Potato Phosphorus Response in Soils with High Value of Phosphorus
Abstract
1. Introduction
2. Materials and Methods
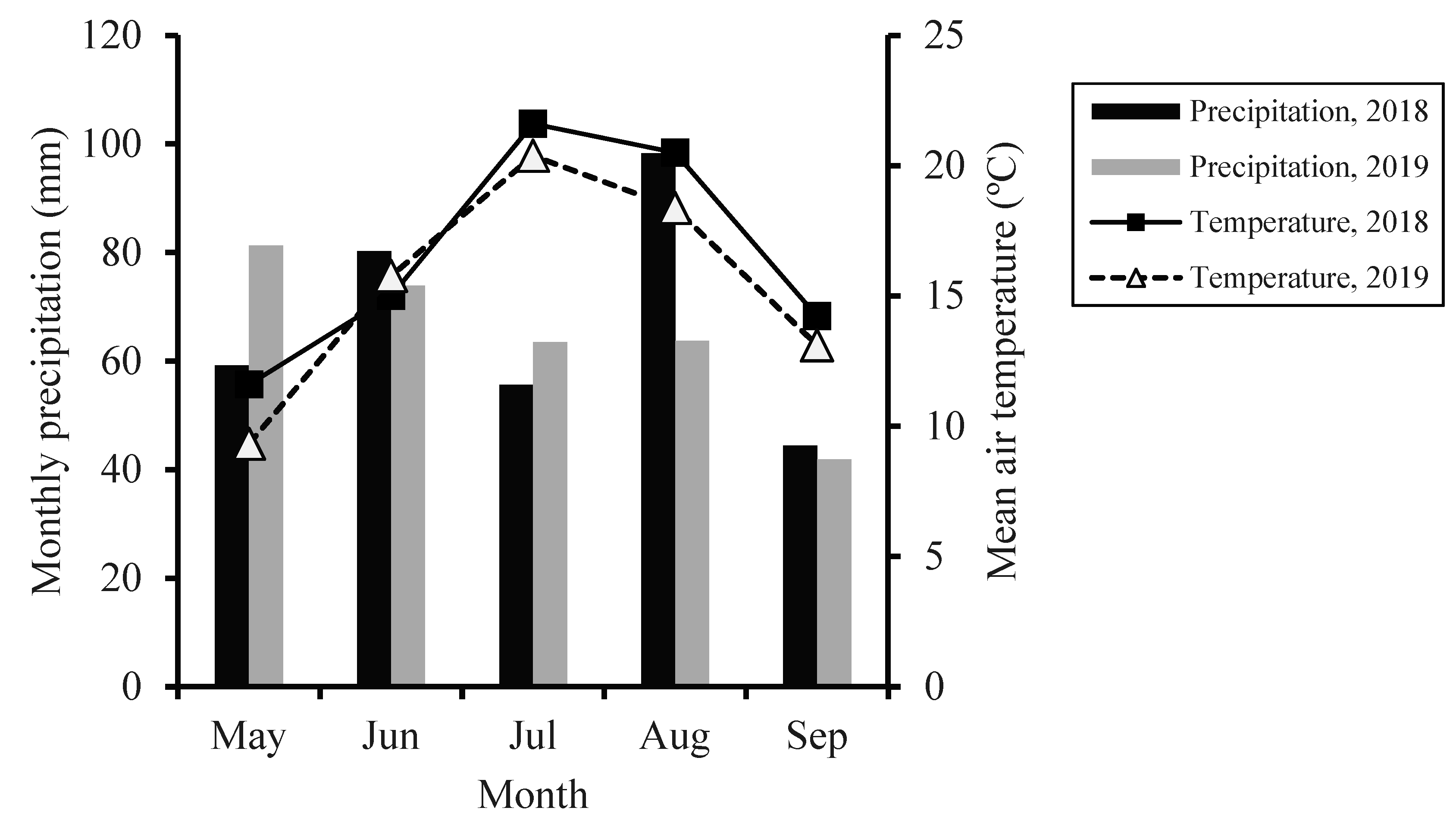
2.1. Site Description
2.2. Experimental Design
2.3. Soil Sample Analysis
2.4. Plant Sampling and Analysis
2.5. Potato Yield Components and Measurements
2.6. Statistical Analysis
3. Results
