Abstract
The probiotic potential and antimicrobial activity of Lactiplantibacillus plantarum, Saccharomyces cerevisiae, and Bifidobacterium longum were investigated against Escherichia coli O157:H7, Salmonella typhimurium and Listeria monocytogenes. Selected strains were subjected to different acid levels (pH 2.5–6.0) and bile concentrations (1.0–3.0%). Strains were also evaluated for their antimicrobial activity by agar spot test. The potential probiotic strains tolerated pH 3.5 and above without statistically significant growth reduction. However, at pH 2.5, a significant (p < 0.05) growth reduction occurred after 1 h for L. plantarum (4.32 log CFU/mL) and B. longum (5.71 log CFU/mL). S. cerevisiae maintained steady cell counts for the entire treatment period without a statistically significant (p > 0.05) reduction (0.39 log CFU/mL). The results indicate at 3% bile concertation, 1.86 log CFU/mL reduction was observed for L. plantarum, while S. cerevisiae, and B. longum growth increased by 0.06 and 0.37 log CFU/mL, respectively. L. plantarum and B. longum demonstrated antimicrobial activity against E. coli O157:H7, S. typhimurium and L. monocytogenes. However, S. cerevisiae did not display any inhibition to any of the pathogens. The results indicate that L. plantarum and B. longum present probiotic potential for controlling E. coli O157:H7, S. and L. monocytogenes in poultry.
1. Introduction
The poultry industry is slowing turning away from antibiotics, owing to the rising public health concern over antibiotic-resistant pathogens. The imprudent use of antibiotics in the poultry industry is associated with the development of antimicrobial resistance [1,2]. Antimicrobial resistance in food animals is a major concern due to the potential dissemination of resistant pathogens to humans via the food chain [3]. The extensive use and misuse of antibiotics in animal farming have contributed to the emergence and spread of antibiotic-resistant Salmonella, Campylobacter, and Listeria monocytogenes [4,5,6]. Additionally, the use of antibiotics in animal production make food unsafe due to the accumulation of residues in edible tissue [7,8]. These residues in meats have been reported to cause allergies in hypersensitive consumers [9]. Antibiotics used at sub therapeutic doses have been restricted in many countries including the EU and USA [10,11].
The ban of antibiotics as antibiotic growth promoters (AGPs) has economically impacted the livestock production systems due to diseases [12]. Antimicrobial resistance and the ban of AGPs have driven the poultry industry to search for substitutes with comparable benefits to antibiotics [13]. Novel choices for antibiotics are necessary to satisfy the increased consumer demand for safe poultry products [14] and to mitigate antimicrobial resistance in the food chain. Probiotics have shown many beneficiary effects in poultry including growth performance [15,16], improved meat quality and flavor [17,18], and the reduction of pathogenic microorganisms [13,19].
Lactobacillus acidophilus, Lactobacillus casei, Bacillus spp., Bifidobacterium bifidum, Lactococcus lactis, and S. cerevisiae have been used as probiotics [20,21]. These strains have beneficial effects including broiler performance [22,23,24], the balance of intestinal microflora, and pathogen inhibition [24,25]. However, the effectiveness of the probiotics largely depends on their viability in the harsh gut environment of poultry [16]. Probiotics in the gastrointestinal tract are compromised by its high acidic and bile salt environment, which could ultimately reduce viable cells to utilize their desired functions [26]. Probiotics utilize their antimicrobial activity by producing antimicrobial substances including bacteriocins, organic acids, antimicrobial peptides, competitive exclusion, and modulating the host immune system [27,28,29,30,31]. Lactiplantibacillus plantarum is among the most frequently used probiotics [32].
It has been documented that bacteriocins-like compounds are responsible for the antimicrobial property of Bifidobacterium [28,33,34]. Bifidobacterium is a well known probiotic for its inhibition ability against many pathogens including Escherichia coli O157:H7, Salmonella typhimurium, L. monocytogenes, and Staphylococcus aureus [35]. Lactic acid bacteria (LAB) and yeasts have also been reported for their antimicrobial effect against many pathogens [36,37,38,39,40].
