Extracts from Artemisia vulgaris L. in Potato Cultivation—Preliminary Research on Biostimulating Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparing the Extracts
2.3. Spectrophotometric Characterization of the Extracts
2.3.1. Total Polyphenol Content
2.3.2. Total Flavonoid Content
2.3.3. Total Anthocyanins Content
2.3.4. Reduction Power
2.4. Extraction of Potato Leaves
2.5. Determination of Proline Content
2.6. Determination of Chlorophyll, Carotenoids and Total Phenolic Compounds Content in Leaves
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zarzecka, K.; Grużewska, A.; Gugała, M.; Yatsyshyn, A. Production and quality of table potato in the opinion of consumers in Poland and Ukraine. Probl. World Agric. 2017, 17, 308–318. [Google Scholar] [CrossRef]
- Mystkowska, I.; Zarzecka, K.; Baranowska, A.; Gugala, M.; Gluszczak, B.; Lipiecki, M. The comparison of profitability of starchy potato cultivation in a family farm. PAAAE 2016, 18, 186–189. [Google Scholar]
- Zarzecka, K.; Gugała, M.; Baranowska, A. Economic effect of mechanical and chemical cultivation of potato. PAAAE 2014, 16, 240–243. [Google Scholar]
- Dzwonkowski, W. Evolution of potato production in Poland and the EU. Probl. World Agric. 2017, 17, 71–80. [Google Scholar]
- Baranowska, A.; Zarzecka, K.; Mystkowska, I.; Gugala, M. Economic effect of mechanical and chemical cultivation of potatoes plantation. PAAAE 2016, 18, 27–32. [Google Scholar]
- Dymkowska-Malesa, M.; Szparaga, A.; Czerwińska, E. Evaluation of polychlorinated biphenyls content in chosen vegetables from Warmia and Mazury region. Rocz. Ochr. Sr. 2014, 16, 290–299. [Google Scholar]
- Hara, P.; Szparaga, A.; Czerwińska, E. Ecological methods used to control fungi that cause diseases of the crop plant. Rocz. Ochr. Sr. 2018, 20, 1764–1775. [Google Scholar]
- Szparaga, A.; Kocira, S. Generalized logistic functions in modelling emergence of Brassica napus L. PLoS ONE 2018, 13, e0201980. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kocira, A.; Czerwińska, E.; Depo, K.; Erlichowska, B.; Deszcz, E. Effect of applying a biostimulant containing seaweed and amino acids on the content of fiber fractions in three soybean cultivars. Legume Res. 2019, 42, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Ribera, A.; Cotoras, M.; Zúñiga, G.E. Effect of extract from in vitro-grown shoots of Quillaja saponaria Mol. on Bortytiscinerea Pers. World J. Microbiol. Biotechnol. 2008, 24, 1803–1811. [Google Scholar] [CrossRef]
- Sas-Piotrowska, B.; Piotrowski, W. Vitality and healthiness of barley (Hordeum vulgare L.) seeds treated with plant extract. J. Plant Prot. Res. 2010, 50, 117–124. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Grange, E.; Mannino, G.; van Arkel, J.; Beekwilder, J.; Karlova, R.; Garabello, C.; Contartese, V.; Bertea, C.M. A biostimulant seed treatment improved heat stress tolerance during cucumber seed germination by acting on the antioxidant system and glyoxylate cycle. Front. Plant Sci. 2020, 11, 836. [Google Scholar] [CrossRef] [PubMed]
- González-González, M.F.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.V.; Casarrubias-Castillo, K.; Becerril-Espinosa, A.; Castañeda-Nava, J.J.; Hernández-Herrera, R.M. Physiological, ecological, and biochemical implications in tomato plants of two plant biostimulants: Arbuscular mycorrhizal fungi and seaweed extract. Front. Plant Sci. 2020, 11, 999. [Google Scholar] [CrossRef] [PubMed]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [Green Version]
- Mannino, G.; Nerva, L.; Gritli, T.; Novero, M.; Fiorilli, V.; Bacem, M.; Bertea, C.M.; Lumini, E.; Chitarra, W.; Balestrini, R. Effects of different microbial inocula on tomato tolerance to water deficit. Agronomy 2020, 10, 170. [Google Scholar] [CrossRef] [Green Version]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019R1009 (accessed on 24 June 2020).
