The Increase in the Arsenic Concentration in Brown Rice Due to High Temperature during the Ripening Period and Its Reduction by Silicate Material Treatment
Abstract
:1. Introduction
2. Materials and Methods
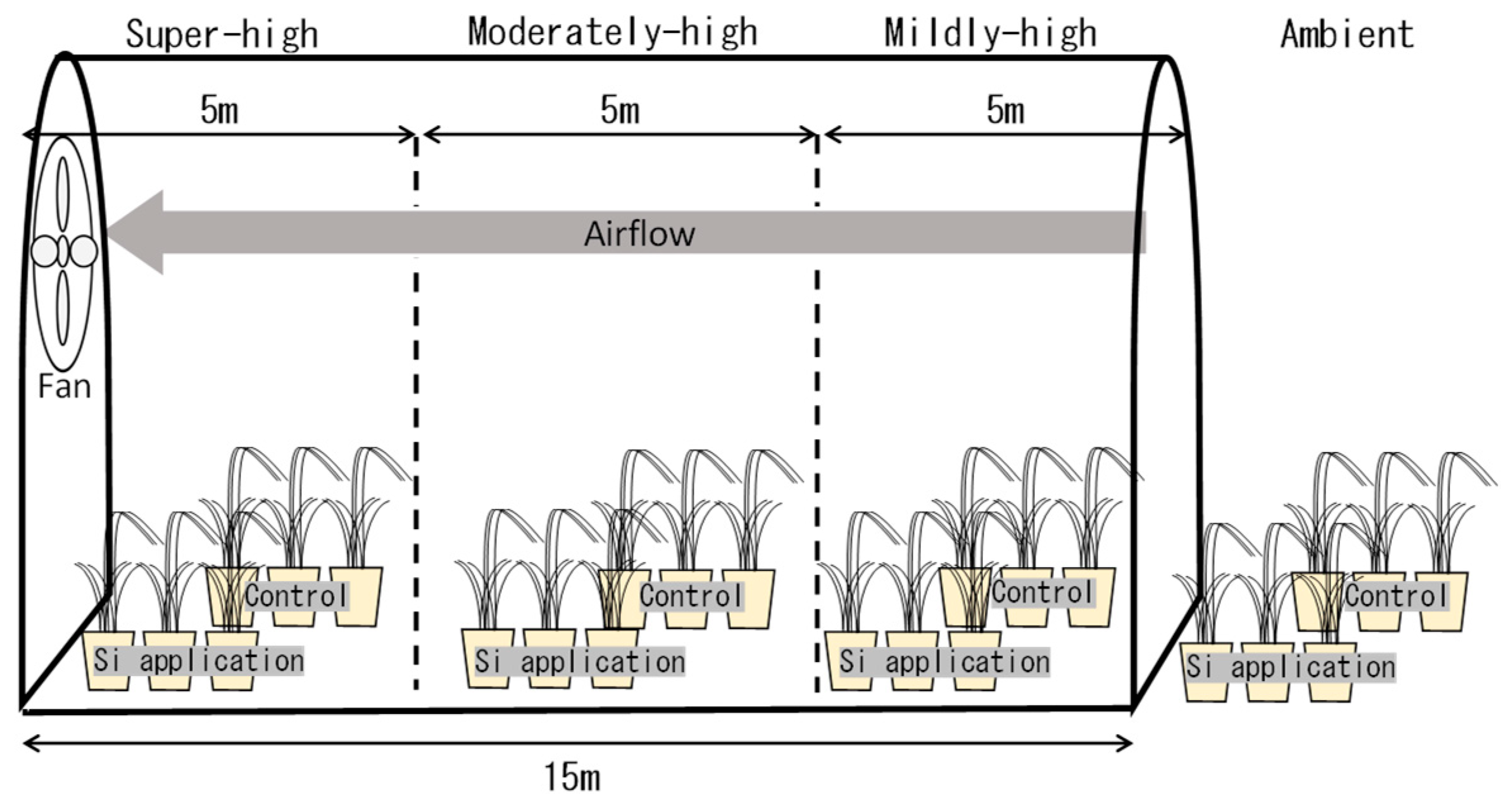