Although probiotics are promising substitutes for antibiotics, there has been several studies that report the inability of several well known probiotics to inhibit the growth of pathogens, for example, Bifidobacterium strains have failed to display antimicrobial activity against E. coli K-12 and Salmonella enterica serovar Typhimurium American Type Culture Collection (ATCC) 14,028 [41]. S. cerevisiae has also been reported not to inhibit the growth of E. coli O157:H7, S. enterica serovar Typhimurium, and Salmonella paratyphi [42]. Hence, it is important to evaluate the antimicrobial properties and ability of bacteria adaption to the host environment before their selection as probiotics. In the present study, we investigated the acid and bile tolerance of L. plantarum, S. cerevisiae, and B. longum and their ability to inhibit the growth of E. coli O157:H7, S. enterica serovar Typhimurium, and L. monocytogenes.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
In this study, L. plantarum (ATCC 8014), B. longum (ATCC 15708) and S. cerevisiae (ATCC 9763) were used as candidate probiotic strains for acid and bile tolerance studies. L. plantarum and B. longum were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and S. cerevisiae from Microbiologics Inc. (St. Cloud, MN, USA). All cultures were stored at −80 °C in 15% (v/v) glycerol and activated by two successive transfers in appropriate broth when needed. L. plantarum and B. longum were cultured in de Man Rogosa Sharpe (MRS) (Oxoid, Basingstoke, Hampshire, England) for 12–18 h at 37 °C anaerobically (; 80% nitrogen, 10% hydrogen and 10% carbon dioxide). S. cerevisiae was cultured in Sabouraud Dextrose (SB) (Becton, Dickinson and Company, Sparks, MD, USA) aerobically at 26 °C for 18 h. When needed, 100 µl each of L. plantarum and B. longum frozen culture was grown in 10 mL of MRS medium whereas S. cerevisiae was cultured in the SB medium.
2.2. Tolerance to Acidic pH Values
To test the acid tolerance levels of the strains, the method described by Jin et al. [43] was used. Briefly, 100 µL of each frozen strain was grown in 10 mL of its appropriate broth (L. plantarum and B. longum in MRS Broth and S. cerevisiae in SB broth). L. plantarum and B. longum were grown anaerobically at 37 °C for 24 h, and S. cerevisiae was grown aerobically at 26 °C for 24 h. Subsequently, 100 µL viable cultures were transferred into freshly prepared 10 mL of their respective broth and incubated for another 24 h. The cultures were centrifuged at 3000 rpm for 10 min at 4 °C, the pellets were washed twice in sterile phosphate buffered saline (PBS), pH 7.2 (Sigma, St Louis, MO, USA) and suspended in 1 mL of PBS (Sigma). For each strain, 0.1 mL of culture suspension was added separately in tubes containing 2 mL of sterile PBS at various pH ranges from 2.5 to 6.0. Hydrochloric acid (2 M) was used to adjust the desired pH values of the PBS. Tubes containing PBS with tested strains were incubated anaerobically at 37 °C for L. plantarum and B. longum and aerobically at 26 °C for S. cerevisiae. During incubation, 1 mL of each culture in PBS was taken every hour for 5 h and the viable number of bacteria were enumerated by spread plate method. Approximately 0.1 mL of diluent was spread plated on MRS agar for L. plantarum and B. longum and SB agar for S. cerevisiae. After incubation, bacterial colonies were counted and recorded. The studies were performed in three independent experiments, and each assay was performed in triplicate to calculate intra-assay variation.
2.3. Bile Tolerance Test
Bile tolerance was tested using the methods described by Jin et al. [43] and Vernazza et al. [44]. L. plantarum and Bifidobacteria longum were cultured in MRS broth (Oxoid, Basingstoke, Hampshire, England) anaerobically at 37° C for 12 h. S. cerevisiae was cultured aerobically in SB. The L. plantarum, B. longum, and S. cerevisiae stored at −80 °C were sub-cultured twice in their respective media. Then, 100 µL from each strain was inoculated into 10 mL fresh tubes of the appropriate broth containing 0–3% bile salts (Oxoid, Basingstoke, Hampshire, England). A sample from each strain was taken every hour for 6 h and subjected to serial dilutions up to 10−8 dilutions, then 100µL of the selected diluent was spread plated onto the respective agar plates to calculate the CFU/mL. After the incubation period, viable bacterial colonies were counted. The bile tolerance assay was performed in triplicate with three replications per assay.
