Nitrogen Recoveries and Nitrogen Use Efficiencies of Organic Fertilizers with Different C/N Ratios in Maize Cultivation with Low-Fertile Soil by 15N Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Incubation Experiment for N Mineralization Test for Organic Amendments
2.2. Plant Cultivation and Analysis of Nitrogen Dynamcs by 15N Tracer
2.3. Plant and Soil Analysis, Fertilizer Nitrogen Use Efficiency, and Its Components
2.4. Total N Uptake by Plant, Plant Uptake N Derived from Fertilizer and Soil
2.5. Agronomic N Use Efficiency, Apparent N Recovery Efficiency, and Physiological Efficiency
2.6. Statistical Analysis
3. Results
3.1. Nitrogen Mineralization under Different Organic Amendments from the Incubation Experiment
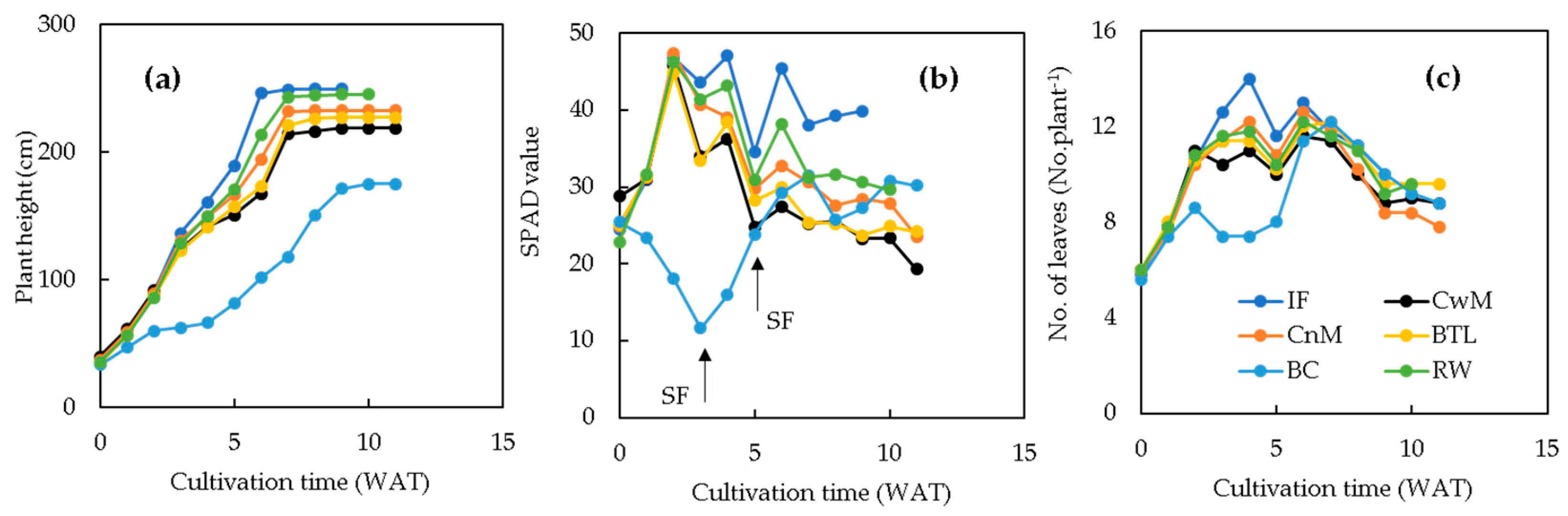
3.2. Plant Growth
3.3. Maize Grain Yield and Total Plant Biomass
3.4. Maize N Dynamics on 15N Tracer Method Analysis
3.5. Fertilizer NUE and Its Components
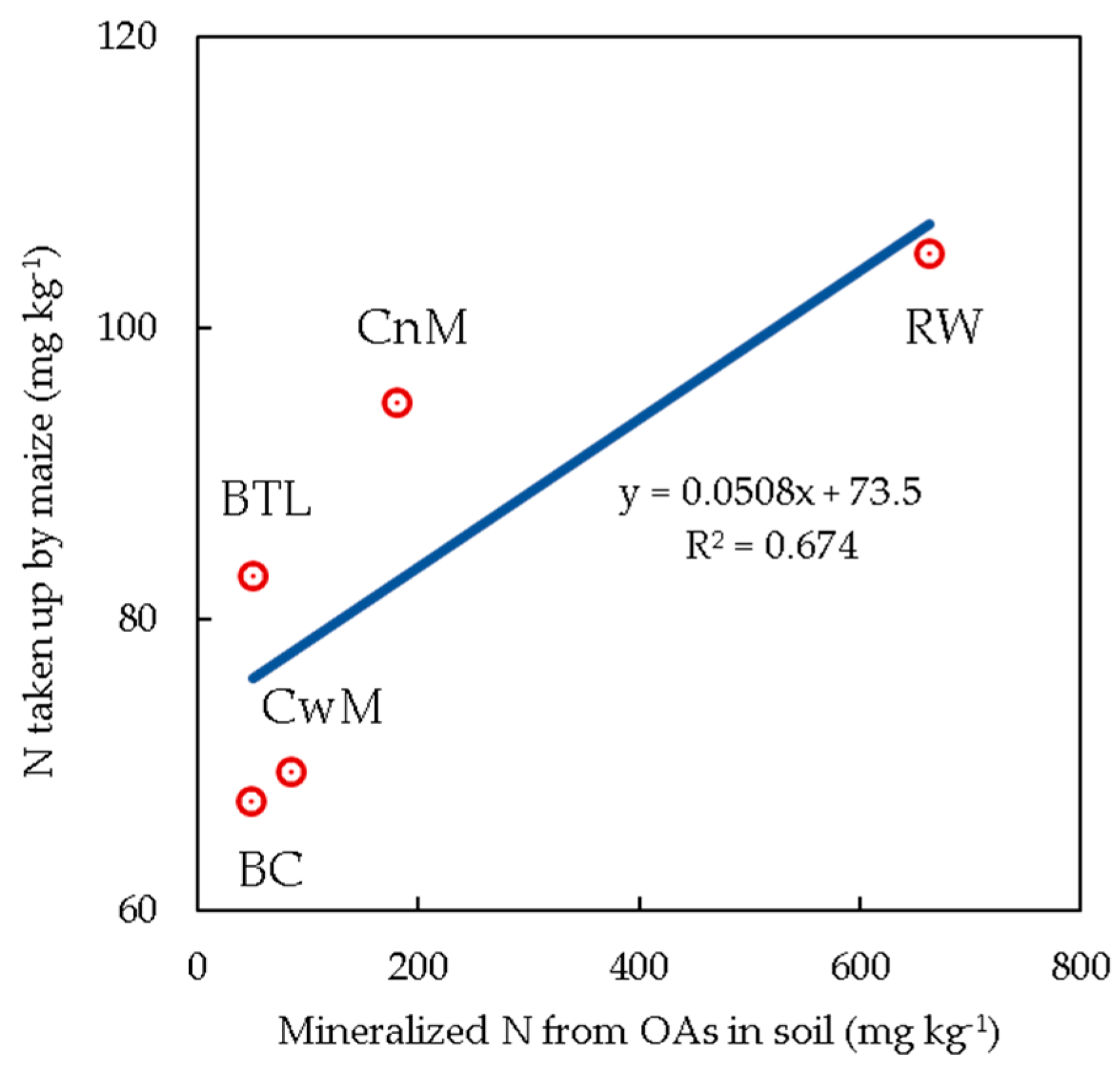
3.6. Relationship between the Incubation Test and Pot Experiment
3.7. Soil Properties after Harvest
4. Discussion
4.1. The Effect of Organic Amendments on Plant Growth and Yield of Maize
4.2. The Effect of Various Fertilizers on NUE, NUE Components, and N Distribution on Maize Plant
4.3. The Effect of Organic Amendmemnts on Soil Chemical Properties
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martens, J. Report of the UN Millennium Project—Investing in Development. In Report of the UN Millennium Project “Investing in Development“; Friedrich-Ebert Foundation: Bonn, Alemanha, 2005; pp. 1–8. [Google Scholar]
- Henao, J.; Baamate, C. Agricultural Production and Soil Nutrient Mining in Africa: Implications for Resource Conservation and Policy Development; IFDC: Muscle Shoals, AL, USA, 2006; pp. 1–13. [Google Scholar]
- Snapp, S.S.; Mafongoya, P.L.; Waddington, S. Organic matter technologies for integrated nutrient management in smallholder cropping systems of southern Africa. Agric. Ecosyst. Environ. 1998, 71, 185–200. [Google Scholar] [CrossRef]
- Lin, H.; Huber, J.; Gerl, G.; Hulsbergen, K. Nitrogen balances and nitrogen-use efficiency of different organic and conventional farming systems. Nutr. Cycl. Agroecosyst. 2016, 105, 1–23. [Google Scholar] [CrossRef]
- Hawkesford, M.J. Reducing the reliance on nitrogen fertilizer for wheat production. J. Cereal Sci. 2014, 59, 276–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaizzi, K.; Byalebeka, J.; Semalulu, O.; Alou, I.N.; Zimwanguyizza, W.; Nansamba, A.; Musinguzi, P.; Ebanyat, P.; Hyuha, T.; Wortmann, C. Sorghum Response to Fertilizer and Nitrogen Use Efficiency in Uganda. Agron. J. 2012, 104, 83–90. [Google Scholar] [CrossRef]