2.1. Soil Preparation, Si Material Treatment and Rice Cultivation
2.2. High-Temperature Treatment during the Ripening Period
2.3. Plant Sampling
2.4. Chemical Analyses of the As and Speciation in the Plant Tissues
2.5. Statistical Analyses
3. Results
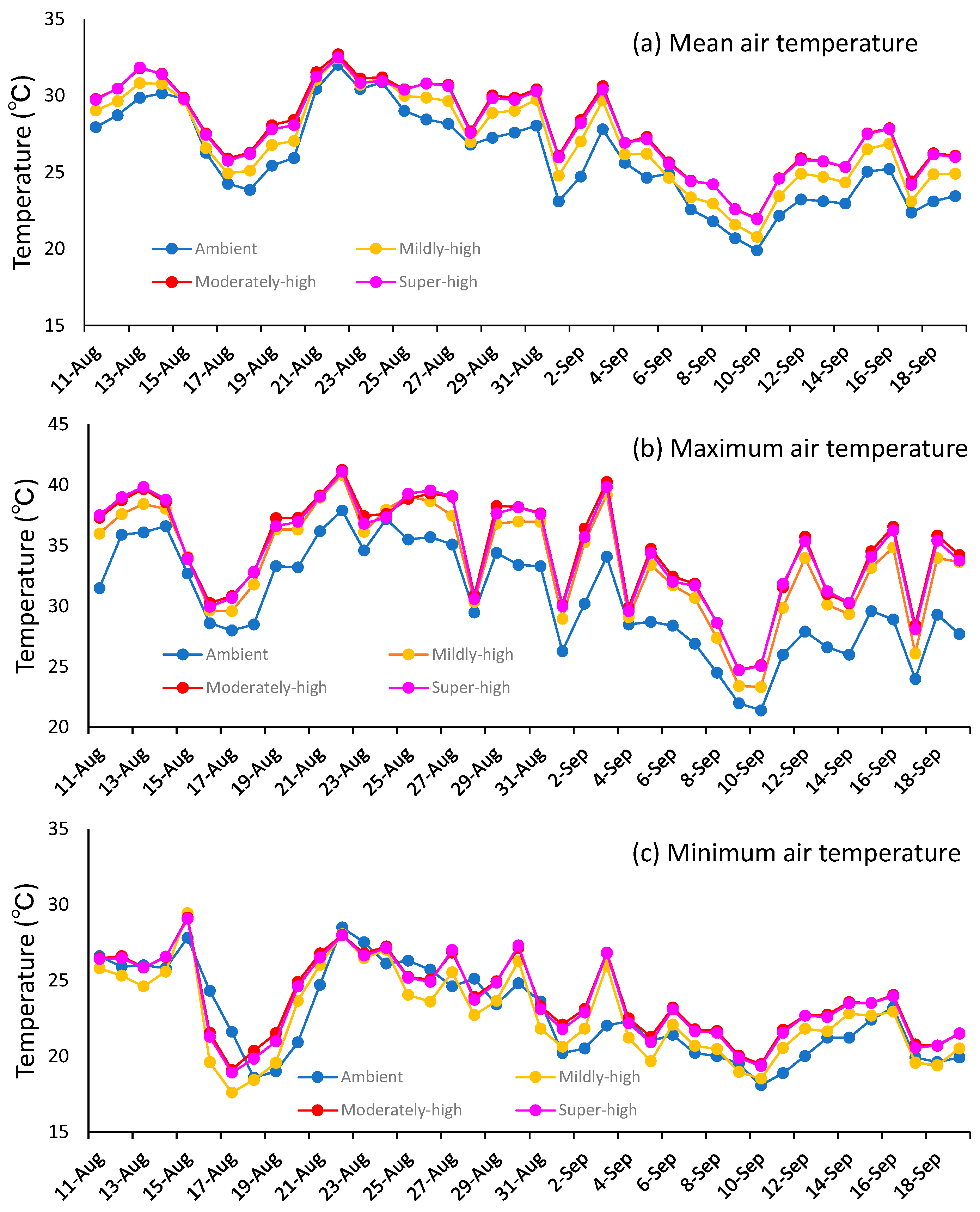
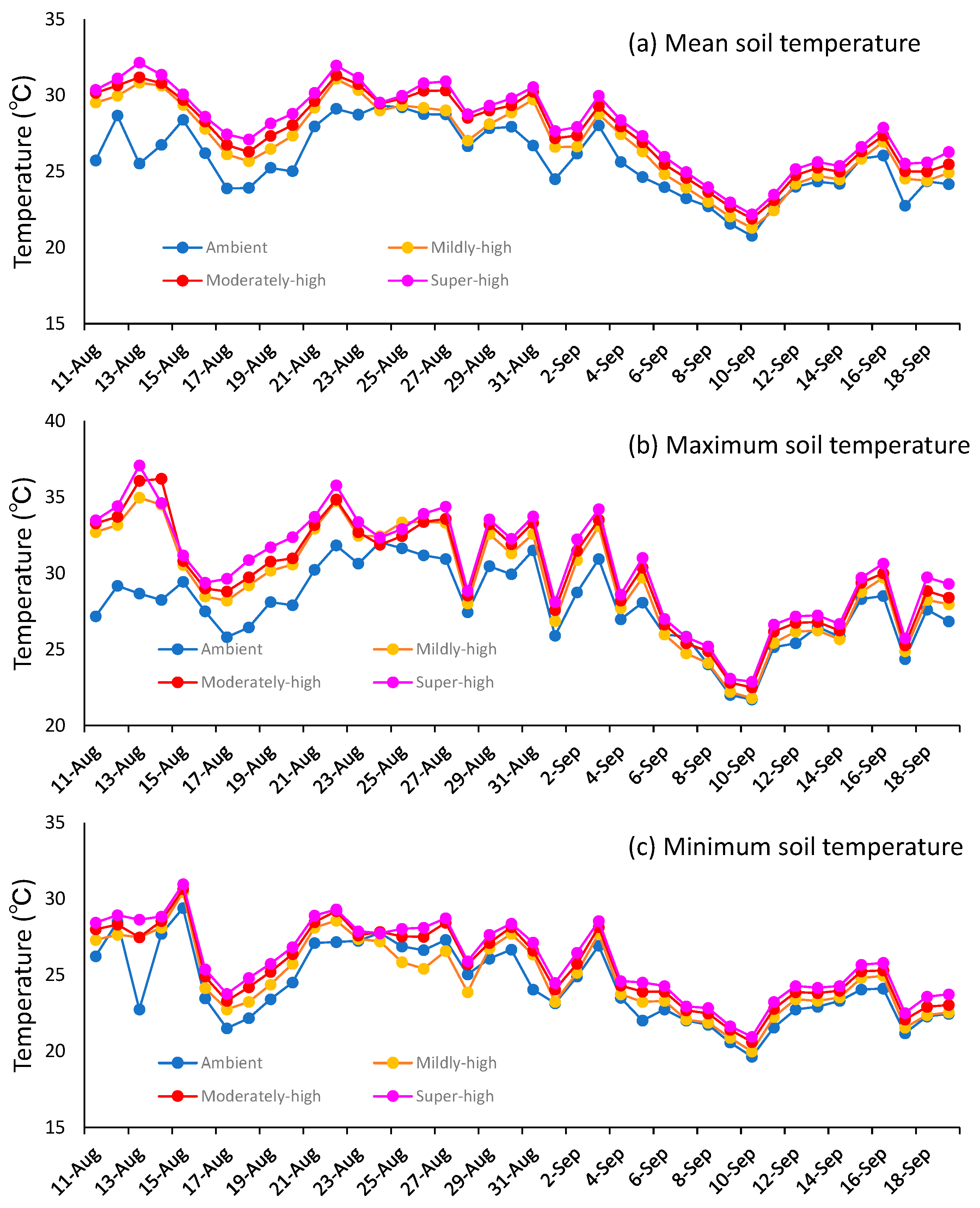
