content of Sesame (Sesamum indicum L.) Using Artificial Neural Network and Multiple Linear Regression Approaches. J. Am. Oil Chem. Soc. 2018, 95, 283–297. [Google Scholar] [CrossRef]
- Mohammadi Torkashvand, A.; Ahmadi, A.; Gómez, P.A.; Maghoumi, M. Using artificial neural network in determining postharvest LIFE of kiwifruit. J. Sci. Food Agric. 2019, 99, 5918–5925. [Google Scholar] [CrossRef] [PubMed]
- Taner, A.; Öztekin, Y.; Tekgüler, A.; Sauk, H.; Duran, H. Classification of Varieties of Grain Species by Artificial Neural Networks. Agronomy 2018, 8, 123. [Google Scholar] [CrossRef] [Green Version]
- Torkashvand, A.M.; Ahmadi, A.; Nikravesh, N.L. Prediction of kiwifruit firmness using fruit mineral nutrient concentration by artificial neural network (ANN) and multiple linear regressions (MLR). J. Integr. Agric. 2017, 16, 1634–1644. [Google Scholar] [CrossRef] [Green Version]
- Kujawa, S.; Dach, J.; Kozłowski, R.J.; Przybył, K.; Niedbała, G.; Mueller, W.; Tomczak, R.J.; Zaborowicz, M.; Koszela, K. Maturity classification for sewage sludge composted with rapeseed straw using neural image analysis. In Proceedings of the SPIE—The International Society for Optical Engineering, Chengu, China, 29 August 2016; Volume 10033, p. 100332H. [Google Scholar]
- Kozłowski, R.J.; Kozłowski, J.; Przybył, K.; Niedbała, G.; Mueller, W.; Okoł, P.; Wojcieszak, D.; Koszela, K.; Kujawa, S. Image analysis techniques in the study of slug behaviour. In Proceedings of the SPIE—The International Society for Optical Engineering, Chengu, China, 29 August 2016; Volume 10033, p. 100332I. [Google Scholar]
- Niedbała, G.; Mioduszewska, N.; Mueller, W.; Boniecki, P.; Wojcieszak, D.; Koszela, K.; Kujawa, S.; Kozłowski, R.J.; Przybył, K. Use of computer image analysis methods to evaluate the quality topping sugar beets with using artificial neural networks. In Proceedings of the SPIE—The International Society for Optical Engineering, Chengu, China, 29 August 2016; Volume 10033, p. 100332M. [Google Scholar]
- Boniecki, P.; Koszela, K.; Świerczyński, K.; Skwarcz, J.; Zaborowicz, M.; Przybył, J. Neural Visual Detection of Grain Weevil (Sitophilus granarius L.). Agriculture 2020, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Ryu, G.-A.; Nasridinov, A.; Rah, H.; Yoo, K.-H. Forecasts of the Amount Purchase Pork Meat by Using Structured and Unstructured Big Data. Agriculture 2020, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Apolo-Apolo, O.E.; Pérez-Ruiz, M.; Martínez-Guanter, J.; Egea, G. A Mixed Data-Based Deep Neural Network to Estimate Leaf Area Index in Wheat Breeding Trials. Agronomy 2020, 10, 175. [Google Scholar] [CrossRef] [Green Version]