- Nyikako, J.; Schierholt, A.; Kessel, B. Genetic variation in nitrogen uptake and utilization efficiency in a segregating DH population of winter oilseed rape. Euphytica 2014, 199, 3–11. [Google Scholar] [CrossRef]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient use efficiency in plants. Commun. Soil Sci. Plant Anal. 2001. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.; Morsy, A. Integrated Impact of Organic and Inorganic Fertilizers on Growth, Yield of Maize (Zea mays L.) and Soil Properties under Upper Egypt Conditions. J. Plant Prod. 2017, 8, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Ministry of the Coordination of the Environmental Action (MICOA). Report of the Environmental Status; MICOA: Maputo, Mozambique, 2002. (In Portuguese) [Google Scholar]
- Onasanya, R.O.; Aiyelari, O.P.; Onasanya, A.; Oikeh, S.; Nwilene, F.E.; Oyelakin, O.O. Growth and yield response of maize (Zea mays L.) to different rates of nitrogen and phosphorus fertilizers in southern Nigeria. World J. Agric. Sci. 2009, 5, 400–407. [Google Scholar]
- Enujeke, E.C. Effects of variety and spacing on yield indices of open-pollinated maize in asaba area of Delta State, Nigeria. Sustain. Agric. Res. 2013, 2, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Abebe, Z.; Feyisa, H. Effects of nitrogen rates and time of application on yield of maize: Rainfall variability influenced time of n application. Int. J. Agron. 2017, 2017, 1545280. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.L.; Chen, D.L. Nitrogen fertilizer use in China—Contributions to food production, impacts on the environment and best management strategies. Nutr. Cycl. Agroecosyst. 2002, 63, 117–127. [Google Scholar] [CrossRef]
- Kaizzi, K.C.; Byalebeka, J.S.; Onesmus, A.I. Maize response to fertilizer and nitrogen use efficiency in Uganda. Agron. J. 2012, 104, 73–82. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Tahir, M.M.; Sadiq, A.; Iqbal, M.; Zafar, M. Yield and nitrogen use efficiency of rainfed maize response to splitting and nitrogen rates in Kashmir, Pakistan. Agron. J. 2012, 104, 448–457. [Google Scholar] [CrossRef]
- Oyedeji, F.N. Analysis of the effect of organic and inorganic fertilizer on growth performance and yield of maize. Stem Cell 2016, 7, 4–9. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.; Helmke, P.A.; Leoppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Summer, M.E. Methods of Soil Analysis: Part 3 Chemical Methods, 5th ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1996; pp. 417–435, 475–490, 1123–1184. [Google Scholar]