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Burgieł, Z.J.; Tomaszkiewicz–Potępa, A.; Vogt, O.; Burgieł, M. Fungistatic properties of extracts from seeds of selected plants belonging to the Apiaceae family. Prog. Plant Prot. 2008, 48, 701–705. [Google Scholar]
- Sas-Piotrowska, B.; Piotrowski, W.; Karczmarek-Cichosz, R. Longevity and healthiness of Oat (Avena sativa L.) seeds treated with plant extracts. J. Plant Prot. Res. 2005, 45, 181–193. [Google Scholar]
- Boligłowa, E.; Gleń, K.; Ropek, D. Preliminary research on an assessment of the effect of mint and eucalyptus oil on selected plant pathogen fungi. Ecol. Chem. Eng. A 2009, 16, 1095–1100. [Google Scholar]
- Górski, R.; Sobieralski, K.; Siwulski, M. Effect of selected natural Essential oils in in vitro development on fungi Trichoderma harzianum fund common mushroom (Agaricus bisporus) cultivation. Ecol. Chem. Eng. A 2010, 17, 177–185. [Google Scholar]
- Gupta, S.; Abu–Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Kocira, S.; Hara, P.; Szparaga, A.; Czerwińska, E.; Beloev, H.; Findura, P.; Bajus, P. Evaluation of the effectiveness of the use of biopreparations as seed dressings. Agriculture 2020, 10, 90. [Google Scholar] [CrossRef] [Green Version]
- Chojnacka, K. An innovative technology of algal extracts. Przem. Chem. 2014, 93, 590–592. [Google Scholar]
- Szparaga, A.; Kocira, S.; Kocira, A.; Czerwińska, E.; Świeca, M.; Lorencowicz, E.; Kornas, R.; Koszel, M.; Oniszczuk, T. Modification of growth, yield, and the nutraceutical and antioxidative potential of soybean through the use of synthetic biostimulants. Front. Plant Sci. 2018, 9, 1401. [Google Scholar] [CrossRef]
- Michałek, W.; Kocira, A.; Findura, P.; Szparaga, A.; Kocira, S. The influence of biostimulant asahi SL on the photosynthetic activity of selected cultivars of Phaseolus vulgaris L. Rocz. Ochr. Sr. 2018, 20, 1286–1301. [Google Scholar]
- Kocira, S.; Szparaga, A.; Kuboń, M.; Czerwińska, E.; Piskier, T. Morphological and biochemical responses of Glycine max (L.) Merr. to the use of seaweed extract. Agronomy 2019, 9, 93. [Google Scholar] [CrossRef] [Green Version]
- Li, X.F.; Wang, J.; Huang, D.; Wang, L.X.; Wang, K. Allelopathic potential of Artemisia frigida and successional changes of plant communities in the northern China steppe. Plant Soil 2011, 341, 383–398. [Google Scholar] [CrossRef]
- Kaur, S.; Batish, D.R. Assessment of allelopathic potential of Artemisia scoparia against some plants. Bioscan 2010, 5, 411–414. [Google Scholar]
- Yun, K.W.; Choi, S. Seasonal variation in allelopathic potential of Artemisia princeps var. orientalis on plants and microbes. J. Plant Biol. 2003, 46, 105. [Google Scholar] [CrossRef]
- Barney, J.N.; Hay, A.G.; Weston, L.A. Isolation and characterization of allelopathic volatiles from mugwort (Artemisia vulgaris). J. Chem. Ecol. 2005, 31, 247–265. [Google Scholar] [CrossRef]
- Kegode, G.O.; Ciernia, M.G.; Vlieger, D.B. Allelopathic potential of Artemisia biennis (biennial wormwood). Agric. Sci. 2012, 3, 582–587. [Google Scholar] [CrossRef] [Green Version]
- Melguizo-Melguizo, D.; Diaz-de-Cerio, E.; Quirantes-Piné, R.; Švarc-Gajić, J.; Segura-Carretero, A. The potential of Artemisia vulgaris leaves as a source of antioxidant phenolic compounds. J. Funct. Foods 2014, 10, 192–200. [Google Scholar] [CrossRef]
- Wróbel, S.; Kęsy, J.; Treder, K. Effect of growth regulators and ethanol on termination of dormancy in potato tubers. Am. J. Potato Res. 2017, 94, 544–555. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lamaison, J.L.C.; Carnet, A. Teneurs en principaux flavonoids des fleurs de Crataegeus monogyna Jacq et de Crataegeus laevigata (Poiret D. C) en fonction de la vegetation. Pharm. Acta Helv. 1990, 65, 315–320. [Google Scholar] [CrossRef]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. 1. Extraction and determination of total anthocyanin in cranberries. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [Green Version]
- Vicas, S.; Laslo, V.; Pantea, S.; Bandici, G. Chlorophyll and carotenoids pigments from Mistletoe (Viscum album) leaves using different solvents. Fasc. Biol. 2010, 17, 213–218. [Google Scholar]
- Carillo, P.; Gibon, Y. Protocol: Extraction and Determination of Proline. 2011. Available online: http://prometheuswiki.org/tiki-index.php?page=Extraction+and+determination+of+proline (accessed on 14 June 2020).
