Impact of Coherent Laser Irradiation on Germination and Mycoflora of Soybean Seeds—Innovative and Prospective Seed Quality Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Experimental Procedures
2.3. Morphological Analysis
2.4. Isolation and Identification of Fungi Colonizing Seeds
2.5. Statistical Analysis
3. Results
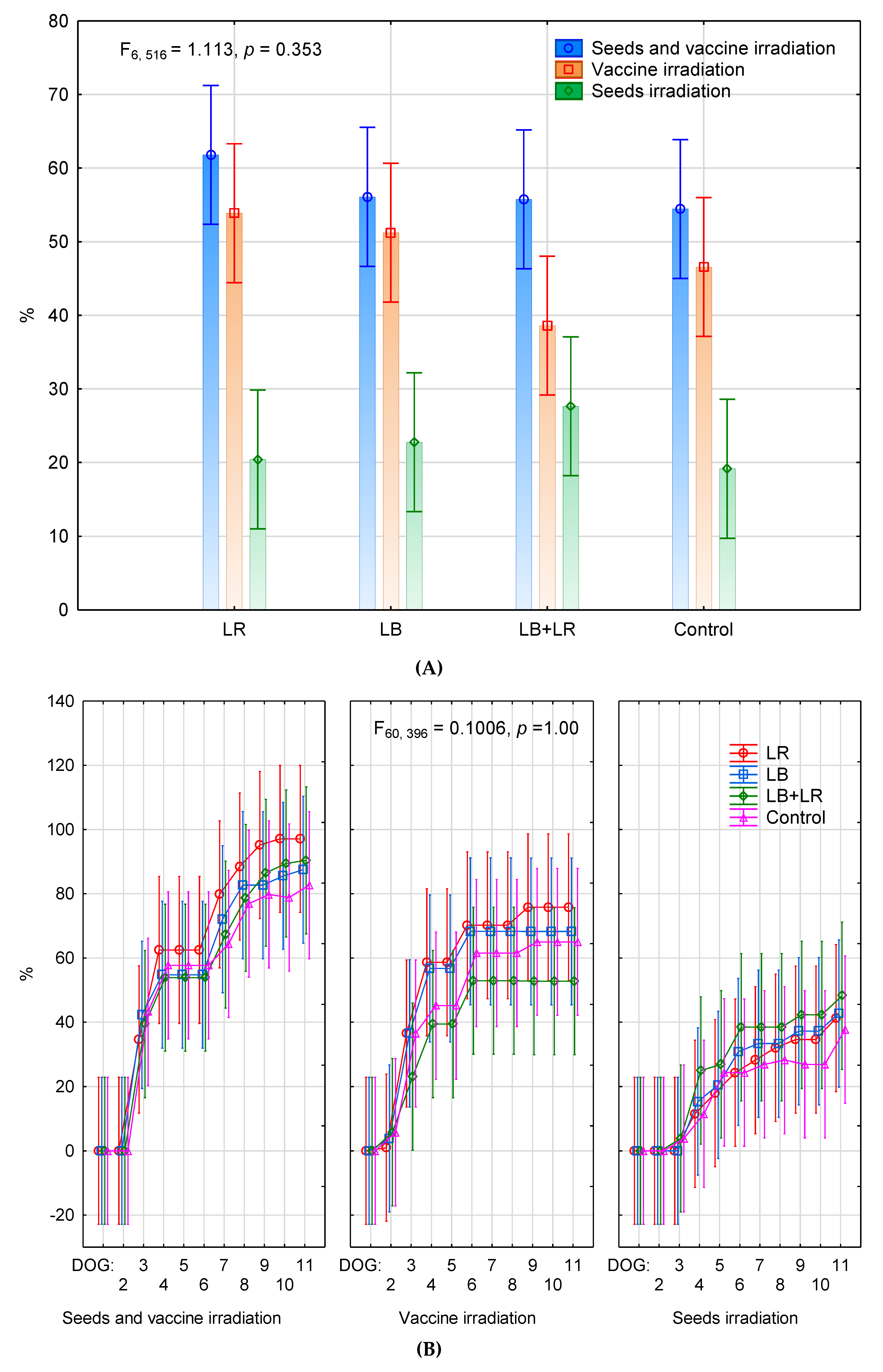
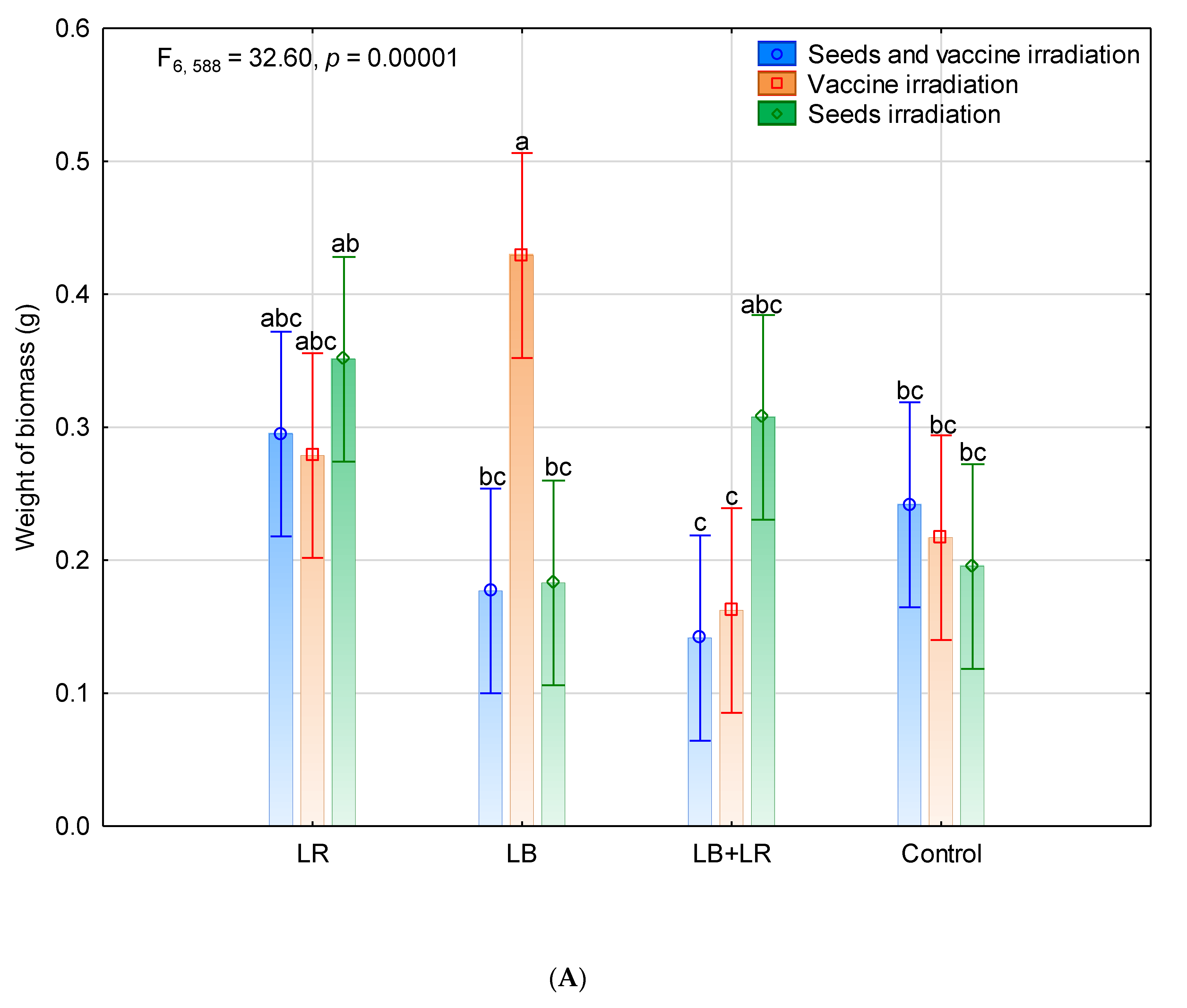
3.1. Seeds Germination and Seedling Biomass
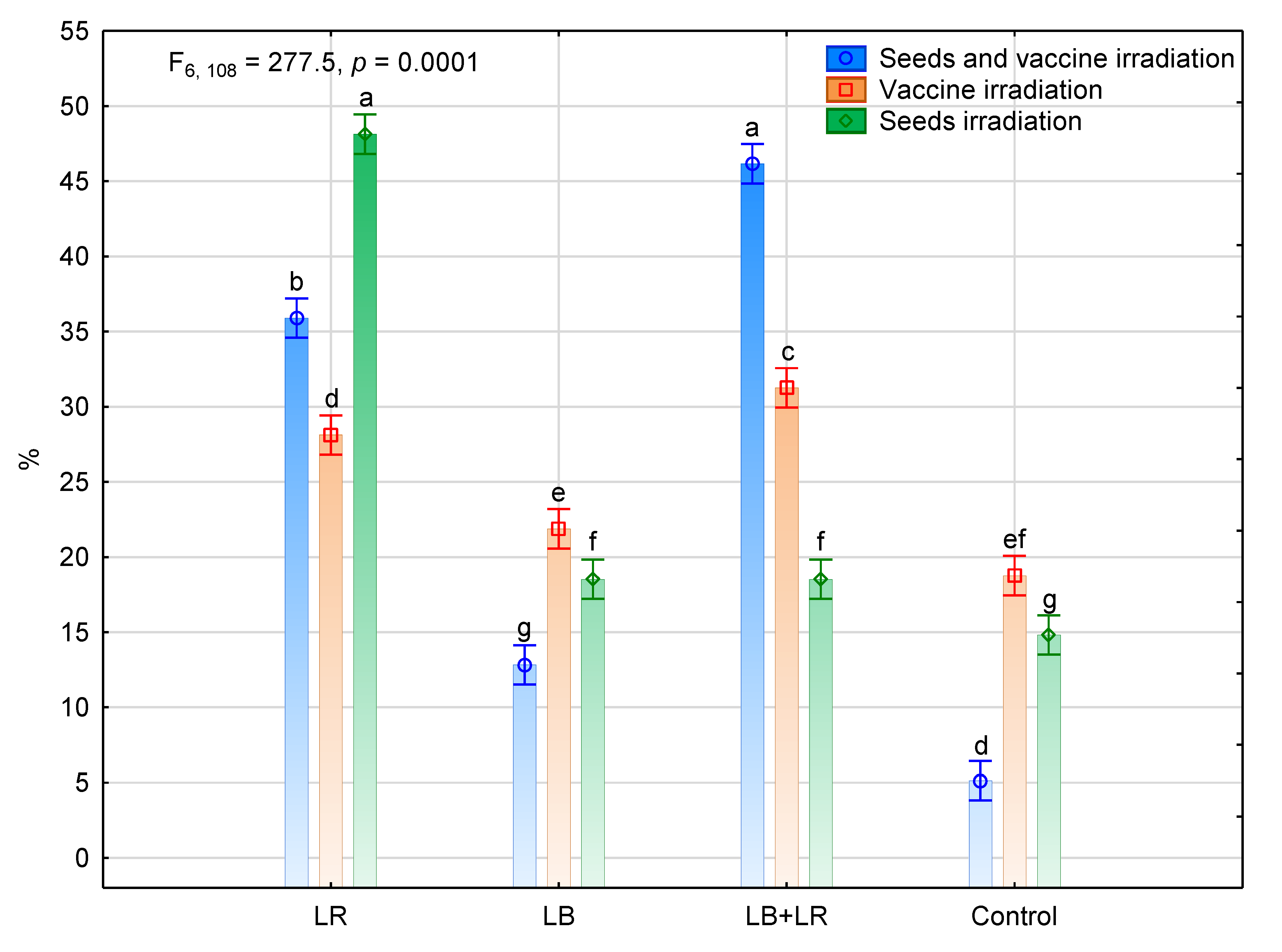
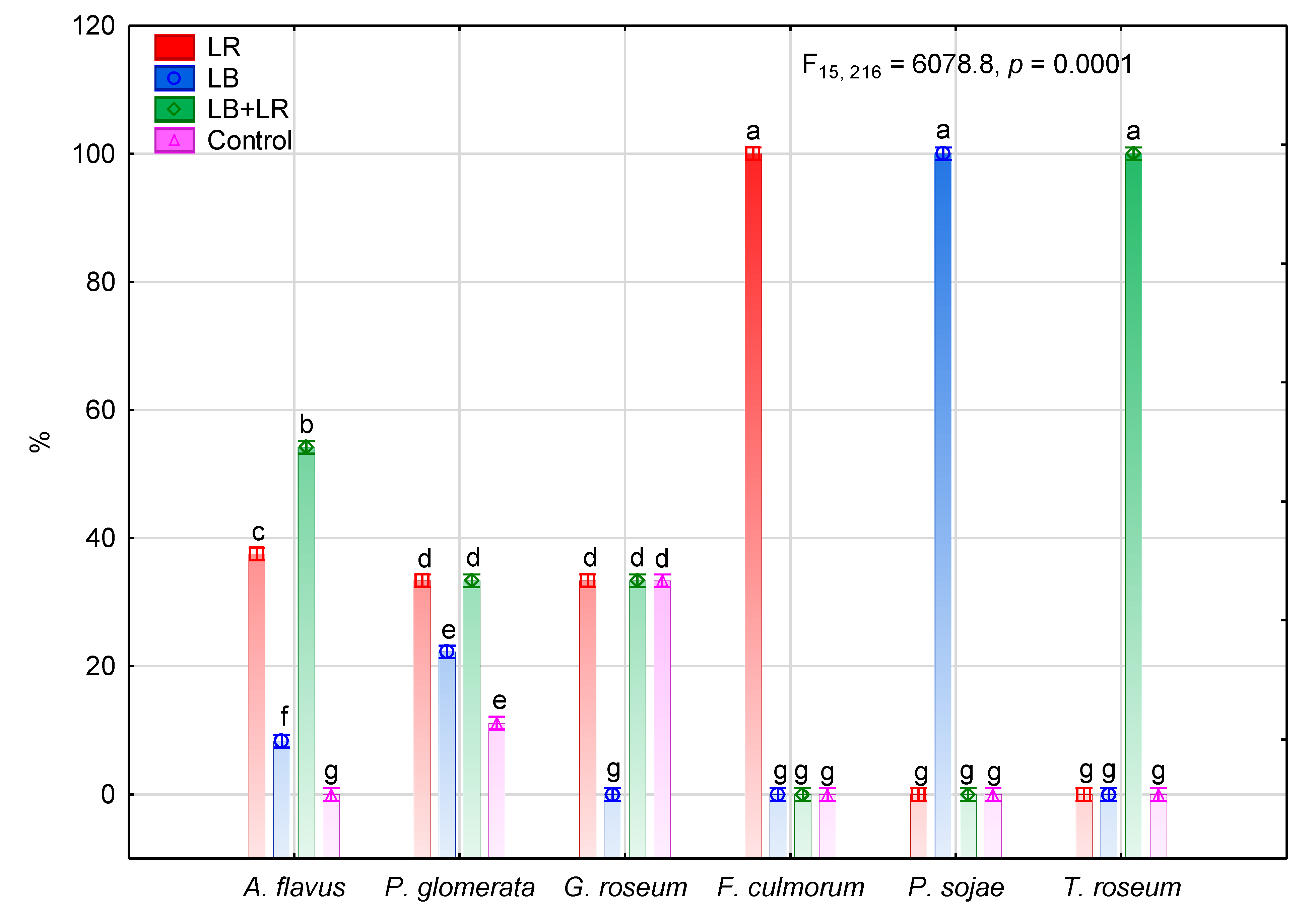
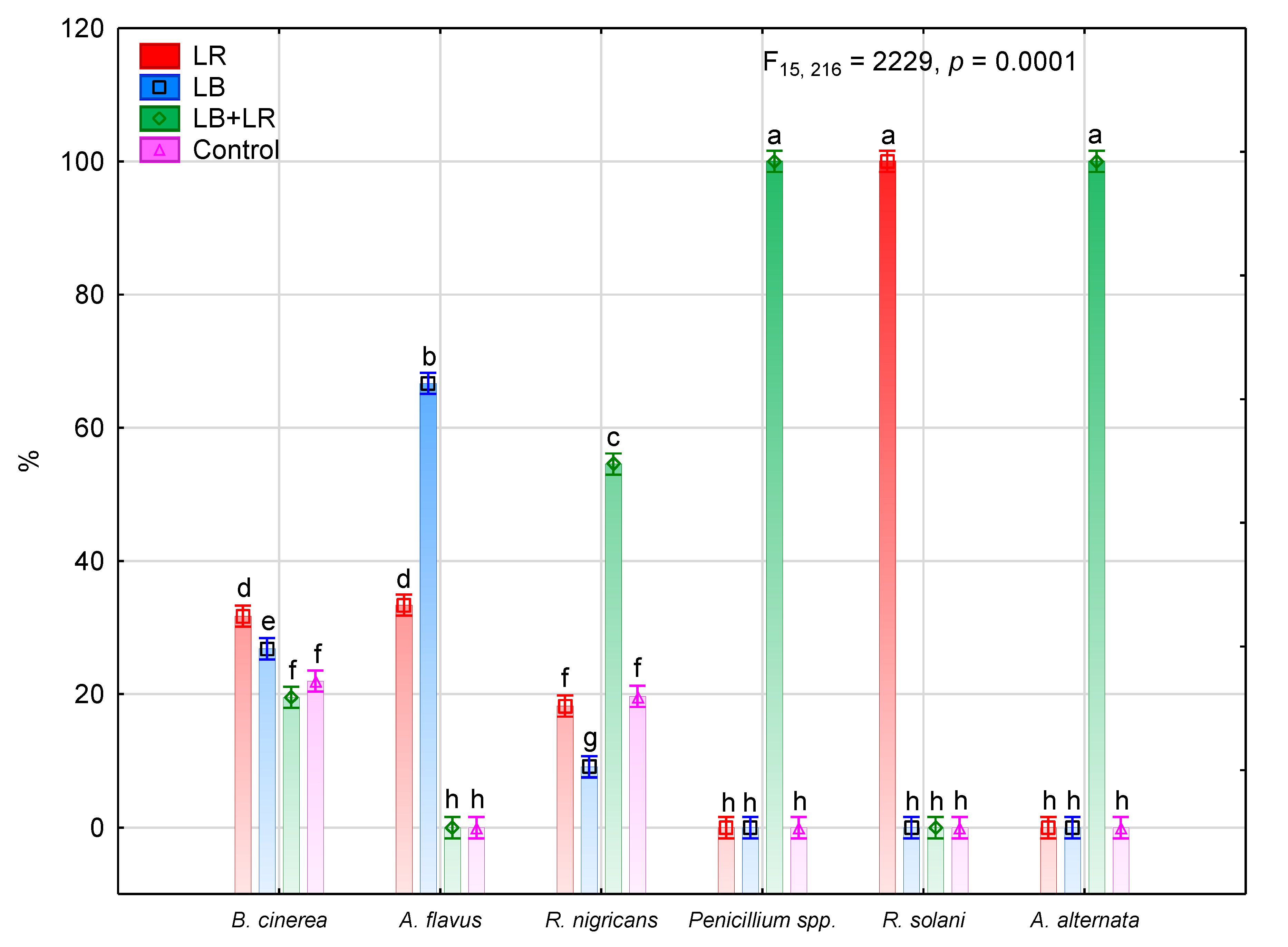
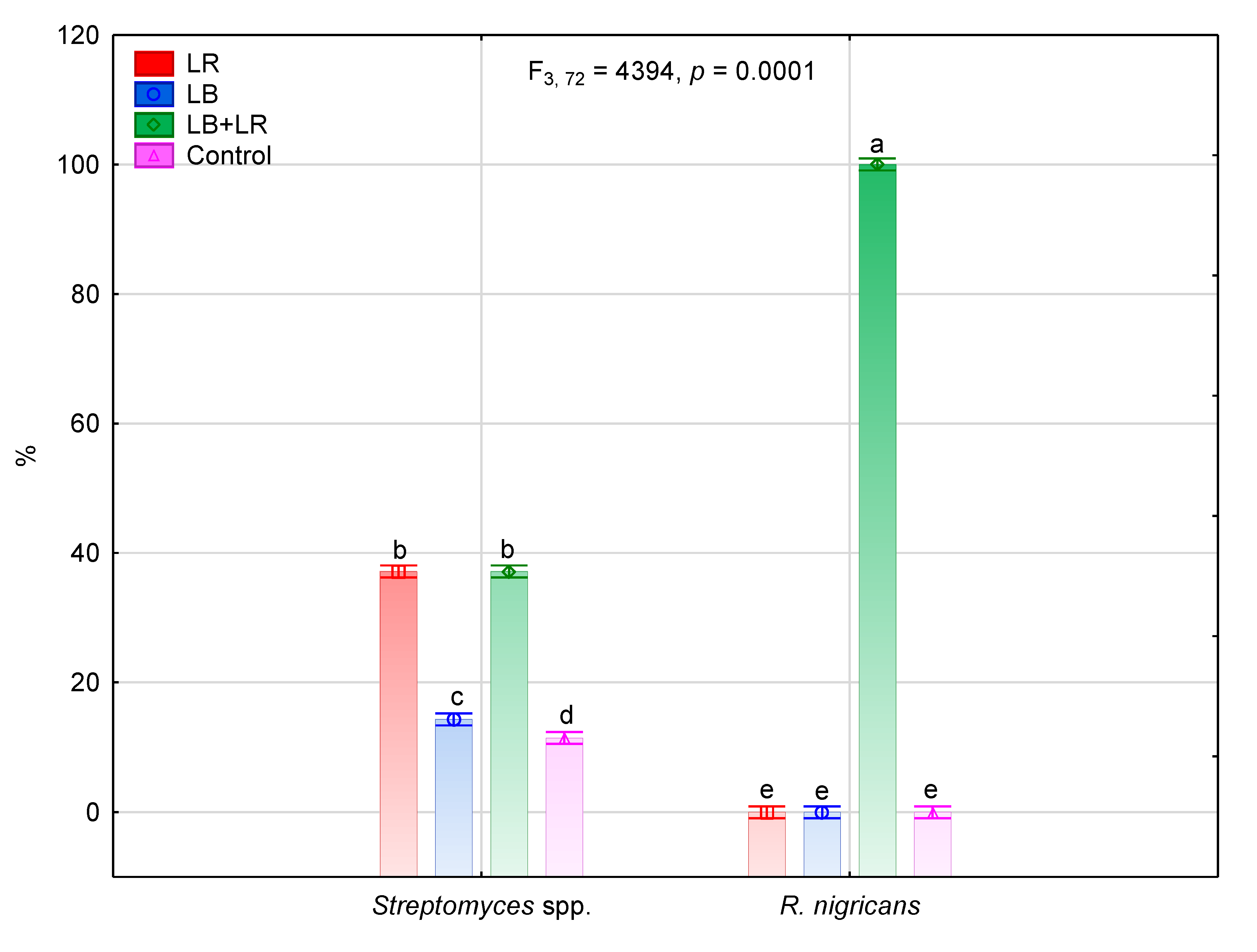
3.2. Isolation and Identification of Fungi Colonizing Seeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dobrowolski, J.W. Laser Biostimulation and Nutritional Prevention of Deficiency of Essential Trace Elements; Hamdard University Press: Sindh, Pakistan, 1986. [Google Scholar]
- Hasan, M.; Hanafiah, M.M.; Taha, Z.A.; Alhilfy, I.H.H.; Said, M.N.M. Laser irradiation effets at different wavelengths on phenology and yield components of pretreated maize seed. Appl. Sci. 2020, 10, 1189. [Google Scholar] [CrossRef] [Green Version]
- Hernández, A.C.; Rodríguez, P.C.L.; Domínguez-Pacheco, F.A.; Hernández, A.A.M.; Cruz-Orea, A.; Carballo, A. Laser light on the microflora content in maize seeds. Afr. J. Biot. 2011, 10, 9280–9288. [Google Scholar] [CrossRef] [Green Version]