- Van Kessel, C. Seasonal accumulation and partitioning of nitrogen by lentil. Plant Soil 1994, 164, 69–76. [Google Scholar] [CrossRef]
- Asagi, N.; Ueno, H. Determination of application effects of sewage sludge on growth, soil properties, and N uptake in Komatsuna by using the indirect 15N isotope method. Commun. Soil. Sci. Plant Anal. 2008, 39, 1928–1942. [Google Scholar] [CrossRef]
- Fageria, N.K. Nitrogen harvest index and its association with crop yields. J. Plant Nutr. 2014, 36, 795–810. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, Q.; Noll, L.; Hu, Y.; Wanek, W. Environmental effects on soil microbial nitrogen use efficiency are controlled by allocation of organic nitrogen to microbial growth and regulate gross N mineralization. Soil Biol. Biochem. 2019, 135, 304–315. [Google Scholar] [CrossRef]
- Odedina, J.; Ojeniyi, S.; Odedina, S. Integrated nutrient management for sustainable cassava production in South Western Nigeria. Arch. Agron. Soil Sci. 2012, 58, S132–S140. [Google Scholar] [CrossRef]
- Hameed, M.A.W.; Khalaf, N.H.; Farhan, H.N. The impact of several animal manure types in comparison with urea on growth and yield of maize (Zea mays L.). Euphrates J. Agric. Sci. 2017, 9, 28–39. [Google Scholar]
- Ayoola, O.T.; Adeniyan, O.N. Influence of poultry manure and NPK fertilizer on yield and yield components of crops under different cropping systems in south west Nigeria. Afr. J. Biotechnol. 2006, 5, 1386–1392. [Google Scholar] [CrossRef]
- Amujoyegbe, B.J.; Opabode, J.T.; Olayinka, A. Effect of organic and inorganic fertilizer on yield and chlorophyll content of maize (Zea mays L.) and sorghum Sorghum bicolour (L.) Moench. Afr. J. Biotechnol. 2007, 6, 1869–1873. [Google Scholar]
- Brady, N.C.; Weils, R.R. The Nature and Properties of Soils, 12th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1999; pp. 74–114. [Google Scholar]
- Sharpley, A.N.; Smith, S.J. Nitrogen and phosphorus forms in soils receiving manure. Soil Sci. 1995, 159, 253. [Google Scholar] [CrossRef] [Green Version]
- Jackson, W.A.; Leonard, R.A.; Wilkinson, S.R. Land disposal of broiler litter—Changes in soil potassium, calcium, and magnesium. J. Environ. Qual. 1975, 4, 202–206. [Google Scholar] [CrossRef]
- Van Delden, A. Yield and growth components of potato and wheat under organic nitrogen management. Agron. J. 2001, 93, 1370–1385. [Google Scholar] [CrossRef] [Green Version]
