Prediction of Soil Oxalate Phosphorus using Visible and Near-Infrared Spectroscopy in Natural and Cultivated System Soils of Madagascar
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Soil Sample Dataset
2.2. Laboratory Analyses
2.3. Spectral Data Acquisition Using Vis-NIRS
2.4. Spectral Analyses and Modeling Approaches
3. Results and Discussion
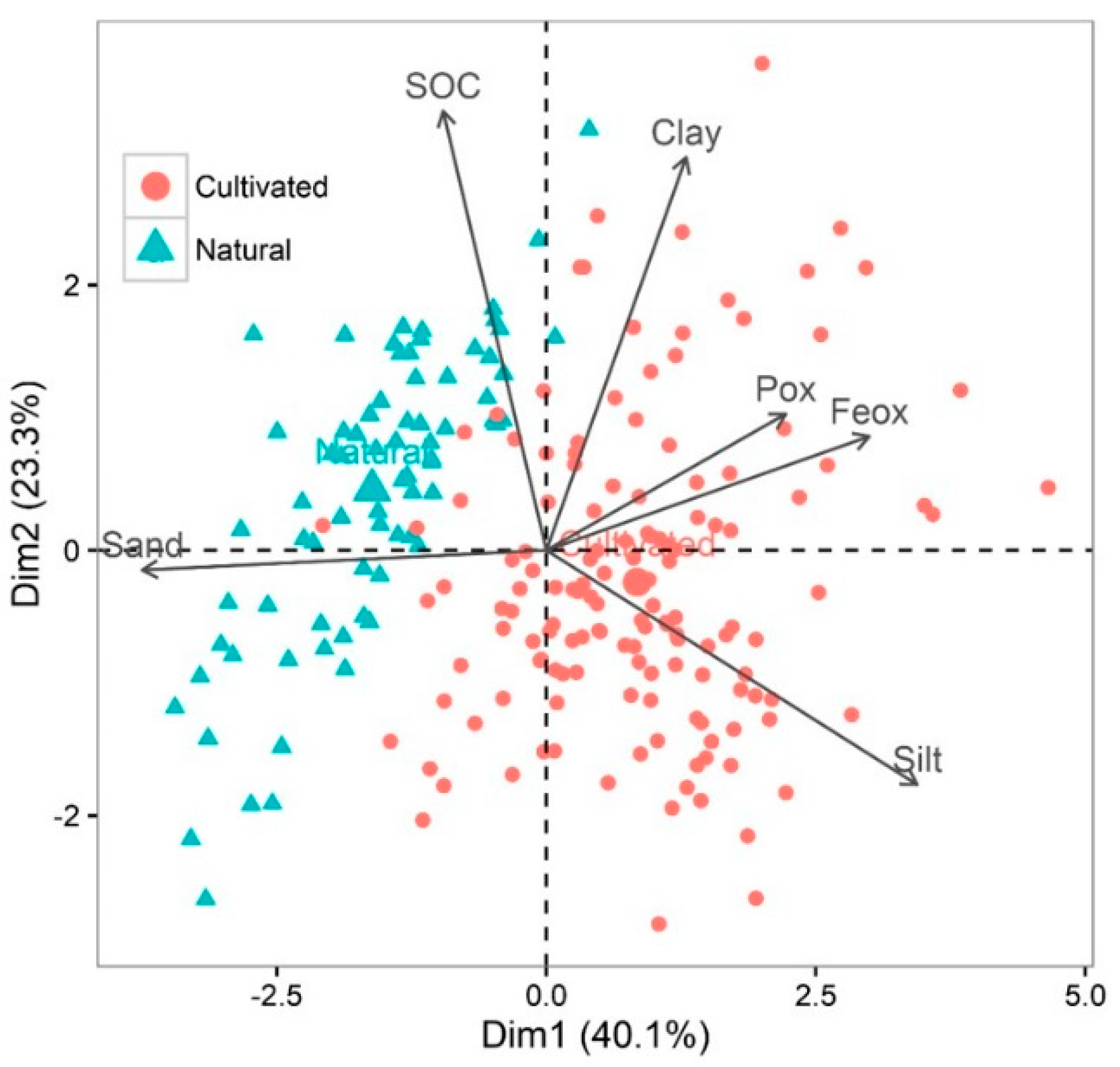
3.1. Soil Characteristics by Chemical Analysis
3.2. Model Prediction Accuracy for Oxalate-Extractable P under Different Land-Use Systems
3.3. Properties of the Prediction-Relevant Wavebands
3.4. Factors Influencing the Prediction Model Accuracy for Oxalate-Extractable P
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andriamananjara, A.; Rakotoson, T.; Razafimbelo, T.; Rabeharisoa, L.; Razafimanantsoa, M.P.; Masse, D. Farmyard manure improves phosphorus use efficiency in weathered P deficient soil. Nutr. Cycl. Agroecosyst. 2019, 115, 407–427. [Google Scholar] [CrossRef]
- Turner, B.L.; Engelbrecht, B. Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry 2011, 103, 297–315. [Google Scholar] [CrossRef]
- Liu, X.; Meng, W.; Liang, G.; Li, K.; Xu, W.; Huang, L.; Yan, J. Available phosphorus in forest soil increases with soil nitrogen but not total phosphorus: Evidence from subtropical forests and a pot experiment. PLoS ONE 2014, 9, e88070. [Google Scholar] [CrossRef]
- Rabeharisoa, L.; Razanakoto, O.R.; Razafimanantsoa, M.P.; Rakotoson, T.; Amery, F.; Smolders, E. Larger bioavailability of soil phosphorus for irrigated rice compared with rainfed rice in Madagascar: Results from a soil and plant survey. Soil Use Manag. 2012, 28, 448–456. [Google Scholar] [CrossRef]
- Bollyn, J.; Castelein, L.; Smolder, E. Fate and bioavailability of phosphorus loaded to iron oxyhydroxide nanoparticles added to weathered soils. Plant Soil 2019, 438, 297. [Google Scholar] [CrossRef]
- Nawara, S.; Van Dael, T.; Merckx, R.; Amery, F.; Elsen, A.; Odeurs, W.; Vandendriessche, H.; Mcgrath, S.; Roisin, C.; Jouany, C.; et al. A comparison of soil tests for available phosphorus in long-term field experiments in Europe. Eur. J. Soil Sci. 2017, 68, 873–885. [Google Scholar] [CrossRef]
- Nishigaki, T.; Tsujimoto, Y.; Rinasoa, S.; Rakotoson, T.; Andriamananjara, A.; Razafimbelo, T. Phosphorus uptake of rice plants is affected by phosphorus forms and physicochemical properties of tropical weathered soils. Plant Soil 2019, 435, 27–38. [Google Scholar] [CrossRef]
- Guo, F.; Yost, R.S. Quantifying the available soil phosphorus pool with the acid ammonium oxalate method. Soil Sci. Soc. Am. J. 1999, 63, 651–656. [Google Scholar] [CrossRef]
- Shahandeh, H.; Hossner, L.R.; Turner, F.T. Phosphorus Relationships in Flooded Rice Soils with Low Extractable Phosphorus. Soil Sci. Soc. Am. J. 1994, 58, 1184–1189. [Google Scholar] [CrossRef]
- Schwertmann, U. The differentiation of iron oxides in soils by extraction with ammonium oxalate solution. Z. Pflanz. Bodenkd. 1964, 105, 194–202. [Google Scholar] [CrossRef]