3.1. Temperature Differences between the Ambient, Mildly-High, Moderately-High, and Super-High Treatments in the TGCs during the Ripening Period
3.2. Effects of Temperature during Ripening Period and Si Application on the Biomass Production and Brown Rice Yield
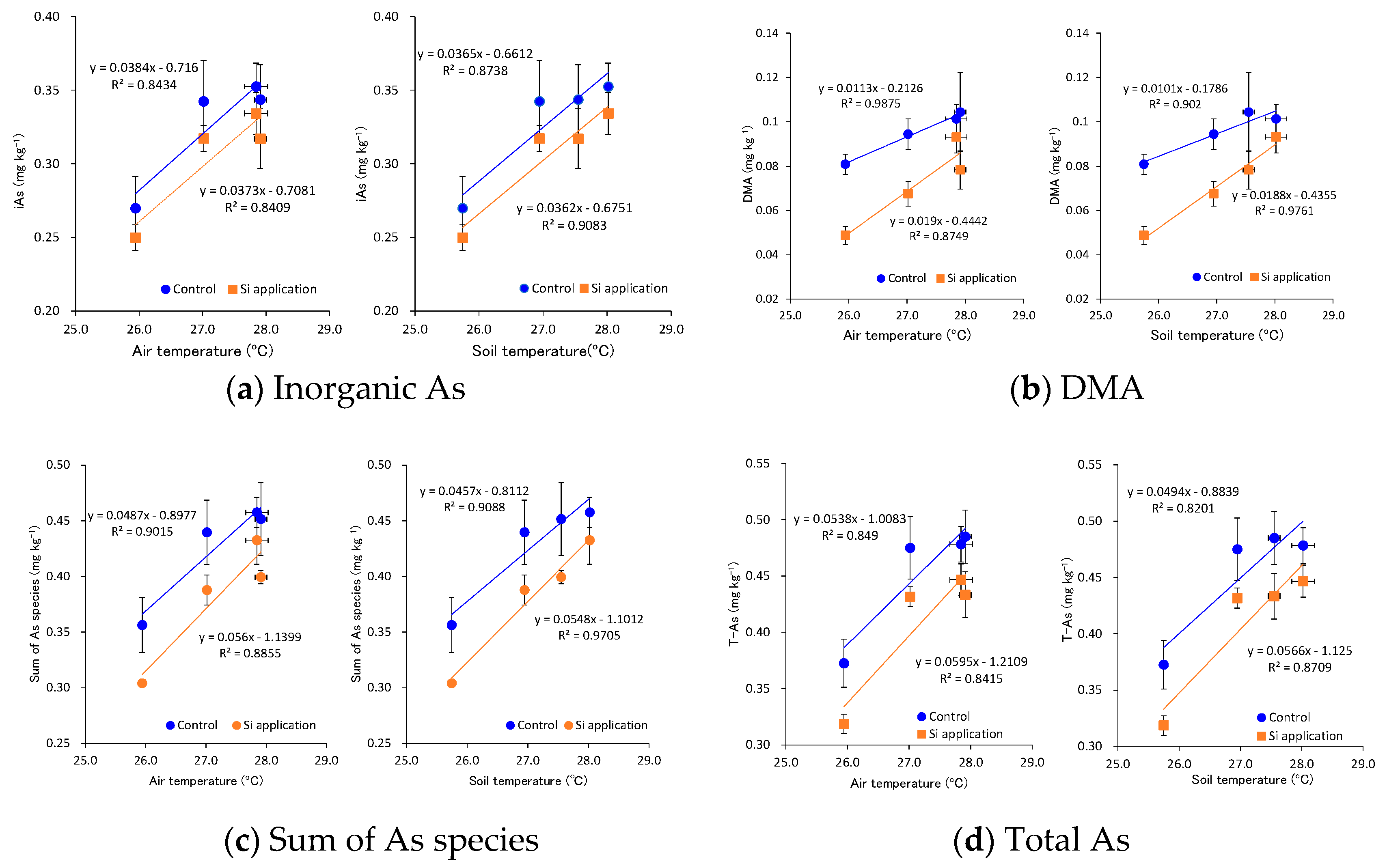
3.3. Effects of Temperature during the Ripening Period and the Si Application on the As Concentration and As Speciation in Brown Rice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/SYR_AR5_FINAL_full.pdf (accessed on 6 April 2020).