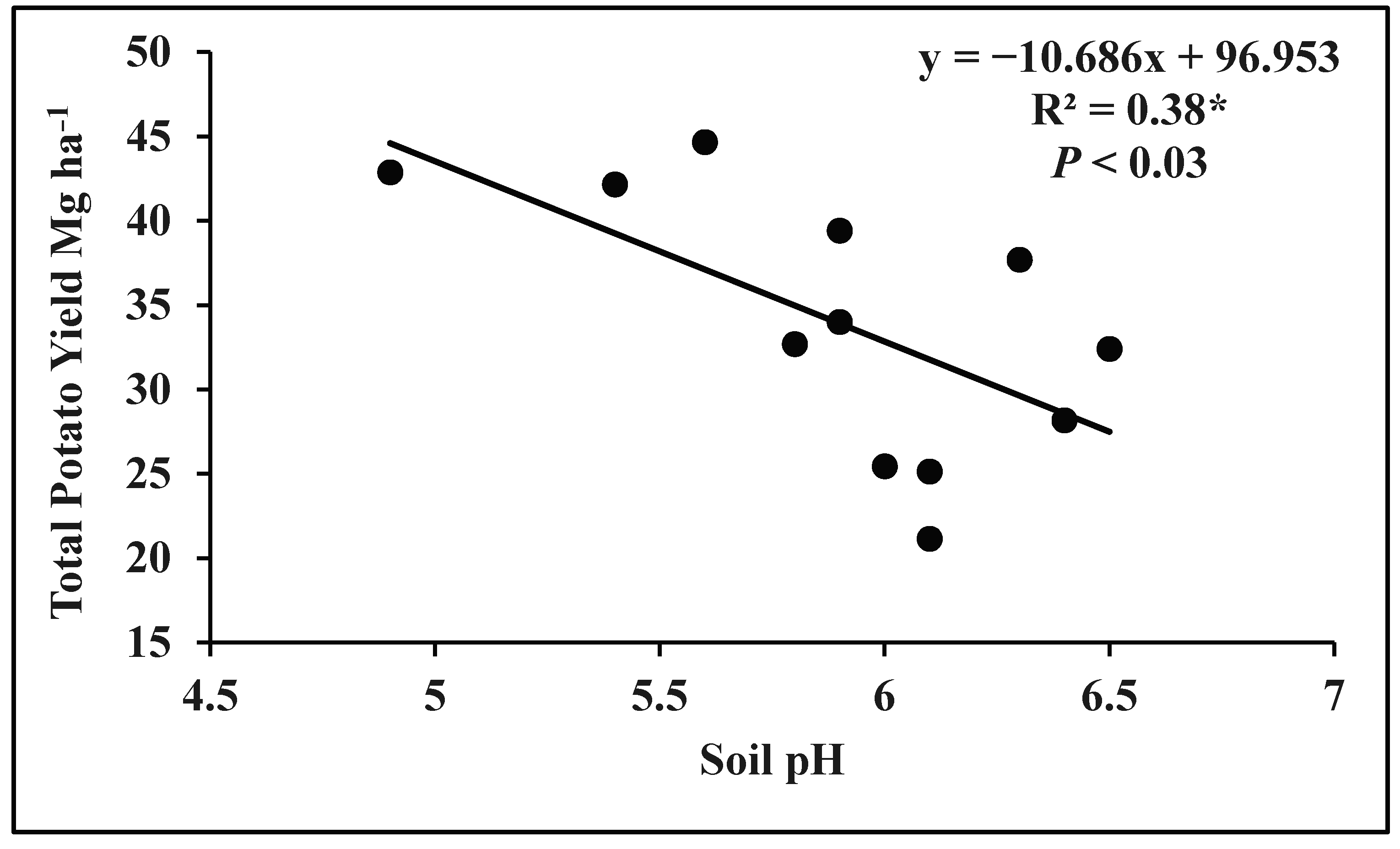
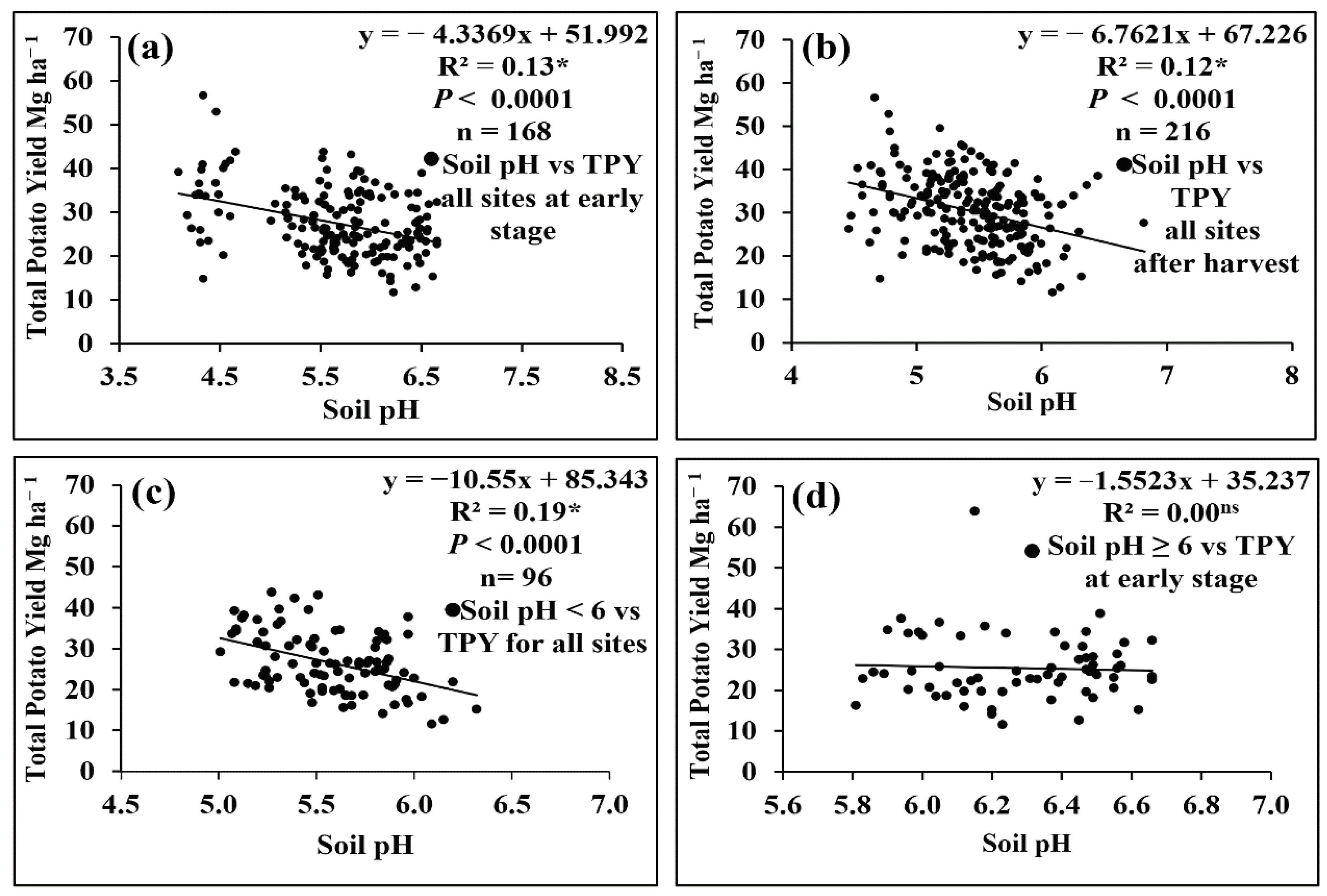
3.1. Correlation of Total Potato Yield with Soil pH
3.2. Phosphorus Effects on Potato Tuber Yield Parameters
3.2.1. The Effect of Phosphorus on Total Potato Tuber Yield, Specific Gravity, Marketable, and Unmarketable Tuber Yield
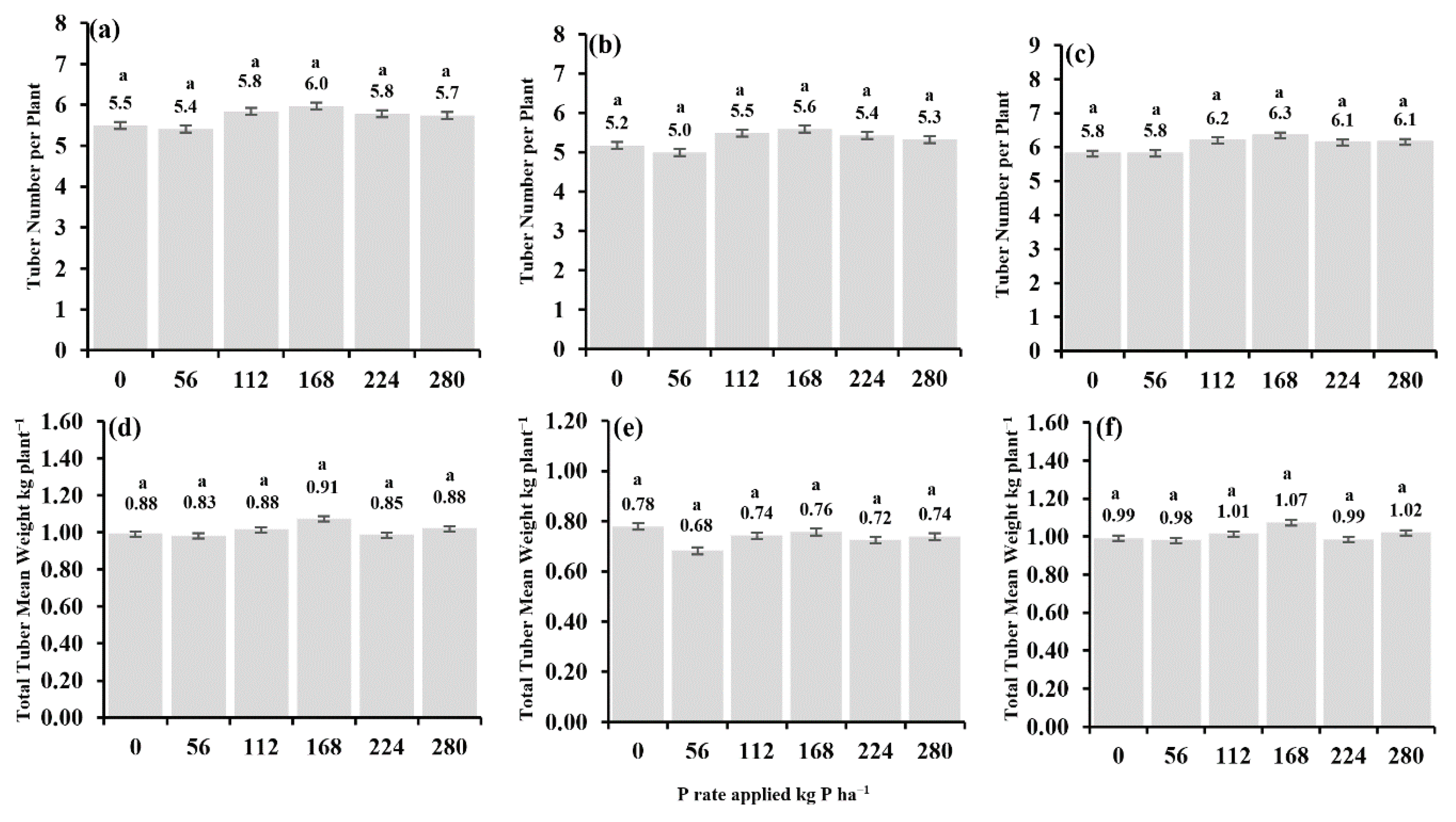
3.2.2. The Effect of Phosphorus on Tuber Number per Plant and Total Tuber Mean Weight
3.3. Petiole P Concentration and its Uptake
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hopkins, B.G.; Hansen, N.C. Phosphorus Management in High-Yield Systems. J. Environ. Qual. 2019, 48, 1265–1280. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. FAO, R. Food and Agriculture Organization. 2019. Available online: http://www.fao.org/faostat/en/#data (accessed on 9 December 2019).
- United States Department of Agriculture National Resources Conservation Service. The PLANTS Database; National Plant Data Center: Baton Rouge, LA, USA, 2018. Available online: https://plants.sc.egov.usda.gov/java/ (accessed on 10 November 2019).