- Board, J.E. Soybean cultivar differences on light interception and leaf area index during seed filling. Agron. J. 2004, 96, 305–310. [Google Scholar] [CrossRef]
- Hokmalipour, S.; Darbandi, M.H. Effects of nitrogen fertilizer on chlorophyll content and other leaf indicate in three cultivars of maize (Zea mays L.). World Appl. Sci. J. 2012, 15, 1780–1785. [Google Scholar]
- Ramesh, K.; Chandrasekaran, B.; Balasubramanian, T.N.; Bangarusamy, U.; Sivasamy, R.; Sankaran, N. Chlorophyll dynamics in rice (Oryza sativa) before and after flowering based on SPAD (chlorophyll) meter monitoring and its relation with grain yield. J. Agron. Crop Sci. 2002, 188, 102–105. [Google Scholar] [CrossRef]
- Bojović, B.; Marković, A. Correlation between nitrogen and chlorophyll content in wheat (Triticum aestivum L.). Kragujev. J. Sci. 2009, 31, 69–74. [Google Scholar]
- Blumenthal, J.D.M.; Baltensperger, D.D.; Cassman, K.G.; Mason, S.C.; Pavlista, A.D. Importance and effect of nitrogen on crop quality and health. Nitrogen Environ. 2008, 51–70. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.E.-M.H. Effect of different nitrogen sources on growth, yield and quality of fodder maize (Zea mays L.). J. Saudi Soc. Agric. Sci. 2011, 10, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Naim, A.H.; Ahmed, K.M.; Ahmed, F.E. Effects of chicken manure on growth and yield of jute mallow (corchorus olitorius L.) under rain-fed conditions of Sudan. Open Access Library J. 2015, 2, 68749. [Google Scholar] [CrossRef]
- Zhang, J.; Blackmer, A.M.; Blackmer, T.M.; Kyveryga, P.M.; Ellsworth, J.W. Nitrogen deficiency and recovery in sustainable corn production as revealed by leaf chlorophyll measurements. Agron. Sustain. Dev. 2007, 27, 313–319. [Google Scholar] [CrossRef]
- Agehara, S.; Warncke, D.D. Soil moisture and temperature effects on nitrogen release from organic nitrogen sources. Soil Sci. Soc. Am. J. 2005, 69, 1844–1855. [Google Scholar] [CrossRef] [Green Version]
- Ladha, J.K.; Pathak, H.; Krupnik, T.J.; Six, J.; van Kessel, C. Efficiency of fertilizer nitrogen in cereal production: Retrospects and prospects. Adv. Agron. 2005, 87, 85–116. [Google Scholar] [CrossRef]
- Yan, X.; Ti, C.; Vitousek, P.; Chen, D.; Leip, A.; Cai, Z.; Zhu, Z. Fertilizer nitrogen recovery efficiencies in crop production systems of China with and without consideration of the residual effect of nitrogen. Environ. Res. Lett. 2014, 9, 095002. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the Twentyfirst century. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130164. [Google Scholar] [CrossRef]