- Morita, S. Prospect for developing measures to prevent high-temperature damage to rice grain ripening. Jpn. J. Crop Sci. 2008, 77, 1–12. [Google Scholar] [CrossRef]
- Peng, S.; Huang, J.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, S.; Nakano, H. Nonstructural carbohydrate content in the stem at full heading contributes to high performance of ripening in heat-tolerant rice cultivar Nikomaru. Crop Sci. 2011, 51, 818–828. [Google Scholar] [CrossRef]
- Arao, T.; Makino, T.; Kawasaki, A.; Akahane, I.; Kiho, N. Effect of air temperature after heading of rice on the arsenic concentration of grain. Soil Sci. Plant Nutr. 2018, 64, 433–437. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission 2016. In Proceedings of the Rep16/cf Joint FAO/WHO Food Standards Programme Codex Alimentarius Commission 39th Session, Rome, Italy, 27 June–1 July 2016; p. 37.
- Kabata‒Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press Inc.: Boca Raton, FL, USA, 2000; pp. 225–230. [Google Scholar]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef] [PubMed]
- Allaway, W.H. Agronomic controls over the environmental cycling of trace elements. Advan. Agron. 1968, 20, 235–274. [Google Scholar]
- Feng, X.; Han, L.; Chao, D.; Liu, D.; Zhang, Y.; Wang, R.; Guo, J.; Feng, R.; Xu, Y.; Ding, Y.; et al. Ionomic and transcriptomic analysis provides new insight into the distribution and transport of cadmium and arsenic in rice. J. Hazard. Mater. 2017, 331, 246–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, Y.; Minamikawa, R.; Hattori, K.H.; Kurishima, K.; Kihou, N.; Yuita, K. Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ. Sci. Technol. 2004, 38, 1038–1044. [Google Scholar] [CrossRef]
- Zavala, Y.J.; Gerads, R.; Gurleyuk, H.; Duxbury, J.M. Arsenic in rice: II. Arsenic speciation in USA grain and implications for human health. Environ. Sci. Technol. 2008, 42, 3861–3866. [Google Scholar] [CrossRef]
- Meharg, A.A.; Williams, P.N.; Adomako, E.; Lawgali, Y.Y.; Deacon, C.; Villada, A.; Cambell, R.C.J.; Sun, G.; Zhu, Y.G.; Feldmann, J.; et al. Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ. Sci. Technol. 2009, 43, 1612–1617. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Umetsu, K.; Ponomarenko, O.; Saito, M.; Aslam, M.; Antipova, O.; Dolgova, N.; Kiani, C.D.; Nehzati, S.; Tanoi, K.; et al. PIN FORMED 2 Modulates the Transport of Arsenite in Arabidopsis thaliana. Plant Comm. 2020, 1, 100009. [Google Scholar] [CrossRef]
- Verma, P.K.; Verma, S.; Tripathi, R.D.; Chakrabarty, D. A rice glutaredoxin regulate the expression of aquaporin genes and modulate root responses to provide arsenic tolerance. Ecotoxico. Environ. Saf. 2020, 195, 110471. [Google Scholar] [CrossRef]
- Yamaji, N.; Ma, J.F. The node, a hub for mineral nutrient distribution in graminaceous plants. Trends Plant Sci. 2014, 19, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Y.; Yamaki, T.; Yamaji, N.; Ko, D.H.; Jung, K.H.; Fujii-Kashino, M.; An, G.; Martinoia, E.; Lee, Y.; Ma, J.F. A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc. Natl. Acad. Sci. USA 2014, 111, 15699–15704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, M.; Hamasaki, T.; Hayashi, T. Temperature gradient chambers for research on global environment change. I. Thermal environment in a large chamber. Biotronics 1995, 24, 85–97. [Google Scholar]