- Dobrowolski, J.W.; Śliwka, M.; Mazur, R. Laser biotechnology for more efficient bioremediation, protection of aquatic ecosystems and reclamation of contaminated areas. J. Chem. Technol. Biotechnol. 2012, 87, 1354–1359. [Google Scholar] [CrossRef]
- Waghmare, V.N.; Mehra, R.B. Indued chlorophyll mutations, mutagenic effectiveness and efficiency in Lathyrus sativus L. Indian J. Genet. 2001, 61, 53–56. [Google Scholar] [CrossRef]
- Ritambhara, S.K.; Girjesh, S.K. Biostimulating effect of laser beam on the Cytomorphological aspects of Lathyrus sativus L. Ann. Plant Sci. 2013, 2, 141–148. [Google Scholar]
- Qi, Z.; Yue, M.; Han, R.; Wang, X.-L. The damage repair role of He-Ne laser on plants exposed to different intensities of ultraviolet-B radiation. Photochem. photobiol. 2002, 75, 680–686. [Google Scholar] [CrossRef]
- Chen, H.; Han, R. Effects of He-Ne laser on nuclear F-actin under UV-B radiation. Russ. J. Plant Phys. 2015, 62, 808–814. [Google Scholar] [CrossRef]
- Podleśna, A.; Gładyszewska, B.; Podleśny, J.; Zgrajka, W. Changes in the germination process and growth of pea in effect of laser seed irradiation. Int. Agrophys. 2015, 29, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, A.C.; Dominguez, P.A.; Cruz- Orea, A.; Ivanov, R.; Carballo, C.A.; Zepeda, B.R. Laser in agriculture (Review). Int. Agrophys. 2010, 24, 407–422. [Google Scholar]