- Schwertmann, U. Use of oxalate for Fe extraction from soils. Can. J. Soil Sci. 1973, 53, 244–246. [Google Scholar] [CrossRef]
- Narteh, L.T.; Sahrawat, K.L. Oxalate and EDTA extractable soil phosphorus and iron in relation to P availability in lowland rice soils of West Africa. Ghana J. Agric. Sci. 1999, 32, 189–198. [Google Scholar] [CrossRef]
- Six, L.; Smolders, E.; Merckx, R. The performance of DGT versus conventional soil phosphorus tests in tropical soils—Maize and rice responses to P application. Plant Soil 2013, 366, 49–66. [Google Scholar] [CrossRef]
- Neyroud, J.A.; Lischer, P. Do different methods used to estimate soil phosphorus availability across Europe give comparable results? J. Soil Sci. Plant Nutr. 2003, 166, 422–431. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.V.; Walvoort, D.J.J.; McBratney, A.B.; Janik, L.J.; Skjemstad, J.O. Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Stenberg, B.; Viscarra Rossel, R.A.; Mouazen, A.M.; Wetterlind, J. Visible and near infrared spectroscopy in soil science. Adv. Agron. 2010, 107, 163–215. [Google Scholar]
- Nawar, S.; Buddenbaum, H.; Hill, J.; Kozak, J.; Mouazen, A.M. Estimating the soil clay content and organic matter by means of different calibration methods of vis-NIR diffuse reflectance spectroscopy. Soil Tillage Res. 2016, 155, 510–522. [Google Scholar] [CrossRef]
- Recena, R.; Fernández-Cabanás, V.M.; Delgado, A. Soil fertility assessment by Vis-NIR spectroscopy: Predicting soil functioning rather than availability indices. Geoderma 2019, 337, 368–374. [Google Scholar] [CrossRef]
- McCarty, G.W.; Reeves, J.B., III. Comparison of near infrared and mid infrared diffuse reflectance spectroscopy for field-scale measurement of soil fertility parameters. Soil Sci. 2006, 171, 94–102. [Google Scholar] [CrossRef]
- Bellon-Maurel, V.; McBratney, A. Near-infrared (NIR) and mid-infrared (MIR) spectroscopic techniques for assessing the amount of carbon stock in soils—Critical review and research perspectives. Soil Biol. Biochem. 2011, 43, 1398–1410. [Google Scholar] [CrossRef]
- Chang, C.-W.; Laird, D.A.; Mausbach, M.J.; Hurburgh, C.R. Near- infrared reflectance spectroscopy—Principal components regression analyses of soil properties. Soil Sci. Soc. Am. J. 2001, 65, 480–490. [Google Scholar] [CrossRef]
- Ludwig, B.; Khanna, P.K.; Bauhus, J.; Hopmans, P. Near infrared spectroscopy of forest soils to determine chemical and biological properties related to soil sustainability. For. Ecol. Manag. 2002, 171, 121–132. [Google Scholar] [CrossRef]
- Zornoza, R.; Guerrero, C.; Mataix-Solera, J.; Scow, K.M.; Arcenegui, V.; Mataix-Beneyto, J. Near infrared spectroscopy for determination of various physical, chemical and biochemical properties in Mediterranean soils. Soil Biol. Biochem. 2008, 40, 1923–1930. [Google Scholar] [CrossRef]