- Alam, S.M. Effects of solution pH on the growth and chemical composition of rice plants. J. Plant Nutr. 1981, 4, 247–260. [Google Scholar] [CrossRef]
- The, C.; Calba, H.; Zonkeng, C.; Ngonkeu, E.L.M.; Adetimirin, V.O.; Mafouasson, H.A.; Meka, S.S.; Horst, W.J. Responses of maize grain yield to changes in acid soil characteristics after soil amendments. Plant Soil 2006, 284, 45–57. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Aihoiu, K.; Aman, S.; Tossah, B.K.; Diels, J.; Lyasse, O.; Hauser, S.; Sanginga, N.; Merckx, R. Nitrogen and phosphorus uptake by maize as affected by particulate organic matter quality, soil characteristics, and land-use history for soils from the West African moist savanna zone. Biol. Fertil. Soils 2000, 30, 400–449. [Google Scholar] [CrossRef]
- Dikinya, O.; Mufwanzala, N. Chicken manure-enhanced soil fertility and productivity: Effects of application rates. J. Soil Sci. Environ. Manag. 2010, 1, 46–54. [Google Scholar]
- Natscher, L.; Schwertmann, U. Proton buffering in organic horizons of acid forest soils. Geoderma 1991. [Google Scholar] [CrossRef]
- Liu, J.; Hue, N.V. Amending subsoil acidity by surface applications of gypsum, lime, and composts. Commun. Soil Sci. Plant Anal. 2001, 32, 2117–2132. [Google Scholar] [CrossRef]
- Escobar, M.E.O.; Hue, N. Temporal changes of selected chemical properties in three manure—Amended soils of Hawaii. Bioresour. Technol. 2008, 99, 8649–8654. [Google Scholar] [CrossRef] [PubMed]


| Measurements | Units | Soil | CnM | CwM | BTL | BC | RW |
|---|---|---|---|---|---|---|---|
| pH | 6.88 | 8.10 | 8.53 | 6.73 | 6.40 | 5.93 | |
| EC | (µS cm−1) | 162 | 7.97 | 4.53 | 0.363 | 0.463 | 2.67 |
| Water content | (%) | 2.26 | 14.6 | 28.6 | 61.8 | 18.8 | 12.6 |
| Total carbon | (g kg−1) | 26.7 | 270 | 321 | 449 | 446 | 414 |
| Total nitrogen | (g kg−1) | 1.13 | 28.6 | 20.7 | 26.1 | 5.48 | 64.3 |
| C/N ratio | 23.6 | 9.44 | 15.5 | 17.2 | 81.4 | 6.44 | |
| Total P | (g kg−1) | 0.7 * | 50.4 | 17.2 | 1.6 | 1.7 | 26.9 |
| NH4+-N | (g kg−1) | 3.51 | 13.3 | 33.4 | 29.5 | 18.0 | 25.0 |
| NO3−-N | (g kg−1) | 17.1 | 49.9 | 106 | 168 | 2.77 | 23.9 |
| CEC | (mmolc 100 g−1 DW) | 12.0 | 33.8 | 36.8 | 49.4 | 37.7 | 37.8 |
| Exchangeable Ca | (mmolc 100 g−1 DW) | 16.0 | - | - | - | - | - |
| Exchangeable Mg | (mmolc 100 g−1 DW) | 4.81 | - | - | - | - | - |
| Exchangeable K | (mmolc 100 g−1 DW) | 0.469 | - | - | - | - | - |