2.4. Antimicrobial Assessment
Antibacterial activity was investigated by an agar spot test by using a colony overlay assay described by Tejero-Sarinena et al. [45]. E. coli O157:H7 (ATTC 35150), S. enterica serovar Typhimurium (ATTC 13311) and L. monocytogenes (ATTC 19115) were used as indicators of antibiotic activity. L. plantarum and B. longum were cultured overnight in MRS broth at 37 °C in the anaerobic chamber, while S. cerevisiae was cultured aerobically in SB broth at 26 °C. Then, overnight cultures (107–109 CFU/mL) of L. plantarum and B. longum were spotted (5µL) on the surface of MRS agar plates and incubated at 37 °C under anaerobic conditions for 24 h. Similarly, 5 µl of S. cerevisiae was spotted on SB agar plates and incubated at 26 °C aerobically for 24 h. After incubation, the plates were overlaid with 10 mL of 0.7% (w/v) nutrient agar, previously inoculated with 100 µL (107–109 CFU/mL) of an overnight culture of the indicator pathogen strains (E. coli O157:H7, S. enterica serovar Typhimurium and L. monocytogenes). All the plates were incubated aerobically at 37 °C for 24 h. Diameter of the zone of inhibition (ZOI) around the colony was examined and measured using a ruler. A ZOI with a diameter of 5 mm or larger was considered as positive inhibition [46]. This assay was performed in triplicates.