- Kruse, J.; Abraham, M.; Amelung, W.; Baum, C.; Bol, R.; Kühn, O.; Lewandowski, H.; Niederberger, J.; Oelmann, Y.; Rüger, C.; et al. Innovative methods in soil phosphorus research: A review. J. Plant Nutr. Soil Sci. 2015, 178, 43–88. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Tsujimoto, Y.; Nishigaki, T.; Andriamanajara, A.; Rabenarivo, M.; Asai, H.; Rakotoson, T.; Razafimbelo, T. Laboratory visible and near-infrared spectroscopy with genetic algorithm-based partial least squares regression for assessing the soil phosphorus content of upland and lowland rice fields in Madagascar. Remote Sens. 2019, 11, 506. [Google Scholar] [CrossRef]
- Badjeck, B.; Ibrahima, N.C.; Slaviero, F. Evaluation de la Sécurité Alimentaire à Madagascar; FAO: Rome, Italy, 2013. [Google Scholar]
- Penot, E.; Domas, R.; Paulin, H.; Durand, C. Rôle et Place du Riz Pluvial Dans les Exploitations Agricoles à Madagascar. Le Cas du Lac Alaotra et du Vakinankaratral; Conference paper; Académie d’Agriculture: Antananarivo, Madagascar, 2011. [Google Scholar]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Andriamaniraka, H. Le Phosphore et la Fertilisation Phosphatée Dans les Sols Ferrallitiques à Madagascar: Amélioration de la fertilité des sols. In Mémoire D’habilitation à Diriger des Recherches; Université d’Antananarivo: Antananarivo, Madagascar, 2016. [Google Scholar]
- Nishigaki, T.; Ikazaki, K.; Tsujimoto, Y.; Andriamananjara, A.; Rakotoson, T.; Razafimbelo, T. Soil survey of the east coast and the central highlands indicates need to update Madagascar soil map. Soil Sci. Plant Nutr. 2020. Accepted. [Google Scholar] [CrossRef]
- Cornet, A. Essai de Cartographie Bioclimatique à Madagascar; Notice Explicative No. 55; ORSTOM: Paris, France, 1974. [Google Scholar]
- Schatz, G.E. Endemism in the Malagasy tree flora. In Diversity and Endemism in Madagascar; Lourenço, W.R., Goodman, S.M., Eds.; Mémoires de la Société de Biogéographie: Paris, France, 2000; pp. 1–9. [Google Scholar]
- Andriamananjara, A.; Hewson, J.; Razakamanarivo, H.; Andrisoa, R.H.; Ranaivoson, N.; Ramboatiana, N.; Razafindrakoto, M.; Ramifehiarivo, N.; Razafimanantsoa, M.P.; Rabeharisoa, L.; et al. Land cover impacts on aboveground and soil carbon stocks in Malagasy rainforest. Agric. Ecosyst. Environ. 2016, 233, 1–15. [Google Scholar] [CrossRef]
- Andriamananjara, A.; Ranaivoson, N.; Razafimbelo, T.; Hewson, J.; Ramifehiarivo, N.; Rasolohery, A.; Andrisoa, R.H.; Razafindrakoto, M.A.; Razafimanantsoa, M.-P.; Rabetokotany, N.; et al. Towards a better understanding of soil organic carbon variation in Madagascar. Eur. J. Soil Sci. 2017, 68, 6. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Kawamura, K.; Tsujimoto, Y.; Rabenarivo, M.; Asai, H.; Andriamananjara, A.; Rakotoson, T. Vis-NIR spectroscopy and PLS regression with waveband selection for estimating the total C and N of paddy soils in Madagascar. Remote Sens. 2017, 9, 1081. [Google Scholar] [CrossRef]
