Abstract
Glyphosate is the main tool for weed management in Brazilian citrus orchards, where weeds, such as Conyza bonariensis and Digitaria insularis, have been found with resistance to this herbicide. Field prospections have allowed the identification of a possible new case of glyphosate resistance. In this work, the susceptibility levels to glyphosate on three Amaranthus viridis L. populations, with suspected resistance (R1, R2, and R-IAC), collected in citrus orchards from the São Paulo State, Brazil, as well as their accumulation rates of shikimic acid, were determined. The fresh weight of the susceptible population (S) was reduced by 50% (GR50) with ~30 g ea ha−1 glyphosate, while the GR50 values of the R populations were between 5.4 and 11.3 times higher than that for S population. The LD50 (herbicide dose to kill 50% of individuals of a weed population) values of the S population were ≤150 g ea ha−1 glyphosate, while the LD50 of the R populations ranged from 600 to 920 g ea ha−1. Based on the reduction of fresh weight and the survival rate, the R1 population showed the highest level of glyphosate resistance, which had GR50 and LD50 values of 248 and 918 g ea ha−1 glyphosate, respectively. The S population accumulated 240 µg shikimic acid at 1000 µM glyphosate, while the R1, R2, and R-IAC populations accumulated only 16, 43, and 33 µg shikimic acid, respectively (between 5.6 to 15 times less than the S population). Enzyme activity assays suggested that at least one target site-type mechanism was involved in resistance. This result revealed the first report of glyphosate resistance in A. viridis reported in the world.
1. Introduction
Brazil is the world’s largest producer and exporter of citrus [1]. The State of São Paulo (SP) accounts for ~77% of the national production of orange (Citrus sinensis (L.) Osbeck), being the main exporter of concentrated orange juice [2]. However, the yield of citrus fruit is not the best in the world, mainly due to improper management of the plantations by growers [3]. The average orange yield is 27.6 tons ha−1, occupying the 13th world rankings [4]. Weed presence can be directly or indirectly responsible for up to 30–52% of yield losses in citrus orchards of young and growing trees [3,5]. In mature citrus orchards in full production, weeds do not have a great impact on yield, but they can hinder agricultural operations [6,7].
Small-scale cultural and ecological practices, such as the use of dead covers of Brachiaria shoot residues, are implemented for weed control in Brazilian citrus [3]; however, a chemical method based on the use of herbicides is the most employed by growers [8]. Glyphosate is the most used herbicide and, in some cases, the only weed control tool, which is applied up to four times a year in high doses (≥720 g ae ha−1) [3]. In susceptible plants, this herbicide inhibits the activity of 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), causing the accumulation of shikimic acid [9,10]. Extensive and excessive use of glyphosate to control weeds has led to select at least 45 species with resistance to this herbicide in the world [9,11]. Specifically, in Brazilian citrus orchards, Conyza bonariensis, Conyza canadensis, and Digitaria insularis [12,13] have been reported as being resistant to glyphosate. However, species of the genera Amaranthus, Bidens, Chloris, Conyza, Eleusine, Lolium, among others, which present high occurrence rates in citrus orchards [8], have a great risk of evolving resistance not only to glyphosate but also to other herbicides [14].
Growth rate, reproductive capacity, genetic variability, and stress tolerance are all high in Amaranthus species [15,16]. These traits give them a large capacity to evolve resistance to herbicides [15], which makes it difficult to control these species. Slender amaranth (Amaranthus viridis L.) is widely spread over tropical and subtropical regions in more than 80 countries [17]. These species, as well as other Amaranthus species, are the most abundant weeds in the Brazilian south and center-west regions [18]. In 2010, this weed was reported with multiple resistance to the acetolactate synthase (ALS) and photosystem II inhibiting herbicides in cotton in the states of Bahia and Mato Grosso, Brazil [19], and in 2015, Amaranthus palmeri was found with resistance to glyphosate and ALS-inhibiting herbicides in soybean in the state of Mato Grosso, Brazil [20].
This study hypothesized that slender amaranth might have evolved glyphosate resistance due to the high survival rates in the field after herbicide applications observed in the last cropping seasons. The objectives were to evaluate glyphosate resistance, monitor the accumulation rates of shikimic acid, and check the activity of the EPSPS in three slender amaranth populations with suspected resistance, which were harvested in lemon and sweet orange orchards of Mogi-Mirim and Cordeirópolis municipalities, SP, aiming to confirm a new unique case of herbicide resistance.
2. Materials and Methods
2.1. Biological Material and Seedling Propagation in Greenhouse
Seeds of three slender amaranth populations with suspected resistance to glyphosate were collected from at least 20 plants that survived the last application of glyphosate (≥720 g ea ha−1) in December 2018. The populations R1 and R2 were collected in sweet orange orchards that were 500-m apart in the municipality of Mogi-Mirim, SP, Brazil (22°25′ S, 47°09′ W). The R-IAC population was collected in the citrus experimental field of the Agronomic Institute (IAC) in Cordeirópolis, SP, Brazil (22°32′ S, 47°27′ W). Seeds of a susceptible population (S-Lim) used as control were collected in an organic orchard (without the use of herbicides) of Tahiti acid lime, also in Mogi Mirim [3].
Seeds of each population were germinated in plastic containers (10 × 20 × 8 cm) containing substrate (Insumax, Nova Europa, SP, Brazil) and sand (1:2, v/v) moistened to field capacity. Some of these seeds were deposited and covered with vermiculite (2-mm), and the containers were hermetically sealed. Germinated seedlings were individualized in 250 mL pots filled with substrate, sand, and vermiculite (2:2:1, v/v/v). Two days after transplanting, seedlings were fertilized with ~100 mg (5–6 granules) of 14-14-14 (Forth Cote, Osmocote, Froth Jardim Ltd., Cerquinho, SP. Brazil) and irrigated as needed until use. Seedlings with 3–5 true leaves were used in all experiments, which were kept under greenhouse conditions (25–32 °C, 60 ± 10% relative humidity, and 16-h photoperiod) from germination to evaluations.