- Abbaspour-Gilandeh, Y.; Molaee, A.; Sabzi, S.; Nabipur, N.; Shamshirband, S.; Mosavi, A. A Combined Method of Image Processing and Artificial Neural Network for the Identification of 13 Iranian Rice Cultivars. Agronomy 2020, 10, 117. [Google Scholar] [CrossRef] [Green Version]
- Abdipour, M.; Younessi-Hmazekhanlu, M.; Ramazani, S.H.R.; omidi, A. hassan Artificial neural networks and multiple linear regression as potential methods for modeling seed yield of safflower (Carthamus tinctorius L.). Ind. Crops Prod. 2019, 127, 185–194. [Google Scholar] [CrossRef]
- Ray, A.; Halder, T.; Jena, S.; Sahoo, A.; Ghosh, B.; Mohanty, S.; Mahapatra, N.; Nayak, S. Application of artificial neural network (ANN) model for prediction and optimization of coronarin D content in Hedychium coronarium. Ind. Crops Prod. 2020, 146, 112186. [Google Scholar] [CrossRef]
- Niazian, M.; Sadat-Noori, S.A.; Abdipour, M. Modeling the seed yield of Ajowan (Trachyspermum ammi L.) using artificial neural network and multiple linear regression models. Ind. Crops Prod. 2018, 117, 224–234. [Google Scholar] [CrossRef]
- Niazian, M.; Sadat-Noori, S.A.; Abdipour, M. Artificial neural network and multiple regression analysis models to predict essential oil content of ajowan (Carum copticum L.). J. Appl. Res. Med. Aromat. Plants 2018, 9, 124–131. [Google Scholar] [CrossRef]
- Niedbała, G. Simple model based on artificial neural network for early prediction and simulation winter rapeseed yield. J. Integr. Agric. 2019, 18, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Niedbała, G. Application of artificial neural networks for multi-criteria yield prediction of winter rapeseed. Sustainability 2019, 11, 533. [Google Scholar] [CrossRef] [Green Version]
- Niedbala, G.; Kozlowski, R.J. Application of Artificial Neural Networks for Multi-Criteria Yield Prediction of Winter Wheat. J. Agric. Sci. Technol. 2019, 21, 51–61. [Google Scholar]
- Niedbała, G.; Nowakowski, K.; Rudowicz-Nawrocka, J.; Piekutowska, M.; Weres, J.; Tomczak, R.J.; Tyksiński, T.; Pinto, A.Á. Multicriteria prediction and simulation of winter wheat yield using extended qualitative and quantitative data based on artificial neural networks. Appl. Sci. 2019, 9, 2773. [Google Scholar] [CrossRef] [Green Version]
- Niedbała, G.; Piekutowska, M.; Weres, J.; Korzeniewicz, R.; Witaszek, K.; Adamski, M.; Pilarski, K.; Czechowska-Kosacka, A.; Krysztofiak-Kaniewska, A. Application of Artificial Neural Networks for Yield Modeling of Winter Rapeseed Based on Combined Quantitative and Qualitative Data. Agronomy 2019, 9, 781. [Google Scholar] [CrossRef] [Green Version]