- Pätzold, S.; Leenen, M.; Frizen, P.; Heggemann, T.; Wagner, P.; Rodionov, A. Predicting plant available phosphorus using infrared spectroscopy with consideration for future mobile sensing applications in precision farming. Prec. Agric. 2019, 1–25. [Google Scholar] [CrossRef]
- Xu, D.; Ma, W.; Chen, S.; Jiang, Q.; He, K.; Shi, Z. Assessment of important soil properties related to Chinese Soil Taxonomy based on vis–NIR reflectance spectroscopy. Comput. Electron. Agric. 2018, 144, 1–8. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, E.J.M. Smoothing and difference of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Summers, D.; Lewis, M.; Ostendorf, B.; Chittleborough, D. Visible near-infrared reflectance spectroscopy as a predictive indicator of soil properties. Ecol. Indic. 2011, 11, 123–131. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Fouad, Y.; Walter, C. Using a digital camera to measure soil organic carbon and iron contents. Biosyst. Eng. 2008, 100, 149–159. [Google Scholar] [CrossRef]
- Abdi, D.; Tremblay, G.F.; Ziadi, N.; Bélanger, G.; Parent, L.É. Predicting soil phosphorus-related properties using reflectance spectroscopy. Soil Sci. Soc. Am. J. 2012, 76, 2318–2326. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Dardenne, P.; Sinnaeve, G.; Baeten, V. Multivariate calibration and chemometrics for near infrared spectroscopy: Which method? J. Near Infrared Spectrosc. 2000, 8, 229–237. [Google Scholar] [CrossRef]
- Mohamed, E.S.; Saleh, A.M.; Belal, A.B.; Abd-Allah, G. Application of near-infrared reflectance for quantitative assessment of soil properties. Egypt. J. Remote Sens. Space Sci. 2018, 21, 1–14. [Google Scholar] [CrossRef]
- Mouazen, A.M.; Karoui, R.; De Baerdemaeker, J.; Ramon, H. Classification of soil texture classes by using soil visual near infrared spectroscopy and factorial discriminant analysis techniques. J. Near Infrared Spectrosc. 2005, 13, 231–240. [Google Scholar] [CrossRef]
- Conforti, M.; Matteucci, G.; Buttafuoco, G. Using laboratory Vis-NIR spectroscopy for monitoring some forest soil properties. J. Soils Sediments 2018, 18, 1009–1019. [Google Scholar] [CrossRef]
- Stevens, A.; Nocita, M.; Toth, G.; Montanarella, L.; van Wesemael, B. Prediction of soil organic carbon at the European scale by visible and near infrared reflectance spectroscopy. PLoS ONE 2013, 8, e66409. [Google Scholar] [CrossRef] [PubMed]
- Segda, Z.; Bonzi, M.; Gnankambary, Z.; Lompo, F.; Sedogo, M.P. Influence of soil fertility management on organic carbon mineralization in irrigated rice. J. Agric. Crop Res. 2014, 2, 32–43. [Google Scholar]
- Balesdent, J.; Chenu, C.; Balabane, M. Relationship of soil organic matter dynamics to physical protection and tillage. Soil Tillage Res. 2000, 53, 215–230. [Google Scholar] [CrossRef]
- Wang, Z.; Kawamura, K.; Sakuno, Y.; Fan, X.; Gong, Z.; Lim, J. Retrieval of chlorophyll-a and total suspended solids using iterative stepwise elimination partial least squares (ISE-PLS) regression based on field hyperspectral measurements in irrigation ponds in Higashi hiroshima, Japan. Remote Sens. 2017, 9, 264. [Google Scholar] [CrossRef]