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Nouman, W.; Anwar, F.; Gull, T.; Newton, A.; Rosa, E.; Domínguez-Perles, R. Profiling of polyphenolics, nutrients and antioxidant potential of germplasm’s leaves from seven cultivars of Moringa oleifera Lam. Ind. Crop. Prod. 2016, 83, 166–176. [Google Scholar] [CrossRef]
- Jafri, L.; Saleem, S.; Ullah, N.; Mirza, B. In vitro assessment of antioxidant potential and determination of polyphenolic compounds of Hedera nepalensis K. Koch. Arab. J. Chem. 2017, 10, S3699–S3706. [Google Scholar] [CrossRef] [Green Version]
- Kamali, H.; Sani, T.A.; Mohammadi, A.; Alesheikh, P.; Khodaverdi, E.; Hadizadeh, F. A comparison between pressurized hot water and pressurized liquid extraction for optimizing phenolic and antioxidants capacity of the wooden layer between of walnut seed. J. Supercrit. Fluids 2018, 133, 535–541. [Google Scholar] [CrossRef]
- Kessler, M.; Ubeaud, G.; Jung, L. Anti-and pro-oxidant activity of rutin and quercetin derivatives. J. Pharm. Pharmacol. 2003, 55, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Souri, E.; Amin, G.; Farsam, H.; Barazandeh, T.M. Screening of antioxidant activity and phenolic content of 24 medicinal plant extracts. DARU 2008, 16, 83–87. [Google Scholar]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, K.; Romanowska-Duda, Z. Positive impact of bio-stimulators on growth and physiological activity of willow in climate change conditions. Int. Agrophys. 2018, 32, 279–286. [Google Scholar] [CrossRef]