- Matsumoto, S.; Kasuga, J.; Taiki, N.; Makino, T.; Arao, T. Reduction of the risk of arsenic accumulation in rice by the water management and material application in relation to phosphate status. J. Plant Interact. 2015, 10, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Li, R.Y.; Stroud, J.L.; Ma, J.F.; Mcgrath, S.P.; Zhao, F.J. Mitigation of arsenic accumulation in rice with water management and silicon fertilization. Environ. Sci. Technol. 2009, 43, 3778–3783. [Google Scholar] [CrossRef]
- Zhao, F.J.; McGrath, S.P.; Meharg, A.A. Arsenic as a Food Chain Contaminant: Mechanisms of Plant Uptake and Metabolism and Mitigation Strategies. Annu. Rev. Plant Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, S.; Kasuga, J.; Taiki, N.; Makino, T.; Arao, T. Inhibition of arsenic accumulation in Japanese rice by the application of iron and silicate materials. Catena 2015, 135, 328–335. [Google Scholar] [CrossRef]
- Matumoto, S.; Kasuga, J.; Makino, T.; Arao, T. Evaluation of the effects of application of iron materials on the accumulation and speciation of arsenic in rice grain grown on uncontaminated soil with relatively high levels of arsenic. Environ. Exp. Bot. 2016, 125, 42–51. [Google Scholar] [CrossRef]
- Tao, F.; Hayashi, Y.; Zhang, Z.; Sakamoto, T.; Yokozawa, M. Global warming, rice production, and water use in China: Developing a probabilistic assessment. Agric. For. Meteorol. 2008, 148, 94–110. [Google Scholar] [CrossRef]
- Van Kiet, H.; Nose, A. Effects of temperature on growth and photosynthesis in the seedling stage of the sheath blight-resistant rice genotype 32R. Plant Prod. Sci. 2016, 19, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Horie, T.; Baker, J.T.; Nakagawa, H.; Matsui, T.; Kim, H.Y. Crop ecosystem responses to climatic change: Rice. In Climate Change and Global Crop Productivity; CABI Publishing: Wallingford, UK, 2000; pp. 81–106. [Google Scholar]
- Jin, Z.; Ge, D.; Chen, H.M.; Fang, J. Effects of climate change on rice production and strategies for adaptation in southern China. In Climate Change and Agriculture: Analysis of Potential International Impacts. Am. Soc. Agron. 1995, 59, 307–323. [Google Scholar]
- Morita, S.; Wada, H.; Matsue, Y. Countermeasures for heat damage in rice grain quality under climate change. Plant Prod. Sci. 2016, 19, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Agostinho, F.B.; Tubana, B.S.; Martins, M.S.; Datnoff, L.E. Effect of different silicon sources on yield and silicon uptake of rice grown under varying phosphorus rates. Plants 2017, 6, 35. [Google Scholar] [CrossRef]
- Matoh, T.; Murata, S.; Takahashi, E. Effect of silicate application on photosynthesis of rice. Jpn. J. Soil Sci. Plant Nutr. 1991, 62, 248–251, (In Japanese with English summary). [Google Scholar]
- Ando, H.; Kakuda, K.I.; Fujii, H.; Suzuki, K.; Ajiki, T. Growth and canopy structure of rice plants grown under field conditions as affected by si application. Soil Sci. Plant Nutr. 2002, 48, 429–432. [Google Scholar] [CrossRef]
- Fujii, H.; Mori, S.; Ando, H. Effects of silicate fertilizer application on the yield of rice plants grown under insufficient light condition. Jpn. J. Soil Sci. Plant Nutr. 2008, 79, 471–477, (In Japanese with English summary). [Google Scholar]
- Kaneta, Y.; Takahashi, D.; Sakaguchi, H.; Kon, K.; Takakai, F.; Sato, T. Effect of silicate fertilizer on leaf temperature, stoma conductance and silicic acid uptake in rice under high temperature during the ripening stage. Jpn. J. Sci. Soil Manure 2010, 81, 504–507. (In Japanese) [Google Scholar]