2.5. Statistical Analysis
Data analysis was carried out by using Duncan′s multiple range test to define the mean differences between the specific treatments. p < 0.05 was considered to indicate a significant difference. All analyses were conducted using SAS software (version 9.1, 2004; SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Acid Tolerance
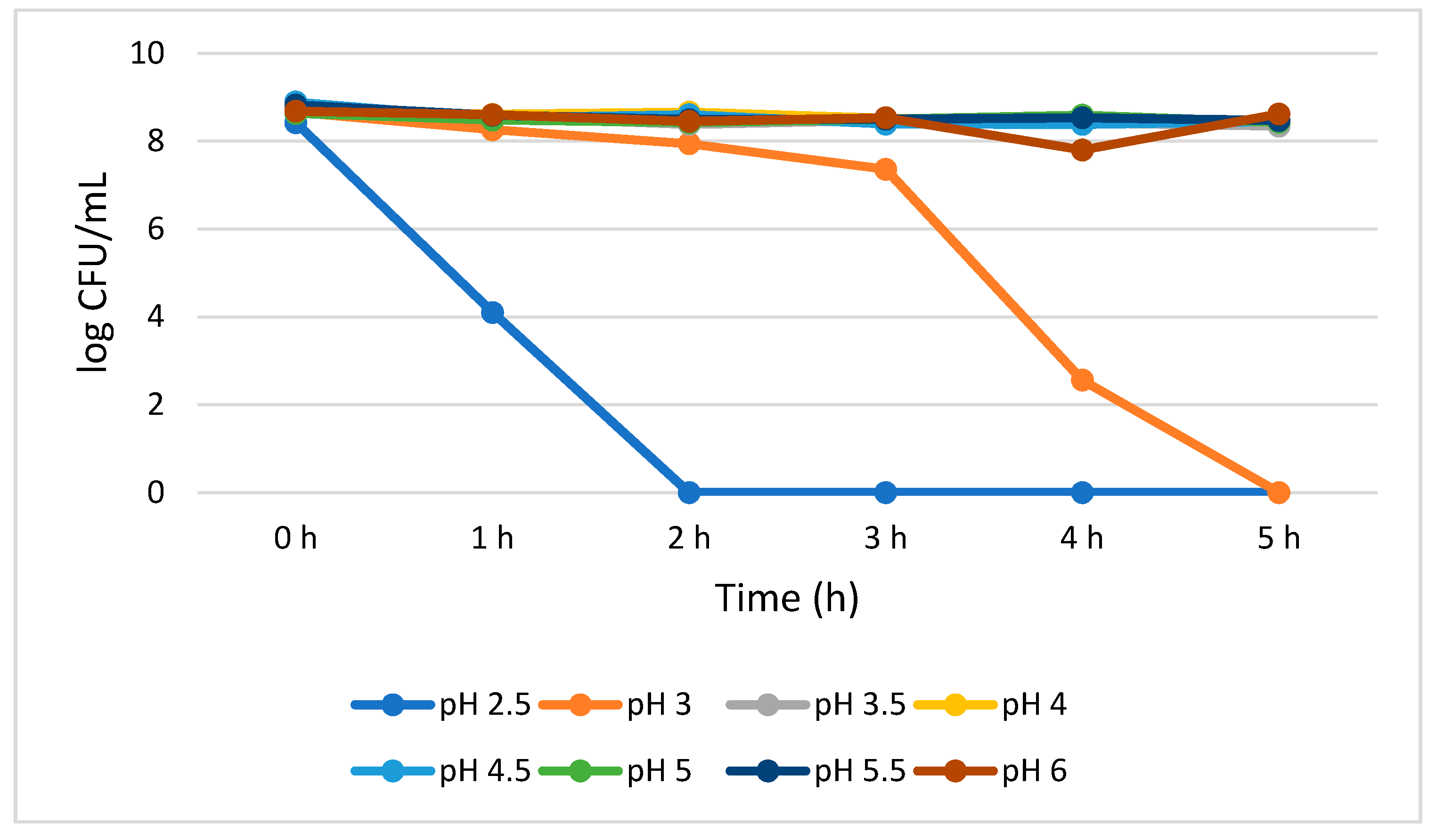
L. plantarum reduction with the progression of time (0–5 h) was not statistically significant (p > 0.05) at pH 3.5 to 6.0. The growth reduction recorded for a 5 h treatment was 0.40, 0.25, 0.44, 0.20, 0.35, 0.06 log CFU/mL at pH 3.5, 4.0, 4.5, 5.0, 5.5, and 6.0, respectively. This insignificant reduction indicates high L. plantarum viability when cultivated at 3.5 to 6.0 pH range. However, at pH 2.5, the growth of L. plantarum significantly (p < 0.05) reduced (4.32 log CFU/mL) at 1 h, and no viable cells were detected from 2 to 5 h. However, L. plantarum successfully survived for 3 h without losing a significant number of viable cells at pH 3.0 as demonstrated by 