- Ouf, S.A.; Abdel-Hady, N.F. Influence of He-Ne Laser Irradiation of Soybean Seeds on Seed Mycoflora, Growth, Nodulation, and Resistance to Fusarium solani. Folia Microbiol. 1999, 44, 388–396. [Google Scholar] [CrossRef]
- Janda, K.; Wolska, J. Study on the quantitative and qualitative of fungi colonizing soybeans (Glycine max L.) (In Polish). Pom. J. Life Sci. 2015, 61, 426–432. [Google Scholar]
- Rajeshwari, M.C.; Hunje, R. Impact of seed mycoflora of soybean on seed quality. J. Oilseeds Res. 2020, 37, 204. [Google Scholar]
- Ahammed, S.K.; Anandam, R.J.; Babu, G.P.; Munikrishnaiah, M.; Gopal, K. Studies on seed mycoflora of soybean and its effect on seed and seedling quality characters. Legume Res. 2006, 29, 186–190. [Google Scholar]
- Anwar, S.A.; Riaz, S.; Ahmad, C.A.; Subhani, M.N.; Chattha, M.B. Mycoflora associated with stored seeds of soybean. Mycopath 2013, 11, 85–90. [Google Scholar]
- Cortina, J.V.; Theodoro, G.F.; Walker, G.R. Identification of fungi on diseased soybean seeds harvested during a high rainfall period in Mato Grosso do Sul, Brazil. Biosci. J. 2013, 29, 386–391. [Google Scholar]
- Ismaiel, A.A.; Papenbrock, J. Mycotoxins: Producing Fungi and Mechanisms of Phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef] [Green Version]
- Wilczek, M.; Koper, M.; Ćwintal, M.; Korniłłowicz-Kowalska, T. Germination capacity and health status of alfalfa seeds after laser treatment. Int. Agrophy 2005, 19, 85–89. [Google Scholar]