- Rosen, C.J.; Kelling, K.A.; Stark, J.C.; Porter, G.A. Optimizing phosphorus fertilizer management in potato production. Am. J. Potato Res. 2014, 91, 145–160. [Google Scholar] [CrossRef]
- Fixen, P.E.; Bruulsema, T.W. Potato management challenges created by phosphorus chemistry and plant roots. Am. J. Potato Res. 2014, 91, 121–131. [Google Scholar] [CrossRef]
- Hopkins, B.G.; Fernelius, K.J.; Hansen, N.C.; Eggett, D.L. AVAIL phosphorus fertilizer enhancer: Meta-analysis of 503 field evaluations. Agron. J. 2018, 110, 389–398. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Soratto, R.P. Response of potato cultivars to phosphate fertilization in tropical soils with different phosphorus availabilities. Potato Res. 2016, 59, 259–278. [Google Scholar] [CrossRef]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers; Pearson Education India: New Delhi, India, 2016. [Google Scholar]
- Faucon, M.-P.; Houben, D.; Reynoird, J.-P.; Mercadal-Dulaurent, A.-M.; Armand, R.; Lambers, H. Advances and Perspectives to Improve the Phosphorus Availability in Cropping Systems for Agroecological Phosphorus Management. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2015; Volume 134, pp. 51–79. [Google Scholar] [CrossRef]
- Ruark, M.D.; Kelling, K.A.; Good, L.W. Environmental concerns of phosphorus management in potato production. Am. J. Potato Res. 2014, 91, 132–144. [Google Scholar] [CrossRef]
- Soratto, R.P.; Pilon, C.; Fernandes, A.M.; Moreno, L.A. Phosphorus uptake, use efficiency, and response of potato cultivars to phosphorus levels. Potato Res. 2015, 58, 121–134. [Google Scholar] [CrossRef]
- Reinhardt, R.L.; Norton, S.A.; Handley, M.; Amirbahman, A. Dynamics of P, Al, and Fe During High Discharge episodic Acidification at the Bear Brook Watershed in Maine, USA. In Biogeochemical Investigations of Terrestrial, Freshwater, and Wetland Ecosystems across the Globe; Springer: Dordrecht, The Netherlands, 2004; pp. 311–323. [Google Scholar] [CrossRef]
- Bruulsema, T.W.; Peterson, H.M.; Prochnow, L.I. The science of 4R nutrient stewardship for phosphorus management across latitudes. J. Environ. Qual. 2019, 48, 1295–1299. [Google Scholar] [CrossRef]
- Hopkins, G.B. Phosphorus. In Handbook of Plant Nutrition, 2nd ed.; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 66–111. Available online: https://www.routledgehandbooks.com/doi/10.1201/b18458-6 (accessed on 18 December 2018).
- Taiz, L.; Zeiger, E. Plant Physiology (2002); Sinauer Associates Inc.: Sunderland, MA, USA, 2002; Volume 3, Available online: https://academic.oup.com/aob/article/91/6/750/211033 (accessed on 20 September 2019).
- Balemi, T. Effect of phosphorus nutrition on growth of potato genotypes with contrasting phosphorus effeciency. Afr. Crop Sci. J. 2009, 17. [Google Scholar] [CrossRef]
- Hailu, G.; Nigussie, D.; Ali, M.; Derbew, B. Nitrogen and phosphorus use efficiency in improved potato (Solanum tuberosum L.) cultivars in southern Ethiopia. Am. J. Potato Res. 2017, 94, 617–631. [Google Scholar] [CrossRef]
- Rosen, C.J.; Bierman, P.M. Potato yield and tuber set as affected by phosphorus fertilization. Am. J. Potato Res. 2008, 85, 110–120. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Soratto, R.P.; Souza, E.d.F.C.d.; Job, A.L.G. Nutrient uptake and removal by potato cultivars as affected by phosphate fertilization of soils with different levels of phosphorus availability. Rev. Bras. de Ciência do Solo 2017, 41. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Cassagne, N.; Remaury, M.; Gauquelin, T.; Fabre, A. Forms and profile distribution of soil phosphorus in alpine Inceptisols and Spodosols (Pyrenees, France). Geoderma 2000, 95, 161–172. [Google Scholar] [CrossRef]
- Grant, C.; Flaten, D.; Tomasiewicz, D.; Sheppard, S. The importance of early season phosphorus nutrition. Can. J. Plant Sci. 2001, 81, 211–224. [Google Scholar] [CrossRef]
- Hodges, S.C. Soil Fertility Basics. In Soil Science Extension; North Carolina State University: Raleigh, NC, USA, 2010; Available online: http://www2.mans.edu.eg/projects/heepf/ilppp/cources/12/pdf%20course/38/Nutrient%20Management%20for%20CCA.pdf (accessed on 12 October 2019).