2.2. Glyphosate Dose-Response Assays
Plants from the R and S populations were treated with the following doses of glyphosate (Roundup Original DI, 370 g acid equivalent (ae) L−1, Monsanto do Brasil Ltd.): 0, 45.25, 92.5, 185, 370, 740 (reference field dose), 1480, and 2960 g ae ha−1. The glyphosate was sprayed using a pneumatic backpack sprayer equipped with an LBD-110015E nozzle (KGF Bicos para Pulverização, Vinhedo, SP) calibrated to deliver 200 L of ha−1 herbicide mixture at 30 psi (pressure measured with a glycerin manometer (Model GCN, Cotergavi Instrumentos de Medição Ltd., São Paulo, Brazil) coupled to the spray bar). Experimental design (completely randomized) included six replicates per glyphosate dose, and they were repeated. When the dose-response experiments were repeated, the 2960 g ae ha−1 glyphosate dose was deleted, but a dose of 22.63 g ae ha−1 was included. In both experiments, the fresh weight by cutting the plants at ground level and the plant mortality rates were determined 21 days after the treatment. Data were expressed as a percentage in relation to the untreated control, and the effective mean doses that reduce the fresh weigh (GR50) and cause plant mortality (LD50) by 50% were determined by non-linear regression analysis.
2.3. Shikimic Acid Accumulation
Shikimic acid accumulation was determined following two approaches. In the first one, the methodology of Cromartie and Polge [21] was followed. A set of plants of each slender amaranth population was treated with 370 g ae ha−1 glyphosate, and another set of untreated plants was reserved as a control to build the calibration curve with known concentrations of shikimic acid (0, 0.01, 0.05, 0.1, and 0.2). The first and second leaf of treated and untreated plants were cut in small segments 96 h after treatment (HAT). Samples of 50 mg of fresh leaf tissue were placed in tubes containing 1 mL of HCl 0.25 N, frozen in liquid nitrogen, and stored at −40 °C for further analysis. Samples were thawed at room temperature and then incubated for 45 min at 37 °C. Aliquots of 50 µL were transferred to new tubes containing 200 µL of periodic acid 0.25% (w/v) + sodium m-periodate 0.25% (w/v) (Solution 1). Samples were incubated at 37 °C for 30 min, following which, 200 µL of 0.6 N sodium hydroxide + 0.22 N sodium sulfite (Solution 2) was added and, finally, they were homogenized. Volumes of 300 µL were transferred to spectrophotometric cuvettes containing 600 µL of distilled water. Absorbance was measured at 380 nm wavelength in a diode-array spectrophotometer (HP 8425A, Palo Alto, CA, USA). The shikimic acid accumulation was determined from the difference between treated and untreated plants, and the results were expressed as µg of shikimic acid g−1 fresh tissue. Five samples with three technical replicates were analyzed per population in a completely randomized design.
In the second approach, shikimic acid was quantified in vivo, according to Dayan et al. [22], with modifications. Different glyphosate concentrations (0, 10, 50, 100, 200, 500, and 1000 µM) were prepared in 10-mM ammonium phosphate monobasic solution (pH adjusted to 4.4 with 0.1 HCl). Samples of 50 mg young leaf segments (4 × 4 mm) were placed in tubes containing 1 mL of the corresponding glyphosate concentration. Tubes were incubated for 24 h in a BOD (biochemical oxygen demand) chamber at 25 °C under fluorescent lights (150 µM m−2 s−1). After incubation, tubes were rapidly frozen in liquid nitrogen, thawed at room temperature, and incubated at 60 °C for 30 min. Samples received 250 µL of 1.25 N HCl, and they were incubated again at 60 °C for 15 min. Volumes of 75 µL of the samples were transferred to spectrophotometric cuvettes containing 300 µL of solution 1 and incubated at 25 °C for 90 min in a BOD chamber. Finally, samples received 300 µL of solution 2, and the absorbance was measured at 380 nm, as described above. The experiment had a completely random design with three replications (three technical replicates each one) per glyphosate concentration. Results were expressed as µg shikimate per mL HCl solution (µg mL−1).
2.4. EPSPS Enzyme Activity Assays
Five grams of fresh leaf tissue from each A. viridis population was harvested, frozen immediately in liquid N2, and stored at −80 °C. EPSPS enzyme extraction was performed following the protocol described by Dayan et al. [22]. The total soluble protein (TPS) in the extract (EPSPS basal activity in the absence of glyphosate) was determined by the Bradford assay [23]. The specific EPSPS activity was assayed in the presence of glyphosate (0, 1, 10, 100, 1000 µM) using the EnzChek Phosphate Assay Kit (Invitrogen, Carlsbad, CA, USA) to determine the amount of inorganic phosphate (μmol) released by μg−1 TSP min−1 in comparison to the control (basal activity). Three replications per glyphosate concentration were assayed. The results were expressed as the inhibition rate of the EPSPS by 50% (I50).
2.5. Statistical Analysis
The GR50 and LD50 values were calculated using a log-logistic model of four-parameters Y = c + {(d − c)/[1 + (x/g)b]} [24], where: Y = response by 50%; d and c are the upper and lower limits of the curve; b is the slope of the line; x is the herbicide dose, and g is the herbicide rate at the point of an inflection curve. SigmaPlot 10.0 (Systat Sofware Inc. San Jose, CA, USA) was used to obtain those parameters. The resistance factors (RF = R/S) were computed as R-to-S GR50 or LD50 ratios. Because the estimated LD50 and GR50 values for population S differed between repetitions of the dose-response experiments, the results were shown separately for each repetition.