- Rassam, Y.Z.; Mashhadani, F.A.; Boya, A.F. Laser treatment may enhance growth and resistance to fungal infection of hard wheat seeds. IOSR J. Agri. Vet. Sci. 2013, 2, 47–51. [Google Scholar] [CrossRef]
- Figueiredo, M.V.B.; Seldin, L.; de Araujo, F.F.; Mariano, R.L.R. Plant Growth Promoting Rhizobacteria: Fundamentals and Applications. In Plant Growth and Health Promoting Bacteria; Maheshwari, D., Ed.; Microbiology Monographs; Springer: Berlin/Heidelberg, Germany, 2010; Volume 18, pp. 21–43. [Google Scholar] [CrossRef]
- Gupta, A.; Gopal, M.; Tilak, K.V.B.R. Mechanism of plant growth promotion by rhizobacteria. Indian J. Exp. Biol. 2000, 38, 856–862. [Google Scholar]
- Al-Ani, R.A.; AdhAb, M.A.; Mahdi, M.H.; Abood, H.M. Rhizobium japonicum as a Biocontrol Agent of Soybean Root Rot Disease Caused by Fusarium solani and Macrophomina phaseolina. Plant Protect. Sci. 2012, 48, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Martyniuk, S.; Oroń, J.; Martyniuk, M. Diversity and numbers of root-nodule bacteria (Rhizobia) in polish soils. Acta Soc. Bot. Pol. 2005, 74, 83–86. [Google Scholar] [CrossRef] [Green Version]
- Narożna, D.; Pudełko, K.; Króliczak, J.; Golińska, B.; Sugawara, M.; Cezary, J.; Mądrzak, C.J.; Sadowsky, M.J. Survival and Competitiveness of Bradyrhizobium japonicum Strains 20 Years after Introduction into Field Locations in Poland. Appl. Environ. Microbiol. 2015, 81, 5552–5559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISTA. International rules for seed testing. Seed Sci. Technol. Proc. Int. Seed Test. Ass 1999, 31, 1–152. [Google Scholar]