- Wojciechowski, T.; Niedbala, G.; Czechlowski, M.; Nawrocka, J.R.; Piechnik, L.; Niemann, J. Rapeseed seeds quality classification with usage of VIS-NIR fiber optic probe and artificial neural networks. In Proceedings of the 2016 International Conference on Optoelectronics and Image Processing, ICOIP 2016, Warsaw, Poland, 10–12 June 2016. [Google Scholar]
- Kaushik, P.; Dhaliwal, M. Diallel Analysis for Morphological and Biochemical Traits in Tomato Cultivated under the Influence of Tomato Leaf Curl Virus. Agronomy 2018, 8, 153. [Google Scholar] [CrossRef] [Green Version]
- Viriyasuthee, W.; Saksirirat, W.; Saepaisan, S.; Gleason, M.L.; Jogloy, S. Jogloy Variability of Alternaria Leaf Spot Resistance in Jerusalem Artichoke (Helianthus Tuberosus L.) Accessions Grown in a Humid Tropical Region. Agronomy 2019, 9, 268. [Google Scholar]
- Perkowski, J.; Wiwart, M.; Buśko, M.; Laskowska, M.; Berthiller, F.; Kandler, W.; Krska, R. Fusarium toxins and total fungal biomass indicators in naturally contaminated wheat samples from north-eastern Poland in 2003. Food Addit. Contam. 2007, 24, 1292–1298. [Google Scholar] [CrossRef]
- Miedaner, T.; Reinbrecht, C.; Lauber, U.; Schollenberger, M.; Geiger, H.H. Effects of genotype and genotype-environment interaction on deoxynivalenol accumulation and resistance to Fusarium head blight in rye, triticale, and wheat. Plant Breed. 2001, 120, 97–105. [Google Scholar] [CrossRef]
- Foroud, N.; Eudes, F. Trichothecenes in Cereal Grains. Int. J. Mol. Sci. 2009, 10, 147–173. [Google Scholar] [CrossRef] [Green Version]
- Mesterházy, Á. Role of Deoxynivalenol in Aggressiveness of Fusarium graminearum and F. culmorum and in Resistance to Fusarium Head Blight. Eur. J. Plant Pathol. 2002, 108, 675–684. [Google Scholar] [CrossRef]
- Miedaner, T.; Schneider, B.; Geiger, H.H. Deoxynivalenol (DON) content and Fusarium head blight resistance in segregating populations of winter rye and winter wheat. Crop Sci. 2003, 43, 519–526. [Google Scholar] [CrossRef]
- Champeil, A.; Doré, T.; Fourbet, J. Fusarium head blight: Epidemiological origin of the effects of cultural practices on head blight attacks and the production of mycotoxins by Fusarium in wheat grains. Plant Sci. 2004, 166, 1389–1415. [Google Scholar] [CrossRef]
- Homdork, S.; Fehrmann, H.; Beck, R. Effects of Field Application of Tebuconazole on Yield, Yield Components and the Mycotoxin Content of Fusarium-infected Wheat Grain. J. Phytopathol. 2000, 148, 1–6. [Google Scholar] [CrossRef]
- Champeil, A.; Fourbet, J..; Doré, T.; Rossignol, L. Influence of cropping system on Fusarium head blight and mycotoxin levels in winter wheat. Crop Prot. 2004, 23, 531–537. [Google Scholar] [CrossRef]
- Rudd, J.C.; Horsley, R.D.; McKendry, A.L.; Elias, E.M. Host Plant Resistance Genes for Fusarium Head Blight. Crop Sci. 2001, 41, 620. [Google Scholar] [CrossRef] [Green Version]