- Nduwamungu, C.; Ziadi, N.; Tremblay, G.F.; Parent, L.-É. Near- infrared reflectance spectroscopy prediction of soil properties: Effects of sample cups and preparation. Soil Sci. Soc. Am. J. 2009, 73, 1896–1903. [Google Scholar] [CrossRef]
- Liu, Y.; Boss, E.; Chase, A.P.; Xi, H.; Zhang, X.; Röttgers, R.; Pan, Y.; Bracher, A. Spectral particulate absorption coefficients and their standard deviation derived from underway AC-S measurements during POLARSTERN cruise PS99.2. PANGAEA 2019. [Google Scholar] [CrossRef]
- Sherman, D.M.; Waite, D.T. Electronic spectra of Fe3+ oxides and oxide hydroxides in the near IR to near UV. Am. Mineral. 1985, 70, 1262–1269. [Google Scholar]
- Mortimore, J.L.; Marshall, L.J.R.; Almond, M.J.; Hollins, P.; Matthews, W. Analysis of red and yellow ochre samples from Clearwell Caves and Catalhoyuk by vibrational spectroscopy and other techniques. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004, 60, 1179–1188. [Google Scholar] [CrossRef]
- Ramaroson, V.H.; Becquer, T.; Sá, S.O.; Razafimahatratra, H.; Delarivière, J.L.; Blavet, D.; Vendrame, P.R.S.; Rabeharisoa, L.; Rakotondrazafy, A.F.M. Mineralogical analysis of ferralitic soils in Madagascar using NIR spectroscopy. Catena 2018, 168, 102–109. [Google Scholar] [CrossRef]
- Blaschek, M.; Roudier, P.; Poggio, M.; Hedley, C.B. Prediction of soil available water-holding capacity from visible near-infrared reflectance spectra. Sci. Rep. 2019, 9, 12833. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.R. Spectral signatures of particulate minerals in visible and near-infrared. Trans. Am. Geophys. Union 1977, 58, 553. [Google Scholar] [CrossRef]
- Clark, R.N.; King, T.V.V.; Klejwa, M.; Swayze, G.A.; Vergo, N. High spectral resolution reflectance spectroscopy of minerals. J. Geophys. Res. 1990, 95, 12653–12680. [Google Scholar] [CrossRef]
- Clark, R.N. Spectroscopy of rocks and minerals and principles of spectroscopy. In Remote Sensing for the Earth Sciences: Manual of Remote Sensing; Rencz, A.N., Ed.; John Wiley & Sons: Chichester, UK, 1999; pp. 3–58. [Google Scholar]
- Ben-Dor, E.; Inbar, Y.; Chen, Y. The reflectance spectra of organic matter in the visible near-infrared and short wave infrared region (400–2500 nm) during a control decomposition process. Remote Sens. Environ. 1997, 61, 1–15. [Google Scholar] [CrossRef]
- Knadel, M.; Viscarra Rossel, R.A.; Deng, F.; Thomsen, A.; Greve, M.H. Visible–near infrared spectra as a proxy for topsoil texture and Glacial boundaries. Soil Sci. Soc. Am. J. 2013, 77, 568. [Google Scholar] [CrossRef]
- Sørensen, L.K.; Dalsgaard, S. Determination of clay and other soil properties by near infrared spectroscopy. Soil Sci. Soc. Am. J. 2005, 69, 159–167. [Google Scholar] [CrossRef]
- Jiang, X.; Bol, R.; Willbold, S.; Vereecken, H.; Klumpp, E. Speciation and distribution of P associated with Fe and Al oxides in aggregate-sized fraction of an arable soil. Biogeosciences 2015, 12, 6443–6452. [Google Scholar] [CrossRef]