- Maguire, J.D. Speed of germination-aid selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; The American Phytopathological Society Press: St. Paul, MN, USA, 1998; p. 218. [Google Scholar]
- Dugan, F. The Identification of Fungi: An illustrated Introduction, with Keys, Glossary, and Guide to Literature; The American Phytopathological Society Press: St. Paul, MN, USA, 2006. [Google Scholar]
- Fassatiova, O. Grzyby Mikroskopowe w Mikrobiologii Technicznej; WNT: Warszawa, Poland, 1983. (In Polish) [Google Scholar]
- Klaus, H.; Domsch, W.; Gams, W.; Anderson, T. Compendium of Soil Fungi, 2nd ed.; IHW-Verlag: Eching, Germany, 2008. [Google Scholar]
- Marcinkowska, J. Oznaczanie Rodzajów Grzybów Ważnych w Patologii Roślin; Fundacja Rozwój SGGW: Warszawa, Poland, 2003. (In Polish) [Google Scholar]
- Roy, K.W.; Baird, R.E.; Abney, T.S. A review of soybean (Glycine max) seed, pod, and flower mycofloras in north america, with methods and a key for identification of selected fungi. Mycopathologia 2001, 150, 15–27. [Google Scholar] [CrossRef]
- Schaal, K.P.; Yassin, A.F.; Stackebrandt, E. The family Actinomycetaceae: The genera Actinomyces, Actinobaculum, Arcanobacterium, Varibaculum, and Mobiluncus. Prokaryotes 2006, 3, 430–537. [Google Scholar] [CrossRef]