1.30 log CFU/mL growth reduction from 0 to 3 h. Eventually, at pH 3.0, statistically significant (p < 0.05) growth reduction (4.80 log CFU/mL) was observed from 3 to 4 h. No detectable L. plantarum viable cells were observed after 5 h (Figure 1).
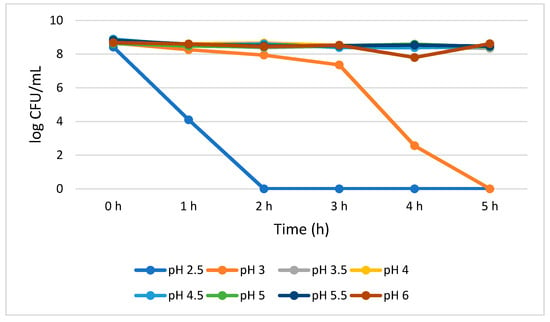
Figure 1.
Acid tolerance of L. plantarum measured at different pH ranges from 2.5 to 6.0.
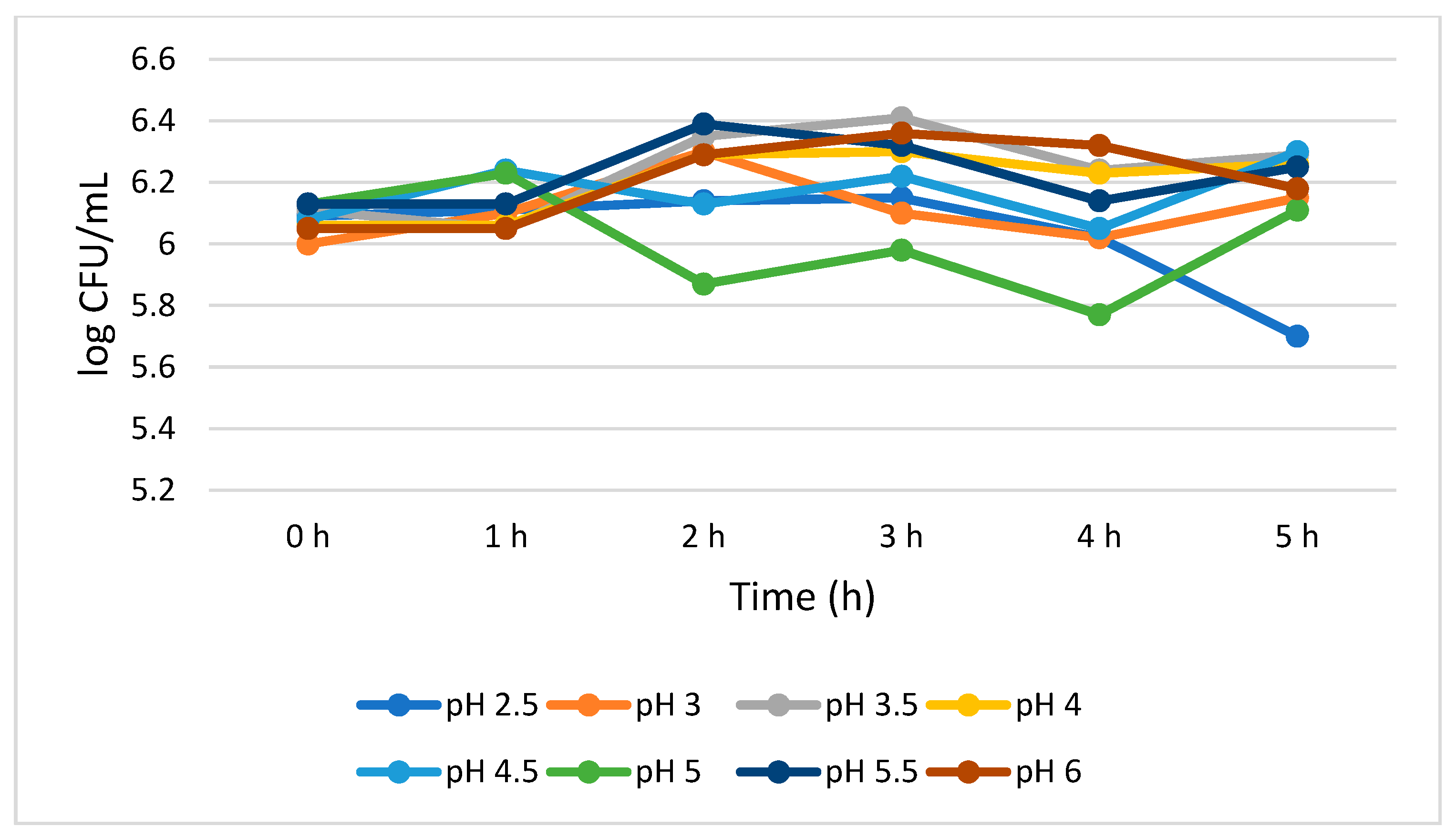
Figure 2 shows that S. cerevisiae demonstrated a better survivability than L. plantarum both at lower and higher pH values. S. cerevisiae showed consistent viability from 0 to 4 h with a 0.07 log CFU/mL growth reduction at pH 2.5. However, at pH 3.0, the growth of S. cerevisiae was insignificantly (p > 0.05) increased (0.15 log CFU/mL) during the 5 h treatment. At pH 4.0, 4.5, 5.5, and 6.0, the total viable cells was 6.26, 6.30, 6.25, and 6.18 log CFU/mL, respectively, at 5 h. At pH 5.0, a mixed trend on the total number of viable cells was observed, but overall, no significant growth reduction (0.02 log CFU/mL) was noted between 0 and 5 h.
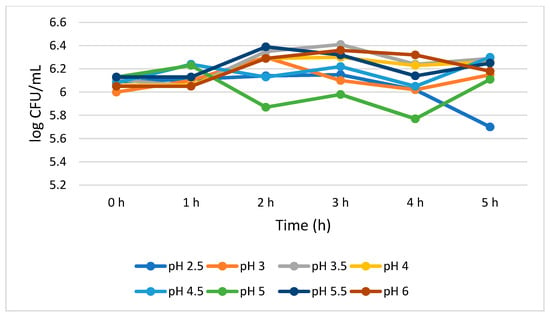
Figure 2.
Acid tolerance of S. cerevisiae measured at different pH ranges from 2.5 to 6.0.