- Wadas, W.; Dziugieł, T. Changes in assimilation area and chlorophyll content of very early potato (Solanum tuberosum L.) cultivars as influenced by biostimulants. Agronomy 2020, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Blunden, G.; Jenkins, T.; Liu, Y.W. Enhanced leaf chlorophyll levels in plants treated with seaweed extract. J. Appl. Phycol. 1996, 8, 535–543. [Google Scholar] [CrossRef]
- Sharma, H.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Netto, A.T.; Campostrini, E.; de Oliveira, J.G.; Bressan-Smith, R.E. Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD-502 readings in coffee leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Jurkow, R.; Pokluda, R.; Sękara, A.; Kalisz, A. Impact of foliar application of some metal nanoparticles on antioxidant system in oakleaf lettuce seedlings. BMC Plant Biol. 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Li, J.; Liu, P.; Duan, J.; Zhao, Y.; Guo, X.; Zhang, H.; Ali, J.; Li, Z. Overexpression of OsMYB48-1, a novel MYB-related transcription factor, enhances drought and salinity tolerance in rice. PLoS ONE 2014, 9, e92913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulgari, R.; Trivellini, A.; Ferrante, A. Effects of two doses of organic extract-based biostimulant on greenhouse lettuce grown under increasing NaCl concentrations. Front. Plant Sci. 2019, 9, 1870. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.F.; Salama, K.H.A. Proline and abiotic stresses: Responses and adaptation. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020. [Google Scholar]