- Mori, S.; Fujii, H. Utilization and research of silicon in recent agriculture: 6. Alleviative effects of silicate application on the rice production by meteorlogical disaster. Jpn J. Soil Sci. Plant Nutr. 2013, 84, 504–507. (In Japanese) [Google Scholar]
- Neumann, R.B.; Seyfferth, A.L.; Teshera-Levye, J.; Ellingson, J. Soil Warming Increases Arsenic Availability in the Rice Rhizosphere. Agric. Environ. Lett. 2017, 2, 170006. [Google Scholar] [CrossRef]
- Weber, F.A.; Hofacker, A.F.; Voegelin, A.; Kretzschmar, R. Temperature dependence and coupling of iron and arsenic reduction and release during flooding of a contaminated soil. Environ. Sci. Technol. 2010, 44, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Tyrovola, K.; Nikolaidis, N.P. Arsenic mobility and stabilization in topsoils. Water Res. 2009, 43, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, K.; Schenk, M.K. Arsenic in rice (Oryza sativa L.) related to dynamics of arsenic and silicic acid in paddy soils. Environ. Sci. Technol. 2008, 42, 7885–7890. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zenng, M.; Tang, J.; Shim, H.; Cai, C. Effect of Silicon on Arsenic Concentration and Speciation in Different Rice Tissues. Pedosphere 2018, 28, 511–520. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wen, S.L.; Chen, P.; Zhang, L.; Cen, K.; Sun, G.X. Mitigation of cadmium and arsenic in rice grain by applying different silicon fertilizers in contaminated fields. Environ. Sci. Pollut. Res. 2016, 23, 3781–3788. [Google Scholar] [CrossRef]
- Swedlundi, P.J.; Webester, J.G. Adsorption and polymerization of silicic acid on ferrihydrite, and its effect on arsenic adsorption. Water Res. 1999, 33, 3413–3422. [Google Scholar] [CrossRef]
- Waltham, C.A.; Eick, M.J. Kinetics of Arsenic Adsorption on Goethite in the Presence of Sorbed Silicic Acid. Soil Sci. Soc. Am. J. 2002, 66, 818–825. [Google Scholar] [CrossRef]
- Luxton, T.P.; Tadanier, C.J.; Eick, M.J. Mobilization of Arsenite by Competitive Interaction with Silicic Acid. Soil Sci. Soc. Am. J. 2006, 70, 204–214. [Google Scholar] [CrossRef]
- Makino, T.; Nakamura, K.; Katou, H.; Ishikawa, S.; Ito, M.; Honma, T.; Miyazaki, N.; Takehisa, K.; Sano, S.; Matsumoto, S.; et al. Simultaneous decrease of arsenic and cadmium in rice (Oryza sativa L.) plants cultivated under submerged field conditions by the application of iron. Soil Sci. Plant Nutri. 2016, 62, 340–348. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.Y.; Su, Y.H.; McGrath, S.P.; Zhao, F.J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An efflux transporter of silicon in rice. Nature 2007, 448, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shon, J.; Lee, C.K.; Yang, W.; Yoon, Y.; Yang, W.H.; Kim, Y.G.; Lee, B.W. Relationship between grain filling duration and leaf senescence of temperate rice under high temperature. Field Crop. Res. 2011, 122, 207–213. [Google Scholar] [CrossRef]

| Temperature | Air Temperature (℃) | Soil Temperature (℃) | ||||
|---|---|---|---|---|---|---|
| Mean | Maximum | Minimum | Mean | Maximum | Minimum | |
| Ambient | 25.9 | 30.6 | 22.7 | 25.7 | 27.9 | 24.3 |
| Mildly-high | 27.0 | 33.6 | 22.6 | 26.9 | 29.5 | 24.9 |
| Moderately-high | 27.9 | 34.6 | 23.7 | 27.5 | 30.0 | 25.5 |
| Super-high | 27.8 | 34.5 | 23.6 | 28.0 | 30.5 | 26.0 |
| Main Factor | Treatment | Dry Matter Production | Brown Rice Yield | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Straw | Panicle | |||||||||
| (g pot−1) | (g pot−1) | (g pot−1) | ||||||||
| Temperature | Ambient | 18.5 | ± | 1.2 | 22.9 | ± | 1.2 | 18.5 | ± | 1.0 |
| Mildly-high | 18.7 | ± | 1.6 | 16.7 | ± | 1.3 | 12.4 | ± | 0.9 | |