- Muszyński, S.; Gadyszewska, B. Representation of He-Ne laser irradiation effect on radish seeds with selected germination indices. Int. Agroph. 2008, 22, 151–157. [Google Scholar]
- Dziwulska-Hunek, A.; Kornarzyński, K.; Matwijczuk, A.; Pietruszewski, S.; Szot, B. Effet of laser and variable magnetic field stimulation on amaranth seeds germination. Int. Agroph. 2009, 23, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Soliman, A.; Harith, M.A. Effects of Laser Biostimulation on Germination of Acacia farnesiana (L.) Willd. XIII International Conference on Medicinal and Aromatic Plants, Cairo, Egypt, 3–5 October 2009; Volume 854, pp. 41–50. [Google Scholar]
- Krawiec, M.; Dziwulska-Hunek, A.; Sujak, A.; Palonka, S. Laser irradiation effects on scorzonera (Scorzonera hispanica L.) seed germination and seedling emergence. Acta Sci. Pol. Hortorum Cultus 2015, 14, 145–158. [Google Scholar]
- Sinclair, J.B. Latent Infection of Soybean Plants and Seeds by Fungi. Plant Dis. 1991, 75, 220–224. [Google Scholar] [CrossRef]
- Kacaniova, M. Feeding soybean colonization by microscopic fungi. Trakya Univ. J. Sci. 2003, 4, 165–168. [Google Scholar]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mykotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxic. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Kumar, V.; Basu, M.S.; Rajendran, T.P. Mycotoxin research and mycoflora in some commercially important agricultural commodities. Crop Prot. 2008, 27, 891–905. [Google Scholar] [CrossRef]