- Snijders, C.H.A. Resistance in wheat to Fusarium infection and trichothecene formation. Toxicol. Lett. 2004, 153, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shewry, P.R.; Ward, J.L. Phenolic Acids in Wheat Varieties in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef] [PubMed]
- Hernández, L.; Afonso, D.; Rodríguez, E.M.; Díaz, C. Phenolic Compounds in Wheat Grain Cultivars. Plant Foods Hum. Nutr. 2011, 66, 408–415. [Google Scholar] [CrossRef]
- Gallardo, C.; Jiménez, L.; García-Conesa, M.-T. Hydroxycinnamic acid composition and in vitro antioxidant activity of selected grain fractions. Food Chem. 2006, 99, 455–463. [Google Scholar] [CrossRef]
- Van Hung, P.; Maeda, T.; Miyatake, K.; Morita, N. Total phenolic compounds and antioxidant capacity of wheat graded flours by polishing method. Food Res. Int. 2009, 42, 185–190. [Google Scholar] [CrossRef]
- Zhou, K.; Laux, J.J.; Yu, L. Comparison of Swiss Red Wheat Grain and Fractions for Their Antioxidant Properties. J. Agric. Food Chem. 2004, 52, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Taddei, F.; Nicoletti, I.; Ciccoritti, R.; Corradini, D.; D’Egidio, M.G. Effects of Genotype and Environment on Phenolic Acids Content and Total Antioxidant Capacity in Durum Wheat. Cereal Chem. J. 2014, 91, 310–317. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [Green Version]
| Symbol | Unit of Measure | Variable Name | The Scope of Data |
|---|---|---|---|
| Quantitative data | |||
| P1-3 | mm | Sum of precipitation from 1 January to 31 March | 90–102 |
| T1-3 | °C | Average air temperature from 1 January to 31 March | −1.6–−1.1 |
| P4 | mm | Sum of precipitation from 1 April to 30 April | 25–29 |
| T4 | °C | Average air temperature from January 1 April to 30 April | 9.9–10 |
| P5 | mm | Sum of precipitation from 1 May to 31 May | 4–70 |
| T5 | °C | Average air temperature from 1 May to 31 May | 15.6–16 |
| P6 | mm | Sum of precipitation from 1 June to 31 June | 102–103 |
| T6 | °C | Average air temperature from 1 June to 31 June | 18.3–18.7 |
| P7 | mm | Sum of precipitation from 1 July to 31 July | 33–58 |
| T7 | °C | Average air temperature from 1 July to 31 July | 19.5–21.4 |
| WH | cm | Wheat height | 67–122 |
| DI | % | Disease index | 0–95 |
| Qualitative data | |||
| VAR | word | Experimental variant | Inoculation Protection Control |
| VOW | word | Variety of wheat | MUSZELKA SMH 8489 KBP 08.17 ARKADIA STH 9011 NAD 08104 STH 9035 AND 394/07 BAMBERKA SMH 8540 KBP 08.8 SVPC 87185 CHD 7143/04 82/2011 TARKUS 91/2011 PRAAG 8 20816 83/2011 FREGATA ERTUS 20818 UNG 136.6.1.1. |
| FERUANN | DONANN | NIVANN | |