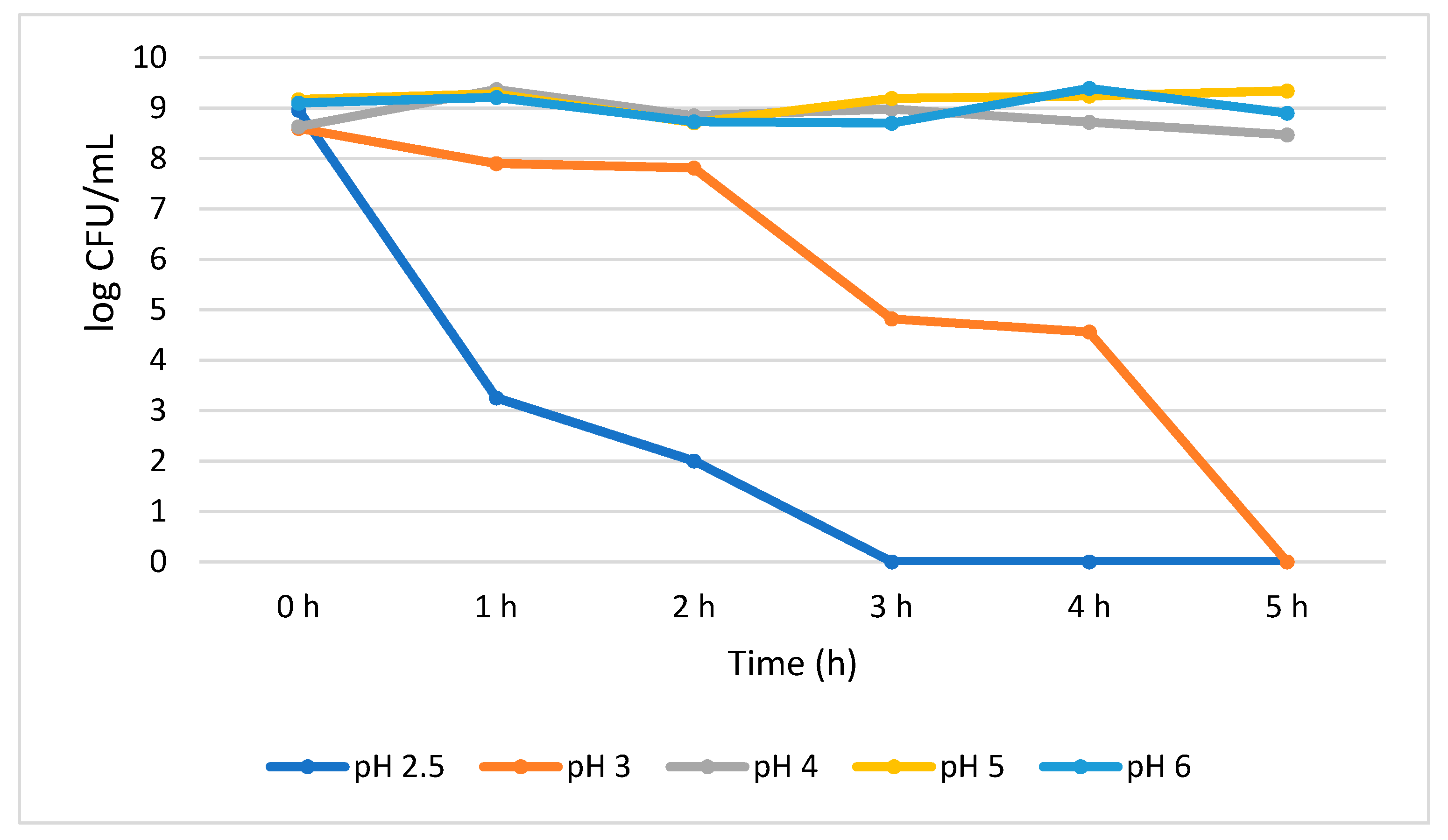
The acid tolerance data for B. longum showed a significant (p < 0.05) growth reduction (5.71 log CFU/mL) when exposed for an hour at pH 2.5. At the same pH, an additional growth reduction of 1.25 log CFU/mL was also observed between 1 h and 2 h; however, from 3 h no viable cells were detected. At pH 3.0, the growth of the B. longum insignificantly decreased (0.70 log CFU/mL) from 0 h to 2 h, but suddenly significantly (p < 0.05) decreased by 2.99 log CFU/mL at 3 h, and no viable cells were detected at 5 h. Our results indicate that B. longum showed better stability at higher pH than lower pH environment. For example, after 5 h treatment at pH 4.0, 5.0, and 6.0, a steady number of population was observed and recorded as 8.47, 9.34, and 8.90 log CFU/mL, respectively, with no significant reduction in viable cells (Figure 3).
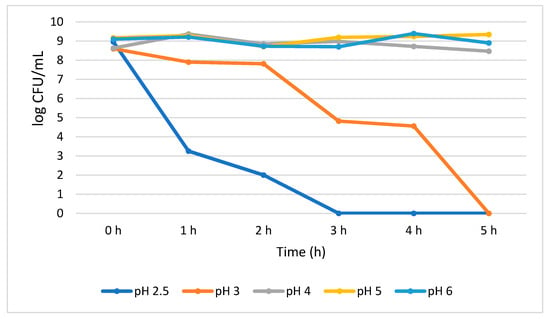
Figure 3.