One-way ANOVA was performed for the analysis of shikimic acid accumulation data. Differences of p < 0.05 were considered significant, and Tukey’s test at α = 0.05 probability was conducted for means comparison. Statistical analyses were performed using the Statistics 9.0 software (Analytical Software, Tallahassee, FL, USA).
3. Results
3.1. Fresh Weight Reduction and Plant Survival
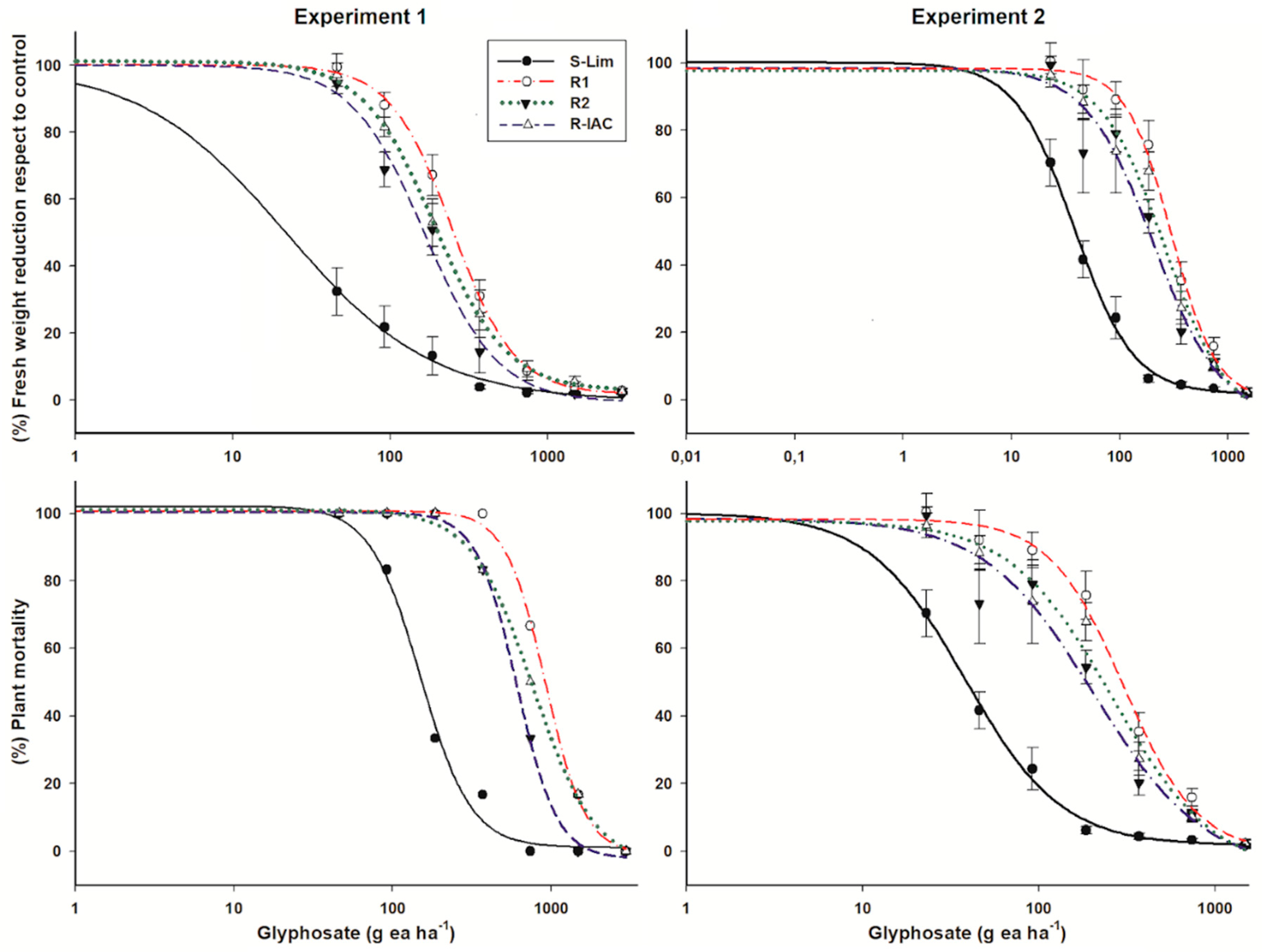
The fresh weight reduction was higher in individuals of the S-Lim slender amaranth population than the R populations. In the first experiment, the estimated GR50 for population S was 22 g ea ha−1 (Table 1). Thus, the RF of the R populations ranged were 7.5, 8.7, and 11.3 for the R2, R-IAC, and R1 populations, respectively. After repetition of dose-response experiments, more representative value of GR50 was estimated for the population S-Lim (38 g ea ha−1) due to the smaller dose of glyphosate included; while the GR50 values of the R populations were similar (according to the confidential intervals) to those estimated in the first experiment. Because the GR50 of the S-Lim population was higher in the second experiment, the ratio of glyphosate resistance level in relation to the R populations decreased and ranged from 5.4 to 7.9 (Figure 1).

Table 1.
Parameters of the sigmoidal equations 1 used to estimate the effective mean dose (g ea ha−1) of glyphosate required to reduce the fresh weight by 50% (GR50) and to cause plant mortality (LD50) by 50% in resistant (R1, R2 and R-IAC) and susceptible (S-Lim) slender amaranth (Amaranthus viridis L.) populations (Pop.) collected in citrus orchards from São Paulo State, Brazil.