- Temraz, A.; El-Tantawy, W.H. Characterization of antioxidant activity of extract from Artemisia vulgaris. Pak. J. Pharm. Sci. 2008, 21, 321–326. [Google Scholar] [PubMed]
- Ertani, A.; Schiavon, M.; Altissimo, A.; Franceschi, C.; Nardi, S. Phenol-containing organic substances stimulate phenylpropanoid metabolism in Zea mays. J. Plant Nutr. Soil Sci. 2011, 174, 496–503. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szparaga, A.; Kuboń, M.; Kocira, S.; Czerwińska, E.; Pawłowska, A.; Hara, P.; Kobus, Z.; Kwaśniewski, D. Towards sustainable agriculture—Agronomic and economic effects of biostimulant use in common bean cultivation. Sustainability 2019, 11, 4575. [Google Scholar] [CrossRef] [Green Version]
- Ashkani, S.; Rafii, M.Y.; Shabanimofrad, M.; Miah, G.; Sahebi, M.; Azizi, P. Molecular breeding strategy and challenges toward improvement of blast disease resistance in rice crops. Front. Plant Sci. 2015, 6, 886. [Google Scholar] [CrossRef] [Green Version]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Matysiak, K.; Kaczmarek, S.; Kierzek, R. Effect of algae Ecklonia maxima (Kelpak SL) on winter oilseed rape. Rośliny Oleiste Oilseed Crop. 2012, 33, 81–88. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.; Colla, G. Plant-and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Hawrylak, B. Physiological reaction of cucumber (Cucumis sativus L.) to salt stress in the presence of selenium. Rocz. Akad. Rol. Pozn. 2007, 383, 483–486. [Google Scholar]