- Pięta, D.; Patkowska, E.; Pastucha, A.; Bełkot, M. Wpływ mikroorganizmów antagonistycznych na ograniczanie porażenia soi przez grzyby chorobotwórcze przeżywające w glebie. Acta Sci. Pol. Hort. Cultus 2002, 1, 23–30. [Google Scholar]
- Wilczek, M.; Koper, R.; Ćwintal, M.; Korniłłowicz-Kowalska, T. Germination capacity and health status of hybrid alfalfa seeds after laser treatment. Int. Agrophys 2005, 19, 257–261. [Google Scholar]
- Wilczek, M.; Koper, M.; Ćwintal, M.; Korniłłowicz-Kowalska, T. Germination capacity and the health status of red clover seeds following laser treatment. Int. Agroph. 2004, 18, 289–293. [Google Scholar]

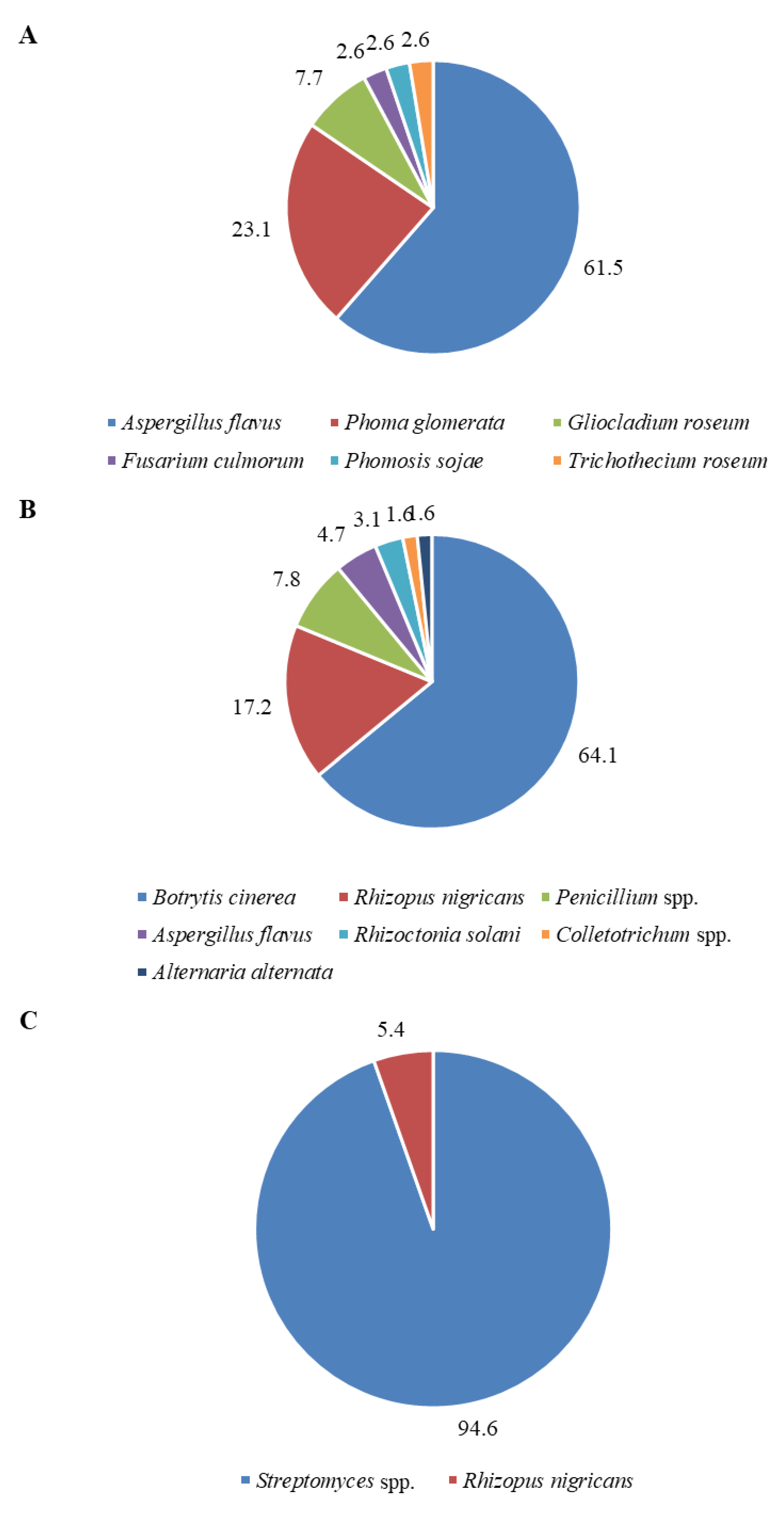
| Microorganism Species | Seed and Vaccine Irradiation | Vaccine Irradiation | Seed Irradiation | Total | Group of Attendance |
|---|---|---|---|---|---|
| Streptomyces spp. | 0.00 | 0.00 | 25.00 | 25.00 | eudominants |
| Pathogenic fungi | |||||
| Botrytis cinerea | 0.00 | 29.29 | 0.00 | 29.29 | eudominants |
| Aspergillus flavus | 17.14 | 2.14 | 0.00 | 19.29 | eudominants |
| Phoma glomerata | 6.43 | 0.00 | 0.00 | 6.43 | dominants |
| Rhizoctonia solani | 0.00 | 1.43 | 0.00 | 1.43 | recedents |
| Fusarium culmorum | 0.71 | 0.00 | 0.00 | 0.71 | subrecedents |
| Phomopsis sojae | 0.71 | 0.00 | 0.00 | 0.71 | subrecedents |