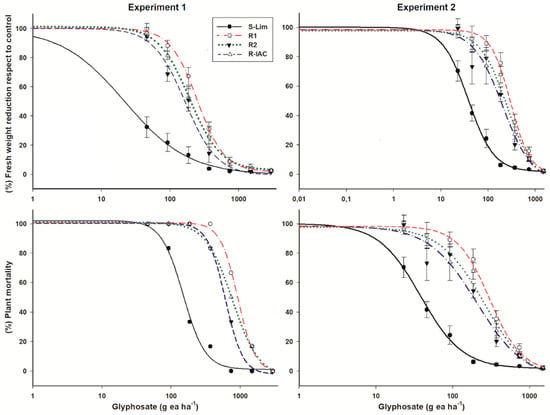
Figure 1.
Glyphosate dose-response curves relative to percentages of fresh weight reduction and plant survival of susceptible and resistant slender amaranth (Amaranthus viridis L.) populations collected in citrus orchards from São Paulo State, Brazil. Vertical bars of the fresh weight reduction plots represent the standard error of the mean (n = 6).
Based on plant survival, the S-Lim population had LD50 values of 149 and 113 g ea ha−1 in the experiments I and II, respectively. However, individuals of this population did not survive glyphosate doses greater than 370 g ea ha−1. In the resistant populations, although their individuals had a large weight loss in relation to their respective untreated controls, around 50% of their individuals survived to the field dose of 720 g ea ha−1. In addition, plants of the R1 and R-IAC populations survived at 1480 g ea ha−1. Thus, the R populations were between 4.1 and 6.4 times more resistant than the S-Lim population. In both experiments, the R1 population was the most resistant based on its lower weight reduction or the highest survival rate of its individuals (Figure 1, Table 1).
3.2. Shikimic Acid Accumulation
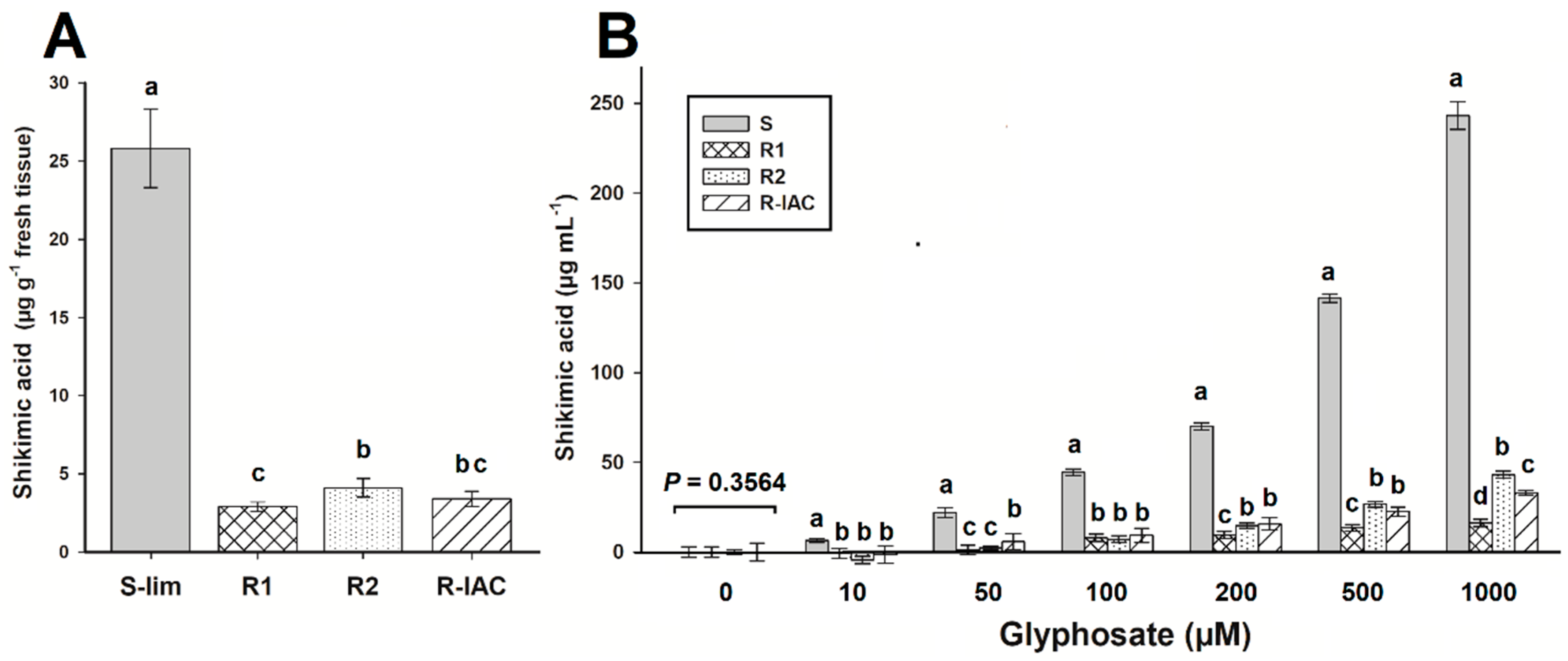
Monitoring the shikimic acid at 96 HAT, the S-Lim population accumulated ~26 µg g−1 fresh weight. This amount of shikimic acid found in the S population was between 6 and 9 times higher than that accumulated in R populations, which accumulated less than 4 µg g−1 fresh weight (Figure 2A). In the in vivo assays, the highest amount of shikimic acid was also found in the S-Lim population that began to accumulate it from the lowest glyphosate concentration (6.4 µg mL−1 at 10-µM glyphosate). The accumulation increased as glyphosate concentrations increased, reaching up ~240-µg shikimic acid mL−1 at 1000 µM glyphosate. R populations began to accumulate shikimic acid only from 50 µM glyphosate; however, such accumulation was much lower than in population S-Lim. At 1000 µM glyphosate, the R1, R2, and R-IAC populations accumulated 16, 43, and 33 µg shikimic acid mL−1, respectively (between 5.6 and 15 times less than the S-Lim population) (Figure 2B).