- Aslam, M.; Saeed, M.S.; Sattar, S.; Sajad, S.; Sajjad, M.; Adnan, M.; Sharif, M.T. Specific role of proline against heavy metals toxicity in plants. Int. J. Pure App. Biosci. 2017, 5, 27–34. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tadayon, M.R.; Nadeem, M.; Cheema, M.; Razmjoo, J. Proline-mediated changes in antioxidant enzymatic activities and the physiology of sugar beet under drought stress. Acta Physiol. Plant. 2019, 41, 23. [Google Scholar] [CrossRef]
- Ali, Q.; Ashraf, M.; Athar, H.U.R. Exogenously applied proline at different growth stages enhances growth of two maize cultivars grown under water deficit conditions. Pak. J. Bot. 2007, 39, 1133–1144. [Google Scholar]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef] [Green Version]
- Shahabivand, S.; Padash, A.; Aghaee, A.; Nasiri, Y.; Rezaei, P.F. Plant biostimulants (Funneliformis mosseae and humic substances) rather than chemical fertilizer improved biochemical responses in peppermint. Iran. J. Plant Physiol. 2018, 8, 2333–2344. [Google Scholar] [CrossRef]

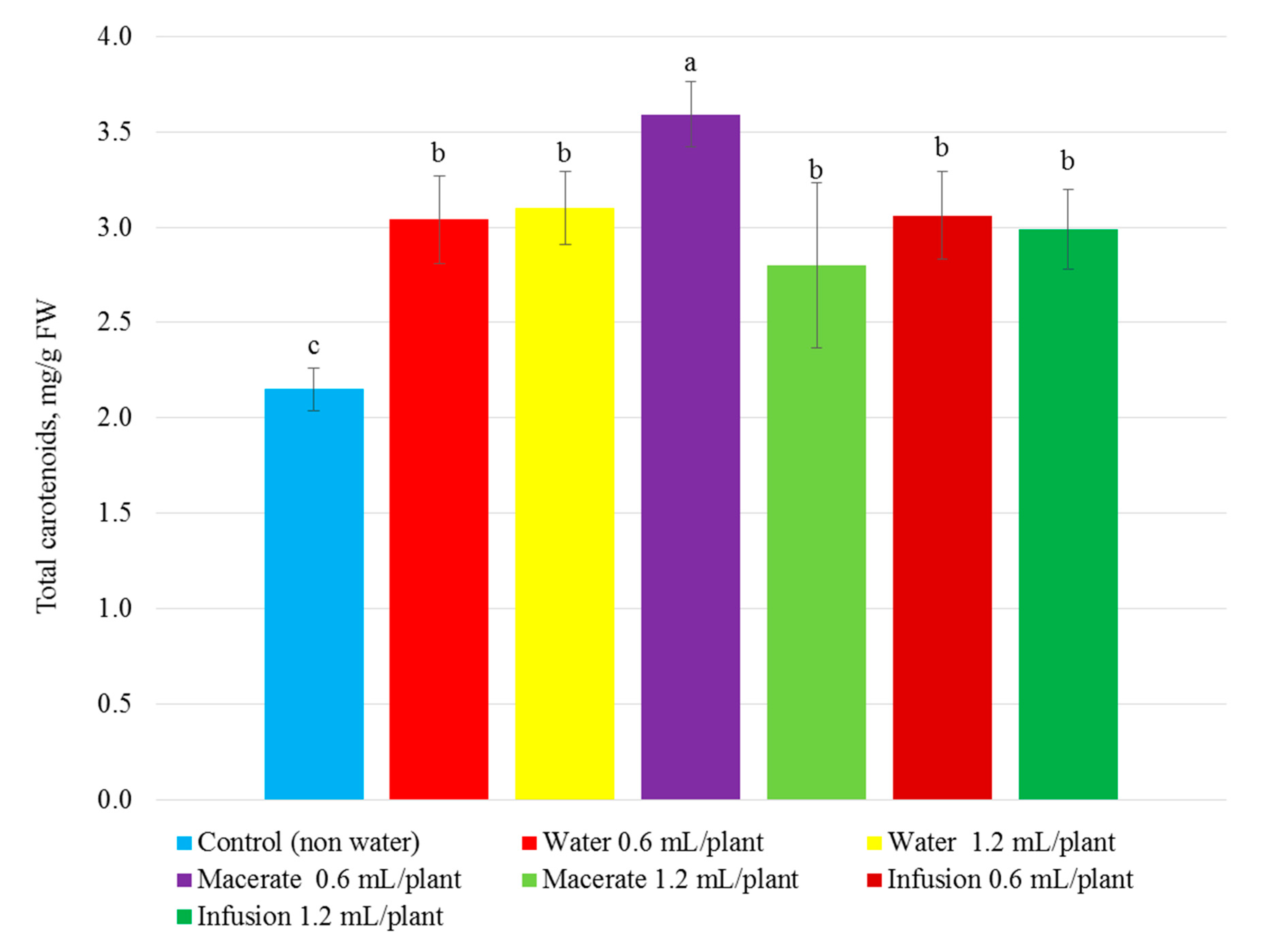
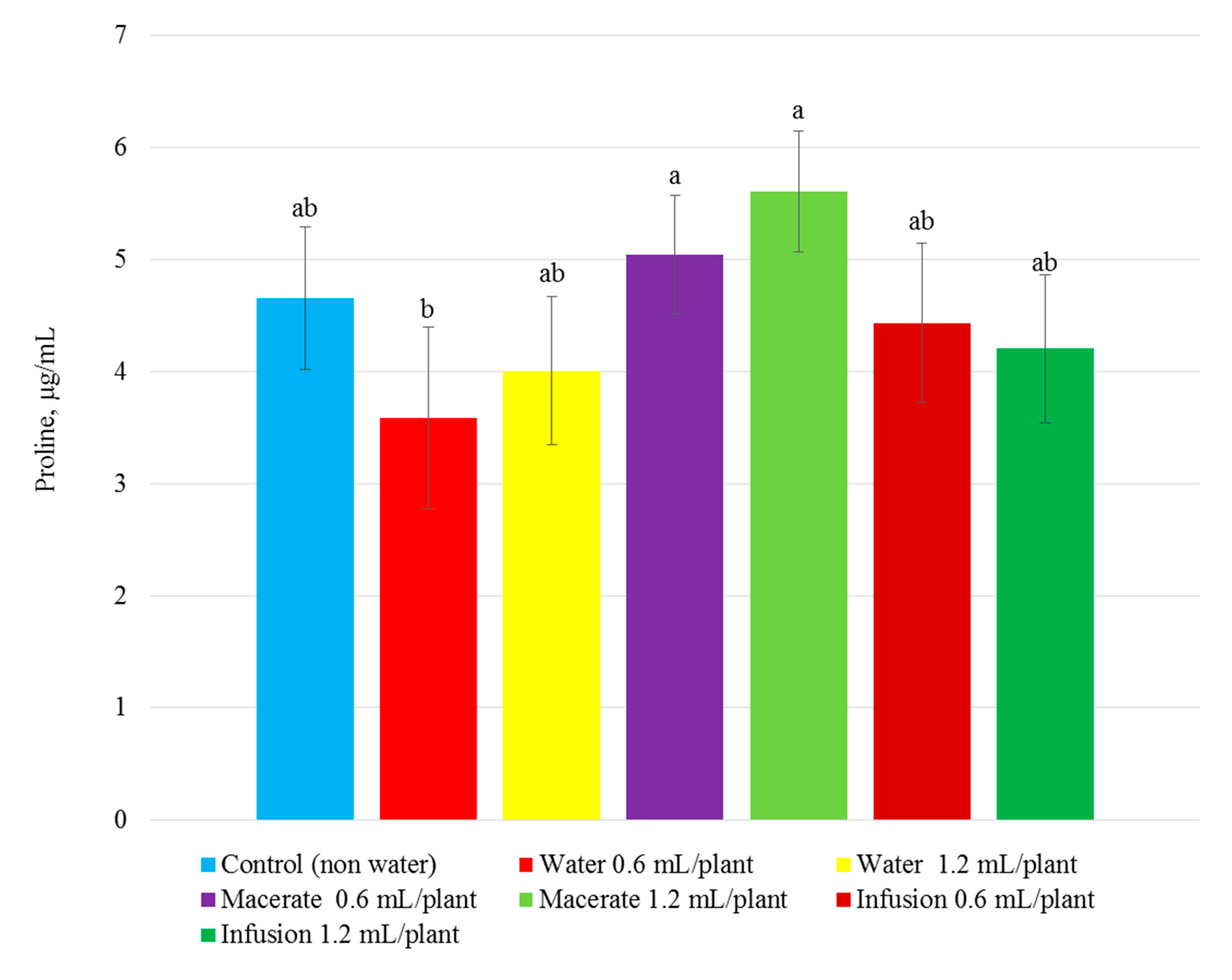
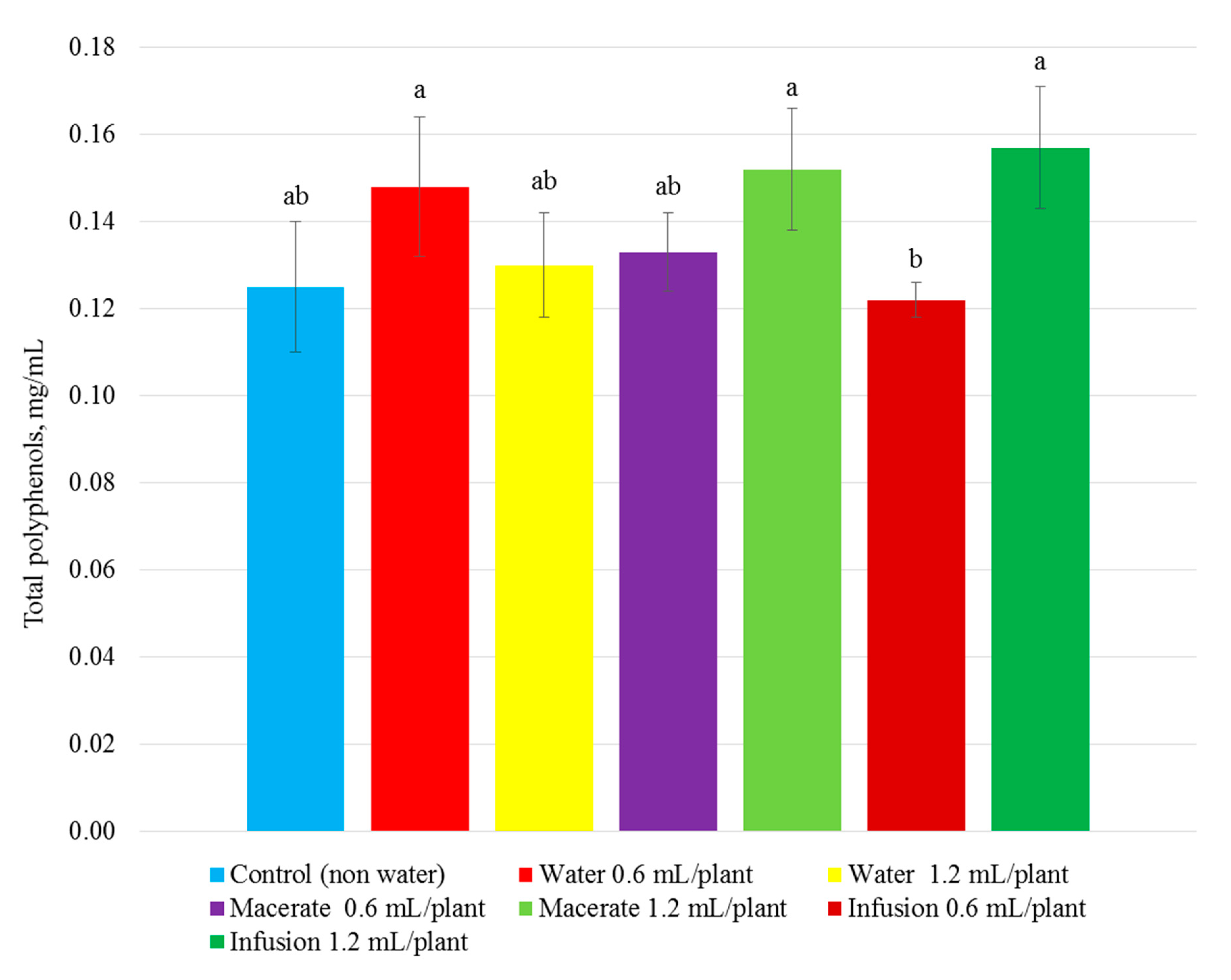
| Total Phenolic Compounds | Total Flavonoid Content | Total Content of Anthocyanins | Reducing Power | ||||
|---|---|---|---|---|---|---|---|
| mg g−1 DM | mg TE g−1 DM | ||||||