| Alternaria alternata | 0.00 | 0.71 | 0.00 | 0.71 | subrecedents |
| Colletotrichum spp. | 0.00 | 0.71 | 0.00 | 0.71 | subrecedents |
| Nonpathogenic fungi | |||||
| Antagonistic fungi | |||||
| Gliocladium roseum | 2.14 | 0.00 | 0.00 | 2.14 | subdominants |
| Saprotrophic fungi | |||||
| Rhizopus nigricans | 0.00 | 7.86 | 1.43 | 9.29 | dominants |
| Penicillium spp. | 0.00 | 3.57 | 0.00 | 3.57 | subdominants |
| Trichothecium roseum | 0.71 | 0.00 | 0.00 | 0.71 | subrecedents |
| Total | 27.8 | 45.7 | 26.4 | 100 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimek-Kopyra, A.; Dłużniewska, J.; Ślizowska, A.; Dobrowolski, J.W. Impact of Coherent Laser Irradiation on Germination and Mycoflora of Soybean Seeds—Innovative and Prospective Seed Quality Management. Agriculture 2020, 10, 314. https://doi.org/10.3390/agriculture10080314
Klimek-Kopyra A, Dłużniewska J, Ślizowska A, Dobrowolski JW. Impact of Coherent Laser Irradiation on Germination and Mycoflora of Soybean Seeds—Innovative and Prospective Seed Quality Management. Agriculture. 2020; 10(8):314. https://doi.org/10.3390/agriculture10080314
Chicago/Turabian StyleKlimek-Kopyra, Agnieszka, Joanna Dłużniewska, Anna Ślizowska, and Jan Wincenty Dobrowolski. 2020. "Impact of Coherent Laser Irradiation on Germination and Mycoflora of Soybean Seeds—Innovative and Prospective Seed Quality Management" Agriculture 10, no. 8: 314. https://doi.org/10.3390/agriculture10080314