- Khalid, R.A.; Patrick, W.H.; Delaune, R.D. Phosphorus sorption characteristics of flooded soils. Soil Sci. Soc. Am. Proc. 1977, 41, 305–310. [Google Scholar] [CrossRef]
- Brown, D.J.; Bricklemyer, R.S.; Miller, P.R. Validation requirements for diffuse reflectance soil characterization models with a case study of VNIR soil C prediction in Montana. Geoderma 2005, 129, 251–267. [Google Scholar] [CrossRef]






| Regions | System | Land Uses | Altitude (m) | MAT (°C) | MAP (mm) | Sampling Year | Number of Samples |
|---|---|---|---|---|---|---|---|
| Central (Vakinankaratra) | Cultivated systems | Upland rice | 1247–1481 | 16.9 | 1381 | 2017–2018 | 8 |
| Lowland rice | 1237–1481 | 2017–2018 | 134 | ||||
| Eastern | Natural systems | Forest | 134–1200 | 18–24 | 2500 | 2014–2015 | 16 |
| Non-Forest | 94–1101 | 2014–2015 | 58 |
| Soil Parameters | Cultivated System Area | Natural System Area |
|---|---|---|
| Sand (%) | 34.6 [10.4–72.5] | 53.6 [30.8–80.6 ] |
| Silt (%) | 32.8 [7.92–63.7] | 14.4 [4.72–23.6] |
| Clay (%) | 32.6 [4.30–52.0] | 32.0 [9.45–53.6] |
| SOC (mg kg−1) | 25.5 [9.47–94.9] | 37.9 [7.29–75.4] |
| Feox (g kg−1) | 7.44 [1.03–19.1] | 2.38 [0.32–9.45] |
| Pox (mg kg−1) | 115.1 [22.3–856.8] | 35.1 [21.9–57.9] |
| System | n | Min | Max | Mean | SD | CV (%) |
|---|---|---|---|---|---|---|
| All systems | 216 | 21.89 | 856.84 | 87.66 | 130.23 | 148.57 |
| Cultivated system | 142 | 22.25 | 856.84 | 115.07 | 153.69 | 133.56 |
| Natural system | 74 | 21.89 | 57.93 | 35.05 | 7.79 | 22.23 |
| Pox | SOC | Sand | Silt | Clay | Feox | |
|---|---|---|---|---|---|---|
| All Systems | ||||||
| Pox | 1.00 | 0.10 | −0.20 | 0.23 | −0.00 | 0.55 |
| SOC | 1.00 | 0.15 | −0.32 | 0.24 | −0.06 | |
| Sand | 1.00 | −0.82 | −0.49 | −0.41 | ||
| Silt | 1.00 | −0.09 | 0.35 | |||
| Clay | 1.00 | 0.18 | ||||
| Feox | 1.00 | |||||
| Natural | ||||||
| Pox | 1.00 | 0.61 | −0.29 | −0.06 | 0.37 | 0.45 |
| SOC | 1.00 | −0.44 | −0.00 | 0.53 | 0.32 | |
| Sand | 1.00 | −0.67 | −0.96 | −0.48 | ||
| Silt | 1.00 | 0.45 | 0.22 | |||
| Clay | 1.00 | 0.50 | ||||
| Feox | 1.00 | |||||
| Cultivated | ||||||
| Pox | 1.00 | 0.30 | −0.03 | 0.05 | −0.03 | 0.51 |
| SOC | 1.00 | −0.05 | −0.05 | 0.15 | 0.33 | |
| Sand | 1.00 | −0.79 | −0.32 | 0.06 | ||
| Silt | 1.00 | −0.33 | −0.15 | |||
| Clay | 1.00 | 0.14 | ||||
| Feox | 1.00 | |||||
| Processing | Systems | n | NLV | R2 | RMSECV | RPD |
|---|---|---|---|---|---|---|
| FS-PLS | All systems | 216 | 13 | 0.48 | 96.58 | 1.34 |
| Cultivated | 142 | 15 | 0.70 | 83.72 | 1.82 | |
| Natural | 74 | 2 | 0.18 | 7.10 | 1.08 | |
| ISE–PLS | All systems | 216 | 13 | 0.70 | 71.87 | 1.81 |
| Cultivated | 142 | 15 | 0.90 | 48.57 | 3.15 | |
| Natural | 74 | 14 | 0.90 | 2.39 | 3.22 |
| Processing | Systems | n | R2 | RMSEP |
|---|---|---|---|---|
| FS-PLS | All systems | 64 | 0.502 ± 0.124 | 89.01 ± 9.21 |
| Cultivated | 42 | 0.678 ± 0.079 | 79.13 ± 8.30 | |