| Infusion | Macerate | Infusion | Macerate | Infusion | Macerate | Infusion | Macerate |
| 48.093 ± 0.978 | 35.274 ± 0.411 | 4.017 ± 0.024 | 16.418 ± 0.033 | nd | nd | 9.170 ± 0.053 | 6.447 ± 0.029 |
| Combination | a + b | a/b |
|---|---|---|
| µg·mL−1 | ||
| Control | 21.86 ± 1.53 b * | 2.11 ± 0.07 b |
| Water 0.6 mL·plant−1 | 22.54 ± 1.88 b | 3.17 ± 0.14 a |
| Water 1.2 mL·plant−1 | 23.12 ± 1.61 b | 3.14 ± 0.08 a |
| Macerate 0.6 mL·plant−1 | 28.46 ± 1.68 a | 2.97 ± 0.03 a |
| Macerate 1.2 mL·plant−1 | 25.27 ± 3.28 ab | 3.08 ± 0.09 a |
| Infusion 0.6 mL·plant−1 | 25.18 ± 3.13 ab | 2.93 ± 0.11 a |
| Infusion 1.2 mL·plant−1 | 25.89 ± 3.83 ab | 2.95 ± 0.28 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Findura, P.; Kocira, S.; Hara, P.; Pawłowska, A.; Szparaga, A.; Kangalov, P. Extracts from Artemisia vulgaris L. in Potato Cultivation—Preliminary Research on Biostimulating Effect. Agriculture 2020, 10, 356. https://doi.org/10.3390/agriculture10080356
Findura P, Kocira S, Hara P, Pawłowska A, Szparaga A, Kangalov P. Extracts from Artemisia vulgaris L. in Potato Cultivation—Preliminary Research on Biostimulating Effect. Agriculture. 2020; 10(8):356. https://doi.org/10.3390/agriculture10080356
Chicago/Turabian StyleFindura, Pavol, Sławomir Kocira, Patryk Hara, Anna Pawłowska, Agnieszka Szparaga, and Plamen Kangalov. 2020. "Extracts from Artemisia vulgaris L. in Potato Cultivation—Preliminary Research on Biostimulating Effect" Agriculture 10, no. 8: 356. https://doi.org/10.3390/agriculture10080356