- Sharma, L.K.; Bali, S.K.; Zaeen, A.A. A case study of potential reasons of increased soil phosphorus levels in the Northeast United States. Agronomy 2017, 7, 85. [Google Scholar] [CrossRef]
- Buob, T.E.; Rochette, E.A. Status of phosphorus in soils of the Connecticut river watershed in New Hampshire. Commun. Soil Sci. Plant Anal. 2003, 34, 1177–1192. [Google Scholar] [CrossRef]
- NOAA National Oceanic and Atmospheric Administration, USA. 2019. Available online: https://www.ncdc.noaa.gov/cdoweb/datasets/GHCND/stations/GHCND:USW00014607/detail (accessed on 15 December 2019).
- McIntosh, J.L. Bray and Morgan Soil Extractants Modified for Testing Acid Soils from Different Parent Materials 1. Agron. J. 1969, 61, 259–265. [Google Scholar] [CrossRef]
- Magdoff, F.; Van Es, H. Building Soils for Better Crops; Sustainable Agriculture Network Beltsville: Brentwood, MD, USA, 2009; Available online: https://www.sare.org/Learning-Center/Books/Building-Soils-for-Better-Crops-3rd-Edition (accessed on 16 June 2019).
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- McLean, E. Soil pH and lime requirement. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1983, 9, 199–224. [Google Scholar]
- Storer, D.A. A simple high sample volume ashing procedure for determination of soil organic matter. Commun. Soil Sci. Plant Anal. 1984, 15, 759–772. [Google Scholar] [CrossRef]
- Page, A.; Miller, R.; Keeney, D. Methods of Soil Analysis. Part 2. American Society of Agronomy; Soil Science Society of America: Madison, WI, USA, 1982; Available online: https://acsess.onlinelibrary.wiley.com/doi/pdf/10.2134/agronmonogr9.2.2ed.frontmatter (accessed on 15 October 2019).
- Dahnke, W.C.; Whitney, D.A. Measurement of soil salinity. Recomm. Chem. Soil Test Proced. North Cent. Reg. 1988, 499, 32–34. [Google Scholar]
- Westermann, D.; Kleinkopf, G. Phosphorus Relationships in Potato Plants 1. Agron. J. 1985, 77, 490–494. [Google Scholar] [CrossRef]
- Kalra, Y.P.; Maynard, D.G. Methods Manual for Forest Soil and Plant Analysis; Minister of Supply and Services Canada 1991; Canadian Forest Service: Edmonton, AB, Canada, 1991; Volume 319. Available online: https://cfs.nrcan.gc.ca/pubwarehouse/pdfs/11845.pdf (accessed on 5 April 2019).
- Chapman, H.D.; Pratt, P.F. Methods of analysis for soils, plants and waters. Soil Sci. 1962, 93, 68. [Google Scholar] [CrossRef]
- Kleinschmidt, G.; Kleinkopf, G.; Westermann, D.; Zalewski, J. Specific Gravity of Potatoes (CIS 609); University of Idaho: Moscow, Russia, 1984; Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C20&q=Kleinschmidt%2C+G.%3B+Kleinkopf%2C+G.%3B+Westermann%2C+D.%3B+Zalewski%2C+J.%2C+Specific+gravity+of+potatoes+%28CIS+609%29.+Moscow%3A+University+of+Idaho+1984.&btnG= (accessed on 21 October 2018).
- SAS. Base SAS 9.4 Procedures Guide; SAS Institute: Cary, NC, USA, 2015; Available online: https://support.sas.com/documentation/cdl/en/procstat/66703/PDF/default/procstat.pdf (accessed on 10 February 2019).
- Sposito, G. The Chemistry of Soils; Oxford University Press: Oxford, UK, 2008; Available online: https://scholar.google.com/scholar?q=Sposito,+G.,+The+chemistry+of+soils.+Oxford+university+press:+2008.&hl=en&as_sdt=0&as_vis=1&oi=scholart (accessed on 18 January 2018).