| Natural | 22 | 0.141 ± 0.096 | 7.15 ± 1.62 | |
| ISE–PLS | All systems | 64 | 0.703 ± 0.115 | 60.48 ± 5.94 |
| Cultivated | 42 | 0.883 ± 0.038 | 57.42 ± 5.57 | |
| Natural | 22 | 0.822 ± 0.051 | 3.26 ± 0.59 |
| Spectra Regions (nm) | Common Selected Wavelength (nm) | Specific Wavelength (nm) for Cultivated | Specific Wavelength (nm) for Natural | Functional Groups | References |
|---|---|---|---|---|---|
| Visible | |||||
| 400–700 | 409, 430, 431, 443, 444,591, 592 | 527–590 | Associated to mineral with Fe (hematite, goethite) SOM: chromophores and darkness of OM | [54,55] | |
| 550 | 550–557 | Chromophore FeOOH in goethite | [55] | ||
| Near Infra-Red (NIR) | |||||
| 751, 825 | 763, 826 | 738–740, 753 | Amine C-H, aromatic C-H | [59] | |
| 860 | 870 | Ferric oxide, Fe3+ | [58] | ||
| 1000 | 1000 | Amine N-H | [59] | ||
| 1100 | 1122–1144 | Aromatic C-H | [59] | ||
| 1170 | 1170 | Alkyl asymmetric-symmetric doublet (C-H) | [59,60] | ||
| 1260 | 1291 | Lignin, starch, protein, | [61] | ||
| 1465, 1470 | 1464–1483 | OH in water, CH2, cellulose, lignin, starch, pectin | [61] | ||
| 2160 | 2160–2164 | Al-OH, Kaolin | [62] | ||
| 2200–2300 | 2200–2270 | Metal-OH, O-H | [15] | ||
| 2300, 2350 | 2302–23062350–2355 | C-H stretch fundamentals | [59,60] | ||
| 2335 | 2330–2334 | Carbonates | [58] | ||
| 850, 1200, 1400, 1900 | 1950–1956 | H2O | [15,58] | ||
| 2200, 2300 | 2200–2270 | Al-OH, O-H | [59] | ||
| 1900 | 1906–1907 | H-O-H | [59] | ||
| Visible-NIR | |||||
| 450, 900 | 453–457 | Fe+3 | [54] | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakotonindrina, H.; Kawamura, K.; Tsujimoto, Y.; Nishigaki, T.; Razakamanarivo, H.; Andrianary, B.H.; Andriamananjara, A. Prediction of Soil Oxalate Phosphorus using Visible and Near-Infrared Spectroscopy in Natural and Cultivated System Soils of Madagascar. Agriculture 2020, 10, 177. https://doi.org/10.3390/agriculture10050177
Rakotonindrina H, Kawamura K, Tsujimoto Y, Nishigaki T, Razakamanarivo H, Andrianary BH, Andriamananjara A. Prediction of Soil Oxalate Phosphorus using Visible and Near-Infrared Spectroscopy in Natural and Cultivated System Soils of Madagascar. Agriculture. 2020; 10(5):177. https://doi.org/10.3390/agriculture10050177
Chicago/Turabian StyleRakotonindrina, Hobimiarantsoa, Kensuke Kawamura, Yasuhiro Tsujimoto, Tomohiro Nishigaki, Herintsitohaina Razakamanarivo, Bruce Haja Andrianary, and Andry Andriamananjara. 2020. "Prediction of Soil Oxalate Phosphorus using Visible and Near-Infrared Spectroscopy in Natural and Cultivated System Soils of Madagascar" Agriculture 10, no. 5: 177. https://doi.org/10.3390/agriculture10050177
APA StyleRakotonindrina, H., Kawamura, K., Tsujimoto, Y., Nishigaki, T., Razakamanarivo, H., Andrianary, B. H., & Andriamananjara, A. (2020). Prediction of Soil Oxalate Phosphorus using Visible and Near-Infrared Spectroscopy in Natural and Cultivated System Soils of Madagascar. Agriculture, 10(5), 177. https://doi.org/10.3390/agriculture10050177