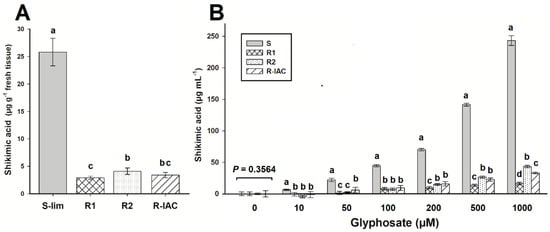
Figure 2.
Shikimic acid accumulation in glyphosate-susceptible and -resistant slender amaranth (Amaranthus viridis L.) populations collected in citrus orchards from São Paulo State, Brazil. (A) Shikimic acid accumulation in sprayed plants with 360 g ae ha−1 at 96 h after treatment. (B) In vivo shikimic acid accumulation at different glyphosate concentrations. Groups of bars with the same letter above them are not different using the Tukey test at 95%. Vertical bars represent the standard error of the mean (n = 9 technical replicates).
3.3. EPSPS Activity
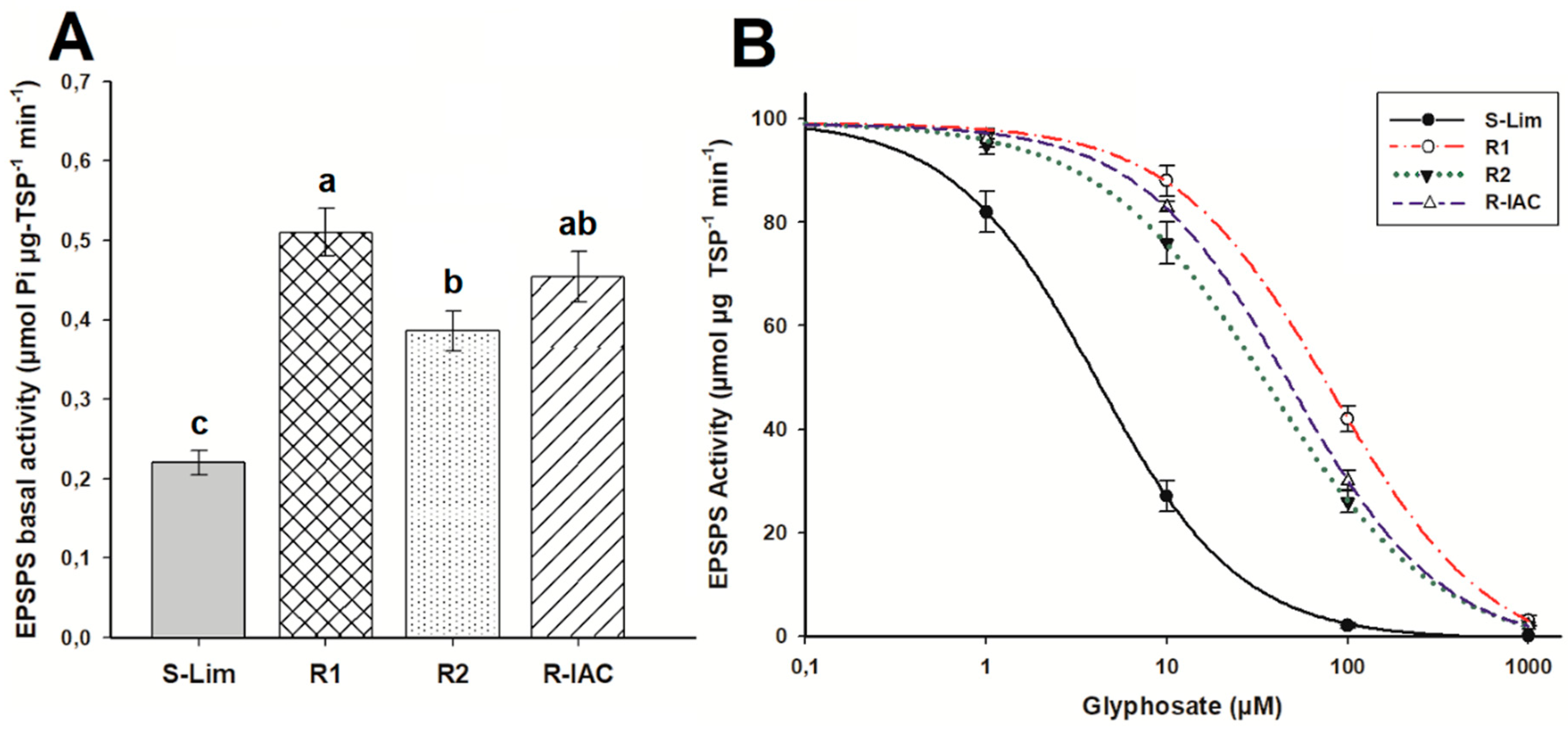
Resistant A. viridis populations presented higher EPSPS basal activity than the S population (0.22 μmol Pi μg−1 TSP min−1), but there were differences between them (ranged from 0.38 to 0.52 μmol Pi μg−1 TSP min−1) (Figure 3A). To inhibit the EPSPS activity in the S-Lim population, only 4 µM glyphosate was necessary. The R1 population was 20 times more resistant than to the S-Lim population, and the R2 and R-IAC populations were 9 and 12 times more resistant, respectively (Figure 3B, Table 2).