|---|---|---|---|
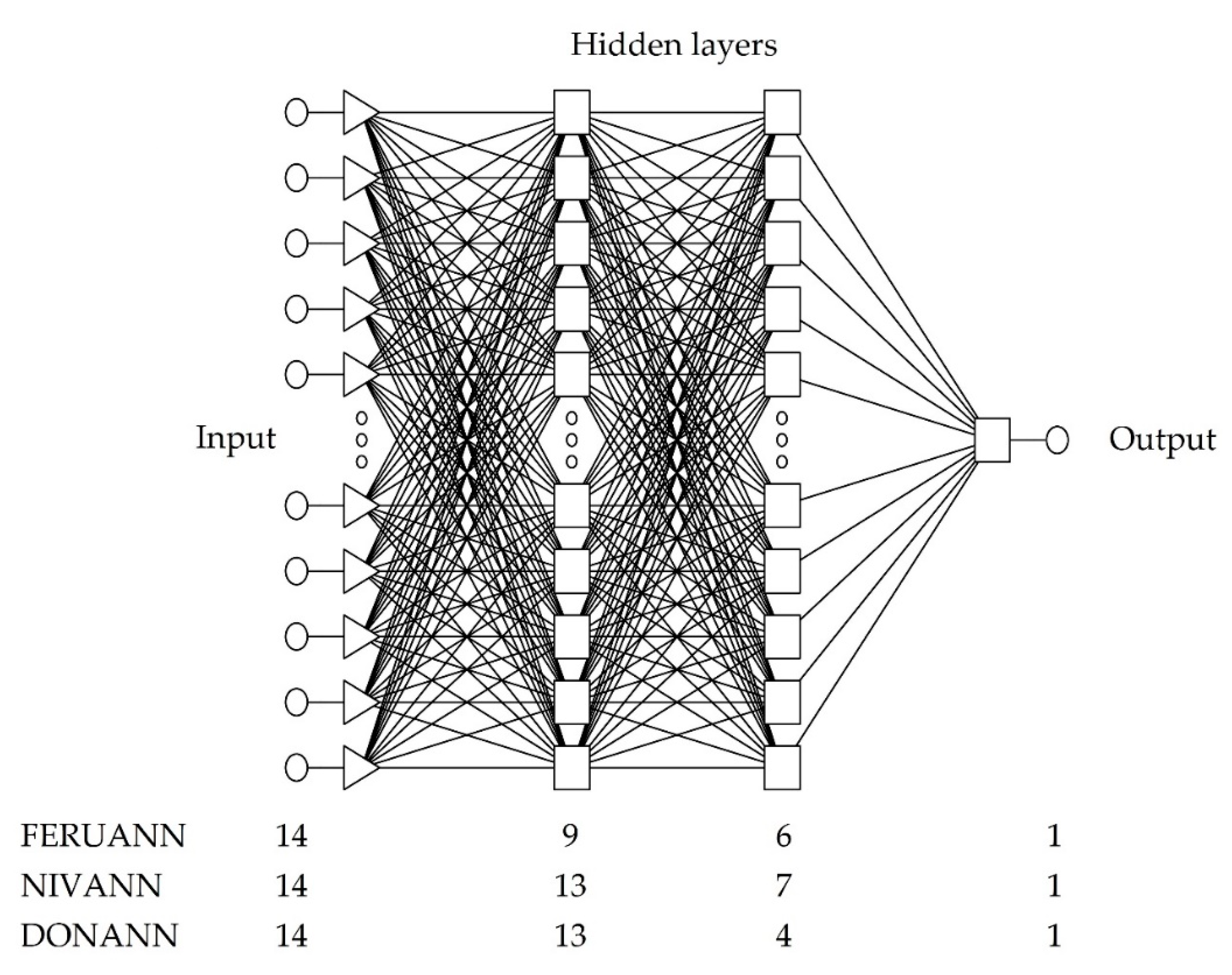
| Neural Network Structure | MLP 14:38-9-6-1:1 | MLP 14:38-13-7-1:1 | MLP 14:38-13-4-1:1 |
| Learning error | 0.0210 | 0.0175 | 0.0244 |
| Validation error | 0.0349 | 0.0308 | 0.0301 |
| Test error | 0.0492 | 0.0356 | 0.2288 |
| Mean | 1646.79 | 2.9708 | 0.0705 |
| Standard deviation | 1034.54 | 4.2031 | 0.1146 |
| Average error | 12.84 | 0.0541 | 0.0076 |
| Deviation error | 156.89 | 0.5336 | 0.0672 |
| Mean Absolute error | 114.34 | 0.3705 | 0.0220 |
| Quotient deviations | 0.1516 | 0.1269 | 0.5861 |
| Correlation | 0.9887 | 0.9919 | 0.8106 |
| Variable | Model | |||||
|---|---|---|---|---|---|---|
| FERUANN | DONANN | NIVANN | ||||
| Quotient | Rank | Quotient | Rank | Quotient | Rank | |
| P1-3 | 1.5973 | 4 | 1.0917 | 3 | 1.1436 | 7 |
| T1-3 | 1.1977 | 9 | 0.9860 | 13 | 1.0629 | 11 |
| P4 | 1.3120 | 7 | 1.0347 | 7 | 1.2597 | 4 |
| T4 | 1.0780 | 11 | 1.0477 | 6 | 1.1246 | 8 |
| P5 | 1.0027 | 14 | 1.0754 | 4 | 1.3281 | 3 |
| T5 | 1.2976 | 8 | 1.0731 | 5 | 1.0381 | 12 |
| P6 | 1.3718 | 5 | 0.9784 | 14 | 1.0977 | 9 |
| T6 | 1.6332 | 3 | 1.0335 | 8 | 1.1894 | 6 |
| P7 | 1.1242 | 10 | 1.0122 | 9 | 1.2163 | 5 |
| T7 | 1.5276 | 5 | 0.9930 | 12 | 1.0853 | 10 |
| WH | 1.0355 | 12 | 0.9963 | 11 | 1.0025 | 14 |
| DI | 1.0163 | 13 | 0.9979 | 10 | 1.0292 | 13 |
| VAR | 7.0823 | 1 | 1.3778 | 1 | 1.4793 | 2 |
| VOW | 3.1471 | 2 | 1.1069 | 2 | 1.6315 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedbała, G.; Kurasiak-Popowska, D.; Stuper-Szablewska, K.; Nawracała, J. Application of Artificial Neural Networks to Analyze the Concentration of Ferulic Acid, Deoxynivalenol, and Nivalenol in Winter Wheat Grain. Agriculture 2020, 10, 127. https://doi.org/10.3390/agriculture10040127
Niedbała G, Kurasiak-Popowska D, Stuper-Szablewska K, Nawracała J. Application of Artificial Neural Networks to Analyze the Concentration of Ferulic Acid, Deoxynivalenol, and Nivalenol in Winter Wheat Grain. Agriculture. 2020; 10(4):127. https://doi.org/10.3390/agriculture10040127
Chicago/Turabian StyleNiedbała, Gniewko, Danuta Kurasiak-Popowska, Kinga Stuper-Szablewska, and Jerzy Nawracała. 2020. "Application of Artificial Neural Networks to Analyze the Concentration of Ferulic Acid, Deoxynivalenol, and Nivalenol in Winter Wheat Grain" Agriculture 10, no. 4: 127. https://doi.org/10.3390/agriculture10040127
APA StyleNiedbała, G., Kurasiak-Popowska, D., Stuper-Szablewska, K., & Nawracała, J. (2020). Application of Artificial Neural Networks to Analyze the Concentration of Ferulic Acid, Deoxynivalenol, and Nivalenol in Winter Wheat Grain. Agriculture, 10(4), 127. https://doi.org/10.3390/agriculture10040127