| Moderately-high | 20.4 | ± | 2.6 | 15.6 | ± | 2.0 | 11.3 | ± | 1.5 | |
| Super-high | 20.2 | ± | 2.3 | 16.4 | ± | 2.3 | 11.9 | ± | 1.7 | |
| Si application | Control | 15.8 | ± | 0.6 | 14.7 | ± | 1.1 | 11.2 | ± | 1.0 |
| Calcium silicate slug | 23.1 | ± | 1.0 | 21.1 | ± | 1.1 | 15.9 | ± | 1.0 | |
| ANCOVA | ||||||||||
| Significance of regression | Mean air temperature | p | = | 0.125 | p | < | 0.001 | p | < | 0.001 |
| Mean soil temperature | p | = | 0.192 | p | < | 0.001 | p | < | 0.001 | |
| Si application | Mean air temperature | p | < | 0.001 | p | < | 0.001 | p | < | 0.001 |
| Mean soil temperature | p | < | 0.001 | p | < | 0.001 | p | < | 0.001 | |
| Main Factor | Treatment | Inorganic As | DMA | MMA | Sum of As Species | Total As | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | ||||||||||||
| Temperature | Ambient | 0.260 | ± | 0.011 | 0.065 | ± | 0.008 | 0.005 | ± | 0.000 | 0.330 | ± | 0.016 | 0.346 | ± | 0.016 |
| Mildly-high | 0.330 | ± | 0.014 | 0.081 | ± | 0.007 | 0.003 | ± | 0.000 | 0.414 | ± | 0.018 | 0.453 | ± | 0.016 | |
| Moderately-high | 0.330 | ± | 0.009 | 0.091 | ± | 0.011 | 0.004 | ± | 0.000 | 0.425 | ± | 0.019 | 0.459 | ± | 0.018 | |
| Super-high | 0.343 | ± | 0.011 | 0.097 | ± | 0.005 | 0.004 | ± | 0.001 | 0.445 | ± | 0.013 | 0.463 | ± | 0.012 | |
| Si application | Control | 0.327 | ± | 0.013 | 0.095 | ± | 0.005 | 0.004 | ± | 0.000 | 0.426 | ± | 0.017 | 0.453 | ± | 0.017 |
| Calcium silicate slug | 0.305 | ± | 0.011 | 0.072 | ± | 0.006 | 0.004 | ± | 0.000 | 0.381 | ± | 0.015 | 0.408 | ± | 0.017 | |
| Ancova | ||||||||||||||||
| Significance of regression | Mean air temperature | p | < | 0.001 | p | < | 0.001 | p | = | 0.071 | p | < | 0.001 | p | < | 0.001 |
| Mean soil temperature | p | < | 0.001 | p | < | 0.001 | p | = | 0.150 | p | < | 0.001 | p | < | 0.001 | |
| Si application | Mean air temperature | p | = | 0.067 | p | < | 0.001 | p | = | 0.458 | p | = | 0.005 | p | = | 0.008 |
| Mean soil temperature | p | = | 0.061 | p | < | 0.001 | p | = | 0.471 | p | = | 0.004 | p | = | 0.007 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhar, P.; Kobayashi, K.; Ujiie, K.; Adachi, F.; Kasuga, J.; Akahane, I.; Arao, T.; Matsumoto, S. The Increase in the Arsenic Concentration in Brown Rice Due to High Temperature during the Ripening Period and Its Reduction by Silicate Material Treatment. Agriculture 2020, 10, 289. https://doi.org/10.3390/agriculture10070289
Dhar P, Kobayashi K, Ujiie K, Adachi F, Kasuga J, Akahane I, Arao T, Matsumoto S. The Increase in the Arsenic Concentration in Brown Rice Due to High Temperature during the Ripening Period and Its Reduction by Silicate Material Treatment. Agriculture. 2020; 10(7):289. https://doi.org/10.3390/agriculture10070289
Chicago/Turabian StyleDhar, Protima, Kazuhiro Kobayashi, Kazuhiro Ujiie, Fumihiko Adachi, Junko Kasuga, Ikuko Akahane, Tomohito Arao, and Shingo Matsumoto. 2020. "The Increase in the Arsenic Concentration in Brown Rice Due to High Temperature during the Ripening Period and Its Reduction by Silicate Material Treatment" Agriculture 10, no. 7: 289. https://doi.org/10.3390/agriculture10070289
APA StyleDhar, P., Kobayashi, K., Ujiie, K., Adachi, F., Kasuga, J., Akahane, I., Arao, T., & Matsumoto, S. (2020). The Increase in the Arsenic Concentration in Brown Rice Due to High Temperature during the Ripening Period and Its Reduction by Silicate Material Treatment. Agriculture, 10(7), 289. https://doi.org/10.3390/agriculture10070289