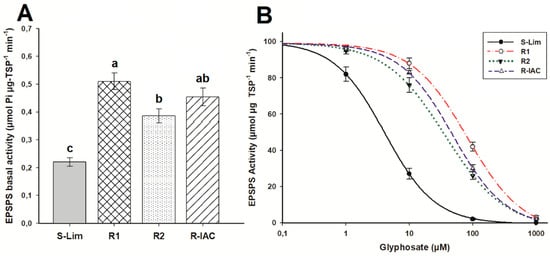
Figure 3.
EPSPS (5-enolpyruvylshikimate-3-phosphate synthase) activity in glyphosate-susceptible and -resistant slender amaranth (Amaranthus viridis L.) populations collected in citrus orchards from São Paulo State, Brazil a. (A) Basal EPSPS activity (absence of glyphosate). Same letter above bars are not different using the Tukey test at 95%. (B) Dose-response curves of the EPSPS enzyme activity, expressed as a percentage of the untreated control, exposed to different glyphosate concentrations (µM). Histograms represent the means, and vertical bars the standard error (n = 3).

Table 2.
Parameters of the sigmoidal equations 1 used to estimate the glyphosate concentration (µM) required to inhibit the EPSPS by 50% (I50) in glyphosate-resistant and -susceptible slender amaranth (Amaranthus viridis L.) populations (Pop.) collected in citrus orchards from São Paulo State, Brazil.
4. Discussion
The GR50 values found for the S-Lim population of slender amaranth were lower than 100 g ae ha−1 and showed the great susceptibility to glyphosate of this species. Susceptible populations of Amaranthus hybridus [16], A. palmeri [25], and Amaranthus tuberculatus [26] also showed GR50 values less than 100 g ae ha−1 (17, 89, and 61 g ae ha−1, respectively). In general, Amaranthus species are very sensitive to glyphosate, so the recommended label doses by manufacturers range from 370 (initial growth stage) to 740 (adult stage) g ae ha−1, while for other weed species, such as Panicum maximum, Richardia brasiliensis, and Sida rhombifolia, the minimum dose is 1850 g ae ha−1 [27]. Taking into account the phenological stage of the plants used in our experiments (3–5 true leaves), the reference dose must be 370 g ae ha−1. However, a dose of 740 g ae ha−1 was considered as the field reference dose because it is the minimum glyphosate dose sprayed by Brazilian citrus growers [3].
One of the main criteria to consider a weed as a new case of herbicide resistance is that individuals have been survived to a dose normally lethal to individuals of a wild (susceptible) population of the same species, and these individuals are able to reproduce sexually, i.e., the resistance must be inheritable [28,29]. The estimated LD50 values for the R slender amaranth populations were close to 740 g ae ha−1, with RFs ranging from 4.1 to 6.4 in relation to the S-Lim population (LD50 ≤ 150 g ae ha−1). These results demonstrated that the field reference dose did not satisfactorily control ~50% of individuals of R populations. Although not arbitrary, the herbicide amount enough to achieve an acceptable control level in a resistant weed population often requires at least twice its LD50 [30]. However, increasing the herbicide dose is not recommended because the selection pressure also increases, as well as the resistance level, depending on the resistance mechanism involved [31].
Glyphosate is a potent inhibitor of the EPSPS, enzyme responsible for the biosynthesis of the chorismate from shikimic acid [9]. Therefore, glyphosate effect can be measured by monitoring the accumulation of shikimic acid [22]. In both experiments, the R plants of slender amaranth accumulated less shikimic acid than the S-Lim plants, showing the low sensitivity of the R populations to this herbicide. The lower accumulation of shikimic acid in resistant plants is due to glyphosate that does not reach the EPSPS in sufficient amount to inhibit this enzyme [32]. The R1 population had a shikimate accumulation pattern different from those observed in the R2 and R-IAC, suggesting that the mechanisms endowing glyphosate resistance among R populations were different from each other.
Enzymatic activity tests did not directly reveal the mechanism that is involved in resistance but could guide whether it is a target-site or non-target-site mechanism. In this case, the three R populations showed high levels of basal enzyme activity than the susceptible population, which might suggest that an EPSPS overexpression participated in the resistance. This mechanism has been the most common resistance mechanism found in glyphosate-resistant Amaranthus sp., such as A. palmeri, A. tuberculatus, and Amaranthus spinosus, which have presented different EPSPS gene copy numbers, ranging from 5 to more than 160 [33,34,35,36]. This mechanism may confer an unpredictable resistance level to glyphosate [37]; however, the higher I50 of the R1 population suggests that another mechanism is also involved in resistance. Single mutations at 106 position in the EPSPS gene confer low levels of glyphosate resistance (2 to 4 times the recommended dose); reduced glyphosate translocation and vacuole sequestration endow moderate resistance levels (4 to 8 times), and double or triple mutations at 102, 103, and 106 positions confer high resistance levels (>10 times) [16,37,38]. Our results did not allow us to confirm the mechanism(s) that endows glyphosate resistance in the R populations of slender amaranth; therefore, biochemical and molecular studies are necessary to unravel them.
5. Conclusions
These results confirmed that slender amaranth (A. viridis) was selected for glyphosate resistance in Brazilian citrus orchards, being the first case in the world reported for this species. At least one target site-type mechanism participated in this glyphosate resistance; however, further experiments are required to elucidate the resistance mechanisms involved.