| Exchangeable Na | (mmolc 100 g−1 DW) | 0.156 | - | - | - | - | - |
| Treatments | Agronomic † N Use Efficiency (g g−1 N) | Apparent N ‡ Recovery Efficiency (g N g−1 N) | Physiological § N Efficiency (g g−1 N) | N Harvest # Index (g N g) |
|---|---|---|---|---|
| IF | 26.0 ± 1.20 a | 0.883 | 21.5 ± 0.996 ab | 0.545 |
| CnM | 21.2 ± 2.88 ab | 0.341 | 39.1 ± 5.30 ab | 0.582 |
| CwM | 12.9 ± 4.25 bc | 0.209 | 32.4 ± 10.7 ab | 0.629 |
| BTL | 15.2 ± 4.09 abc | 0.294 | 32.0 ± 8.63 ab | 0.585 |
| BC | 3.30 ± 2.86 c | 0.253 | 8.59 ± 7.42 b | 0.727 |
| RW | 25.7 ± 0.402 ab | 0.393 | 42.8 ± 0.669 a | 0.561 |
| Treatment | pH | EC | NH4-N | NO3-N | CEC | Exchangeable Cations | |||
|---|---|---|---|---|---|---|---|---|---|
| Ca | Mg | K | Na | ||||||
| (S cm−1) | (mg kg−1) | (mmolc 100 g−1 DW) | |||||||
| IF | 7.04 b | 177 b | 24.2 ab | 20.0 a | 11.5 a | 18.8 b | 3.58 b | 0.633 b | 0.305 ab |
| CnM | 7.38 a | 214 a | 31.2 a | 13.2 ab | 14.8 a | 29.0 a | 5.79 a | 0.998 a | 0.468 a |
| CwM | 7.46 a | 162 b | 27.3 a | 9.11 b | 12.0 a | 19.3 b | 3.99 b | 0.629 b | 0.328 ab |
| BTL | 7.32 a | 175 b | 18.9 b | 8.10 b | 11.8 a | 19.6 b | 4.15 b | 0.664 b | 0.298 ab |
| BC | 7.04 b | 168 b | 30.3 a | 10.3 ab | 12.2 a | 19.4 b | 3.85 b | 0.714 ab | 0.253 b |
| RW | 7.22 ab | 181 ab | 26.1 ab | 9.34 ab | 11.8 a | 19.6 b | 4.72 ab | 0.643 b | 0.401 ab |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamele, R.A.; Ueno, H.; Toma, Y.; Morita, N. Nitrogen Recoveries and Nitrogen Use Efficiencies of Organic Fertilizers with Different C/N Ratios in Maize Cultivation with Low-Fertile Soil by 15N Method. Agriculture 2020, 10, 272. https://doi.org/10.3390/agriculture10070272
Tamele RA, Ueno H, Toma Y, Morita N. Nitrogen Recoveries and Nitrogen Use Efficiencies of Organic Fertilizers with Different C/N Ratios in Maize Cultivation with Low-Fertile Soil by 15N Method. Agriculture. 2020; 10(7):272. https://doi.org/10.3390/agriculture10070272
Chicago/Turabian StyleTamele, Rosalina Armando, Hideto Ueno, Yo Toma, and Nobuki Morita. 2020. "Nitrogen Recoveries and Nitrogen Use Efficiencies of Organic Fertilizers with Different C/N Ratios in Maize Cultivation with Low-Fertile Soil by 15N Method" Agriculture 10, no. 7: 272. https://doi.org/10.3390/agriculture10070272
APA StyleTamele, R. A., Ueno, H., Toma, Y., & Morita, N. (2020). Nitrogen Recoveries and Nitrogen Use Efficiencies of Organic Fertilizers with Different C/N Ratios in Maize Cultivation with Low-Fertile Soil by 15N Method. Agriculture, 10(7), 272. https://doi.org/10.3390/agriculture10070272