- Pehrson, L.; Mahler, R.; Bechinski, E.; Williams, C. Nutrient management practices used in potato production in Idaho. Commun. Soil Sci. Plant Anal. 2011, 42, 871–882. [Google Scholar] [CrossRef]
- Luz, J.M.Q.; Queiroz, A.A.; Borges, M.; Oliveira, R.C.; Leite, S.S.; Cardoso, R.R. Influence of phosphate fertilization on phosphorus levels in foliage and tuber yield of the potato cv. Ágata. Semin. Ciências Agrárias 2013, 34, 649–656. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Soratto, R.P.; Gonsales, J.R. Root morphology and phosphorus uptake by potato cultivars grown under deficient and sufficient phosphorus supply. Sci. Hortic. 2014, 180, 190–198. [Google Scholar] [CrossRef]
- Laboski, C.A.; Kelling, K.A. Influence of fertilizer management and soil fertility on tuber specific gravity: A review. Am. J. Potato Res. 2007, 84, 283–290. [Google Scholar] [CrossRef]
- Stark, J.C.; Love, S. Tuber Quality. In Potato Production Systems; Stark, J.C., Love, S.L., Eds.; Univ of Idaho Extension: Moscow, ID, Russia, 2003; Available online: https://www.extension.uidaho.edu/detail.aspx?IDnum=1636 (accessed on 21 June 2019).
- Human, J. The Effect of Fertilizer Levels on Yield and Specific Gravity of Potatoes; Holetta Guenet Research Station Progress Report; IAR (Institute of Agricultural Research): Addis Ababa, Ethiopia, 1961; p. 278.
- Shibabaw, A.; Alemayehu, G.; Adgo, E.; Asch, F.; Freyer, B. Effects of organic manure and crop rotation system on potato (Solanum tuberosum L.) tuber yield in the highlands of Awi Zone. Ethiop. J. Sci. Technol. 2018, 11, 1–18. [Google Scholar] [CrossRef]
- Zelalem, A.; Tekalign, T.; Nigussie, D. Response of potato (Solanum tuberosum L.) to different rates of nitrogen and phosphorus fertilization on vertisols at Debre Berhan, in the central highlands of Ethiopia. Afr. J. Plant Sci. 2009, 3, 16–24. [Google Scholar]
- Fernandes, A.M.; Soratto, R.P. Phosphorus fertilizer rate for fresh market potato cultivars grown in tropical soil with low phosphorus availability. Am. J. Potato Res. 2016, 93, 404–414. [Google Scholar] [CrossRef]
- Benjannet, R.; Nyiraneza, J.; Khiari, L.; Fuller, K.; Bizimungu, B.; Savoie, D.; Jiang, Y.; Rodd, V.; Mills, A. An Agro-Environmental Phosphorus Model for Potato in the Canadian Maritime Provinces. Agron. J. 2018, 110, 2566–2575. [Google Scholar] [CrossRef]
- Parsad, R.; Power, J.F. Soil Fertility Management for Sustainable Agriculture; CRC Press: Boca Raton, FL, USA, 1997; Available online: https://www.google.com/books/edition/_/otAyc8tJGbwC?hl=en&gbpv=1 (accessed on 11 September 2019).