Author Contributions
Conceptualization, R.A.-d.l.C. and M.F.d.G.F.d.S.; methodology, R.A.-d.l.C., G.d.S.A., G.M.d.O., and L.B.d.C.; investigation, R.A.-d.l.C., G.d.S.A., G.M.d.O., and L.R.R.; resources, R.A.-d.l.C., F.A.d.A., and M.F.d.G.F.d.S.; writing—original draft preparation, R.A.-d.l.C., G.M.d.O., and G.d.S.A.; writing—review and editing, all authors; funding acquisition, R.A.-d.l.C. and M.F.d.G.F.d.S.; Supervision, M.F.d.G.F.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
The RAC, GMO and MFGFS thank the “Fundação de Amparo à Pesquisa do Estado de São Paulo” for the financial support (main-grant: 2014/50918-7, sub-grants: 2018/15910-6 and 2019/15527-0).
Acknowledgments
Authors thank Pamela Freitas dos Santos for her technical assistance in the greenhouse experiments.
Conflicts of Interest
No conflicts of interest have been declared.
References
- U.S. Department of Agriculture [USDA]. Citrus: World Markets and Trade. Available online: https://apps.fas.usda.gov/psdonline/circulars/citrus.pdf (accessed on 17 August 2019).
- Instituto Brasileiro de Geografia e Estatística [IBGE]. Produção Agrícola Municipal. 2017. Available online: http://www.sidra.ibge.gov.br/bda/tabela/protabl.asp?c=1613&z=p&o=18&i=p (accessed on 14 August 2019).
- Martinelli, R.; Monquero, P.A.; Fontanetti, A.; Conceição, P.M.; Azevedo, F.A. Ecological mowing: An option for sustainable weed management in young citrus orchards. Weed Technol. 2017, 31, 260–268. [Google Scholar] [CrossRef]
- Food and Agriculture Organization [FAO]. FAOSTAT: Statistical Database. Available online: http://faostat.fao.org/site/567/default.aspx (accessed on 17 August 2019).
- Singh, M.; Sharma, S.D. Benefits of triazine herbicides and other weed control technology in citrus management. In The Triazine Herbicides—50 Years Revolutionizing Agriculture, 1st ed.; Lebaron, H.M., McFarland, J.E., Burnside, O.C., Eds.; Elsevier: San Diego, CA, USA, 2008; pp. 199–209. [Google Scholar]
- La Cruz, R.A.-D.; Domínguez-Martínez, P.A.; Da Silveira, H.M.; Cruz-Hipólito, H.E.; Palma-Bautista, C.; Vazquez-Garcia, J.G.; Domínguez-Valenzuela, J.A.; De Prado, R. Management of glyphosate-resistant weeds in Mexican citrus groves: Chemical alternatives and economic viability. Plants 2019, 8, 325. [Google Scholar] [CrossRef]
- Bracamonte, E.; Da Silveira, H.M.; La Cruz, R.A.-D.; Domínguez-Valenzuela, J.A.; Cruz-Hipolito, H.E.; De Prado, R. From tolerance to resistance: Mechanisms governing the differential response to glyphosate in Chloris barbata. Pest Manag. Sci. 2018, 74, 1118–1124. [Google Scholar] [CrossRef]
- Caetano, R.S.X.; Christoffoleti, P.J.; Victoria-Filho, R. Weed seed bank of a ’Pera’ citrus orchard. Sci. Agric. 2001, 58, 509–517. [Google Scholar] [CrossRef]
- Duke, S.O. The history and current status of glyphosate. Pest Manag. Sci. 2018, 74, 1027–1034. [Google Scholar] [CrossRef]
- Vázquez-García, J.G.; Castro, P.; Torra, J.; Alcántara-de la Cruz, R.; De Prado, R. Resistance evolution to EPSPS inhibiting herbicides in false barley (Hordeum murinum) harvested in Southern Spain. Agronomy 2020, 10, 992. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: http://www.weedscience.org (accessed on 30 June 2020).
- Moreira, M.S.; Nicolai, M.; Carvalho, S.J.P.; Christoffoleti, P.J. Glyphosate-resistance in Conyza canadensis and C. Bonariensis. Planta Daninha 2008, 25, 157–164. [Google Scholar] [CrossRef]
- De Carvalho, L.B.; Cruz-Hipólito, H.; González-Torralva, F.; Alves, P.; Christoffoleti, P.; De Prado, R. Detection of sourgrass (Digitaria insularis) biotypes resistant to glyphosate in Brazil. Weed Sci. 2011, 59, 171–176. [Google Scholar] [CrossRef]
- Alcántara-de la Cruz, R.; Oliveira, G.M.; Carvalho, L.B.; Silva, M.F.G.F. Herbicide resistance in Brazil: Status, impacts, and future challenges. In Herbicides-Current Research and Case Studies in Use, 2nd ed.; Ferreira, K.M., Ed.; IntechOpen: Londod, UK, 2020. [Google Scholar] [CrossRef]
- Sellers, B.A.; Smeda, R.J.; Johnson, W.G.; Kendig, J.A.; Ellersick, M.R. Comparative growth of six Amaranthus species in Missouri. Weed Sci. 2003, 51, 329–333. [Google Scholar] [CrossRef]
- García, M.J.; Palma-Bautista, C.; Rojano-Delgado, A.M.; Bracamonte, E.; Portugal, J.; La Cruz, R.A.-D.; De Prado, R. The triple amino acid substitution TAP-IVS in the EPSPS gene confers high glyphosate resistance to the superweed Amaranthus hybridus. Int. J. Mol. Sci. 2019, 20, 2396. [Google Scholar] [CrossRef]
- Thomas, W.E.; Burke, I.C.; Spears, J.F.; Wilcut, J.W. Influence of environmental factors on slender amaranth (Amaranthus viridis) germination. Weed Sci. 2006, 54, 316–320. [Google Scholar] [CrossRef]
- Raimondi, M.; Oliveira, J.R.; Constantin, J.; Rios, F.; Gemelli, A.; Raimondi, R. Dose-response curve to soil applied herbicides and susceptibility evaluation of different Amaranthus species using model identity. Planta Daninha 2015, 33, 157–164. [Google Scholar] [CrossRef]
- Francischini, A.C.; Constantin, J.; Oliveira, R.S., Jr.; Santos, G.; Braz, G.B.P.; Dan, H.A. First report of Amaranthus viridis resistence to herbicides. Planta Daninha 2014, 32, 571–578. [Google Scholar] [CrossRef]
- Küpper, A.; Borgato, E.A.; Patterson, E.; Netto, A.G.; Nicolai, M.; De Carvalho, S.J.P.; Nissen, S.J.; Gaines, T.A.; Christoffoleti, P. Multiple resistance to glyphosate and acetolactate synthase inhibitors in Palmer amaranth (Amaranthus palmeri) identified in Brazil. Weed Sci. 2017, 65, 317–326. [Google Scholar] [CrossRef]
- Cromartie, T.H.; Polge, N. Method of Detecting Shikimic Acid. U.S. Patent 006482654B1, 8 February 2002. Available online: https://patents.google.com/patent/US6482654B1/en (accessed on 30 June 2020).