| Site | Year | Latitude | Longitude | † Soil Type | Slope | Planting Date | Harvest Date | Varieties |
|---|---|---|---|---|---|---|---|---|
| Frenchville (FV) | 2018 | 47.2170080 | −68.4112920 | Coarse-loamy, isotic, frigid Oxyaquic Haplorthods | 2–8% | 22 May | 12 September | RB |
| New Sweden-1 (NS1) | 2018 | 46.9511590 | −68.1479550 | Fine-loamy, mixed, frigid Typic Haplorthods | 2–8% | 29 May | 15 October | RB |
| New Sweden-2 (NS2) | 2018 | 46.9529336 | −68.1454612 | Fine-loamy, mixed, frigid Aquic Haplorthods | 2–8% | 30 May | 15 October | RB |
| WoodLand (WL) | 2018 | 46.8850498 | −68.1256605 | Fine-loamy, mixed, frigid Typic Haplorthods | 2–8% | 15 May | 01 October | RB |
| Caribou-1 (CA1) | 2018 | 46.8842966 | −68.0292126 | Fine-loamy, mixed, frigid Typic Haplorthods | 2–8% | 17 May | 13 September | RB |
| Aroostook Farm-1 (AF1) | 2018 | 46.6601582 | −68.0216085 | Fine-loamy, mixed, frigid Typic Haplorthods | 2–8% | 24 May | 14 September | SH |
| Aroostook Farm-2 (AF2) | 2018 | 46.6619155 | −68.0209886 | Fine-loamy, mixed, frigid Typic Haplorthods | 2–8% | 24 May | 14 September | RB |
| Aroostook Farm-3 (AF3) | 2019 | 46.66011944 | −68.02125 | Fine-loamy, mixed, frigid Typic Haplorthods | 2–8% | 31 May | 20 September | SP |
| Aroostook Farm-4 (AF4) | 2019 | 46.46.6601694 | −68.01650278 | Fine-loamy, mixed, frigid Typic Haplorthods | 2–8% | 31 May | 20 September | RB |
| Caribou -2 (CA2) | 2019 | 46.89628611 | −68.07754722 | Fine-loamy, mixed, frigid Typic Haplorthods | 2–8% | 14 May | 30 September | RB |
| Caribou-3 (CA3) | 2019 | 46.89180556 | −68.04066667 | Fine-loamy, mixed, frigid Typic Haplorthods | 2–15% | 28 May | 30 September | RB |
| Limestone (LM) | 2019 | 46.96186944 | −67.83323056 | Fine-loamy, mixed, frigid Typic Haplorthods | 2–8% | 29 May | 01 October | RB |
| Site | FV | NS1 | NS2 | WL | CA1 | AF1 | AF2 | AF3 | AF4 | CA2 | CA3 | LM | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growing Years | 2018 | 2019 | |||||||||||
| pH | 5.9 | 4.9 | 5.6 | 5.8 | 6.5 | 6.1 | 6.4 | 6.0 | 6.1 | 5.4 | 6.3 | 5.9 | |
| Organic Matter | % | 4.9 | 3.1 | 5.3 | 4.1 | 3.7 | 2.6 | 4.0 | 2.3 | 3.1 | 2.6 | 3.3 | 1.6 |
| P-MME | mg nutrient kg−1 soil | 20 | 12 | 10 | 16 | 21 | 13 | 21 | 11 | 17 | 18 | 24 | 18 |
| P-M3 | 379 | 469 | 257 | 356 | 421 | 440 | 421 | 341 | 423 | 586 | 537 | 558 | |
| N-NO3− | 5 | 62 | 2 | 15 | 6 | 4 | 12 | 5 | 6 | 8 | 7 | 3 | |
| N-NH4+ | 1 | 28 | 25 | 5 | 1 | 15 | 17 | 4 | 6 | 10 | 7 | 19 | |
| K-M3 | 237 | 221 | 151 | 256 | 376 | 300 | 225 | 234 | 160 | 348 | 292 | 230 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasim, A.; Sharma, L.K.; Zaeen, A.; Bali, S.K.; Buzza, A.; Alyokhin, A. Potato Phosphorus Response in Soils with High Value of Phosphorus. Agriculture 2020, 10, 264. https://doi.org/10.3390/agriculture10070264
Jasim A, Sharma LK, Zaeen A, Bali SK, Buzza A, Alyokhin A. Potato Phosphorus Response in Soils with High Value of Phosphorus. Agriculture. 2020; 10(7):264. https://doi.org/10.3390/agriculture10070264
Chicago/Turabian StyleJasim, Ahmed, Lakesh K. Sharma, Ahmed Zaeen, Sukhwinder K. Bali, Aaron Buzza, and Andrei Alyokhin. 2020. "Potato Phosphorus Response in Soils with High Value of Phosphorus" Agriculture 10, no. 7: 264. https://doi.org/10.3390/agriculture10070264
APA StyleJasim, A., Sharma, L. K., Zaeen, A., Bali, S. K., Buzza, A., & Alyokhin, A. (2020). Potato Phosphorus Response in Soils with High Value of Phosphorus. Agriculture, 10(7), 264. https://doi.org/10.3390/agriculture10070264