- Dayan, F.; Owens, D.K.; Corniani, N.; Silva, F.M.L.; Watson, S.B.; Howell, J.; Shaner, D.L. Biochemical markers and enzyme assays for herbicide mode of action and resistance studies. Weed Sci. 2015, 63, 23–63. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Fernández-Escalada, M.; Gil-Monreal, M.; Zabalza, A.; Royuela, M. Characterization of the Amaranthus palmeri physiological response to glyphosate in susceptible and resistant populations. J. Agric. Food Chem. 2016, 64, 95–106. [Google Scholar] [CrossRef]
- Lorentz, L.; Gaines, T.A.; Nissen, S.J.; Westra, P.; Strek, H.J.; Dehne, H.W.; Ruiz-Santaella, J.P.; Beffa, R. Characterization of glyphosate resistance in Amaranthus tuberculatus populations. J. Agric. Food Chem. 2016, 62, 8134–8142. [Google Scholar] [CrossRef]
- Agência de Defesa Agropecuária do Paraná [ADAPAR]. Trade Label of Roundup Original DI. Available online: http://www.adapar.pr.gov.br/arquivos/File/defis/DFI/Bulas/Herbicidas/rounduporiginaldi190118.pdf (accessed on 30 June 2020).
- Heap, I. Criteria for Confirmation of Herbicide-Resistant Weeds—With Specific Emphasis on Confirming Low Level Resistance. Available online: http://www.weedscience.org/Documents/ResistanceCriterion.pdf (accessed on 30 June 2020).
- Takano, H.K.; Oliveira, R.S., Jr.; Constantin, J.; Braz, G.B.P.; Gheno, E.A. Goosegrass resistant to glyphosate in Brazil. Planta Daninha 2017, 35, e017163071. [Google Scholar] [CrossRef]
- Alcántara-de la Cruz, R.; Fernández-Moreno, P.T.; Ozuna, C.V. Target and non-target site mechanisms developed by glyphosate-resistant hairy beggarticks (Bidens pilosa L.). Front. Plant Sci. 2016, 7, 1492. [Google Scholar] [CrossRef] [PubMed]
- Salas, R.A.; Scott, R.C.; Dayan, F.E.; Burgos, N.R. EPSPS Gene amplification in glyphosate-resistant Italian ryegrass (Lolium perenne ssp. multiflorum) populations from Arkansas (United States). J. Agric. Food Chem. 2015, 63, 5885–5893. [Google Scholar] [PubMed]
- Shaner, D.L.; Nadler-Hassar, T.; Henry, W.B.; Koger, C.H. A rapid in vivo shikimate accumulation assay with excised leaf discs. Weed Sci. 2005, 53, 769–774. [Google Scholar] [CrossRef]
- Gaines, T.A.; Zhang, W.; Wang, D.; Bukun, B.; Chisholm, S.T.; Shaner, D.L.; Nissen, S.J.; Patzoldt, W.L.; Tranel, P.J.; Culpepper, A.S.; et al. Gene amplification confers glyphosate resistance in Amaranthus palmeri. Proc. Natl. Acad. Sci. USA 2010, 107, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Nandula, V.K.; Ray, J.D.; Ribeiro, D.N.; Pan, Z.; Reddy, K.N. Glyphosate resistance in tall waterhemp (Amaranthus tuberculatus) from Mississippi is due to both altered target-site and nontarget-site mechanisms. Weed Sci. 2013, 61, 374–383. [Google Scholar] [CrossRef]
- Riggins, C.W.; Peng, Y.H.; Stewart, C.N.; Tranel, P.J. Characterization of de novo transcriptome for waterhemp (Amaranthus tuberculatus) using GS-FLX 454 pyrosequencing and its application for studies of herbicide target-site genes. Pest Manag. Sci. 2010, 66, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Gaines, T.A.; Patterson, E.L.; Neve, P. Molecular mechanisms of adaptive evolution revealed by global selection for glyphosate resistance. New Phytol. 2019, 223, 1770–1775. [Google Scholar] [CrossRef]
- Giacomini, D.A.; Westra, P.; Ward, S.M. Variable inheritance of amplified EPSPS gene copies in glyphosate-resistant Palmer Amaranth (Amaranthus palmeri). Weed Sci. 2019, 67, 176–182. [Google Scholar] [CrossRef]
- Yu, Q.; Jalaludin, A.; Han, H.; Chen, M.; Sammons, R.D.; Powles, S.B. Evolution of a double amino acid substitution in the 5-enolpyruvylshikimate-3-phosphate synthase in Eleusine indica conferring high-level glyphosate resistance. Plant Physiol. 2015, 167, 1440–1447. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).