Acid tolerance of B. longum measured at different pH ranges from 2.5 to 6.0.
3.2. Bile Tolerance
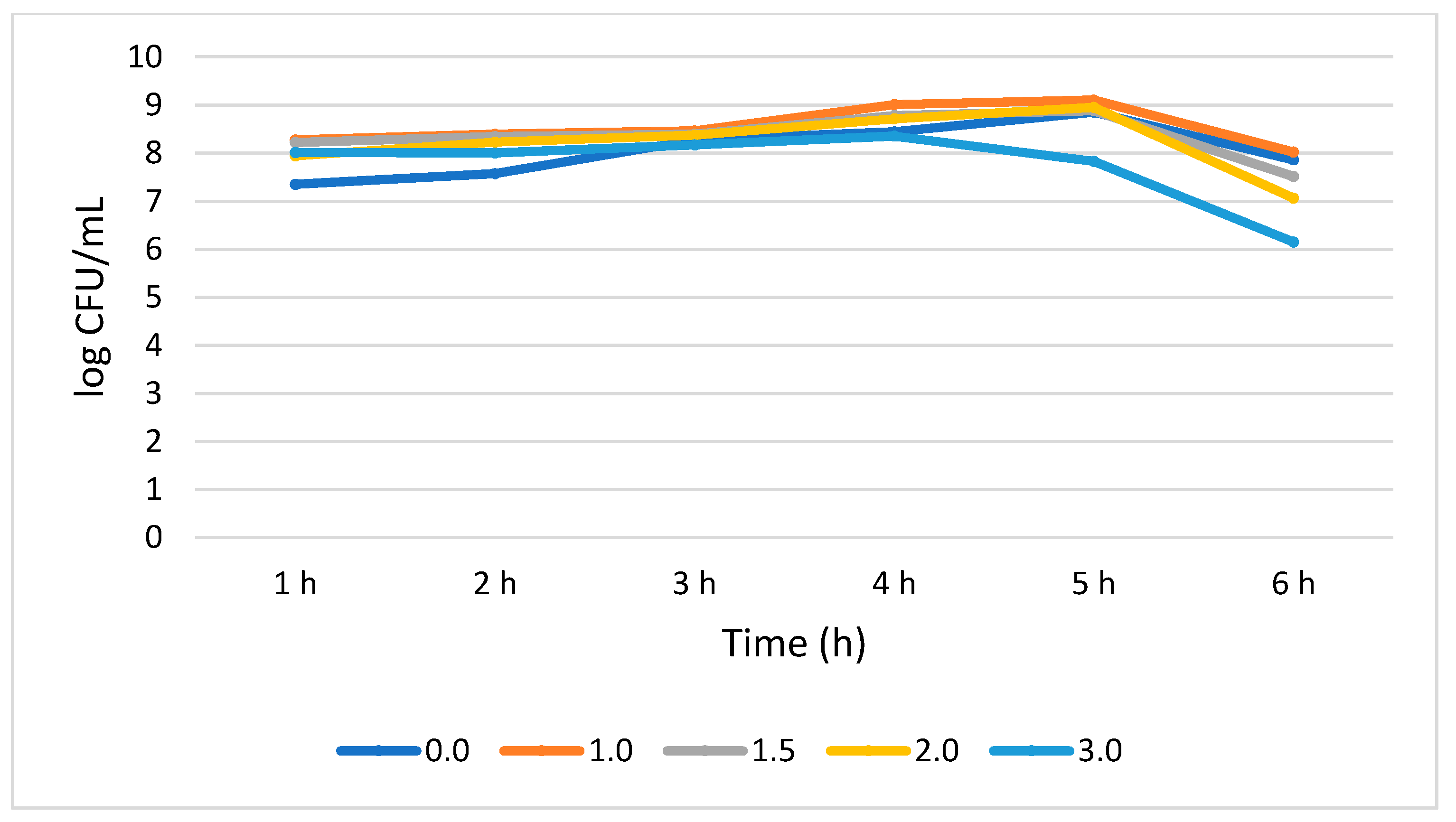
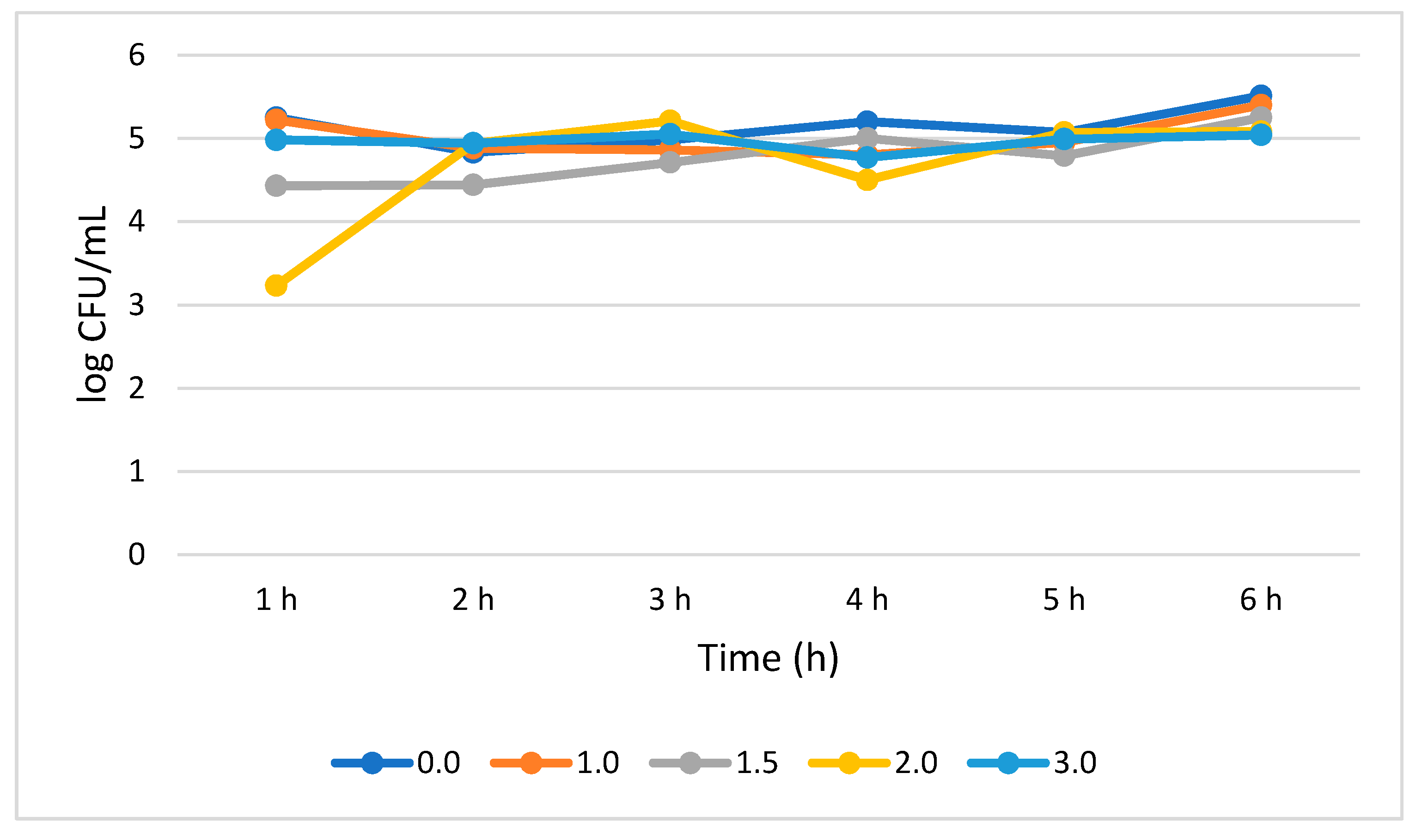
Figure 4 shows that L. plantarum tolerated different concentrations of bile salt without compromising its growth during the 6 h treatment period. A 0.51 log CFU/mL growth increase (p > 0.05) was observed for the bile salt control (0%) at 6 h, while a not statistically significant (0.05) growth reduction of 0.25, 0.71, 0.88, and 1.86 log CFU/mL was recorded at 1.0%, 1.5%, 2.0, and 3.0% bile concentrations, respectively. Interestingly, in all the tested bile concentrations, the highest number of viable cells was observed at 5 h while the lowest was observed at 6 h.
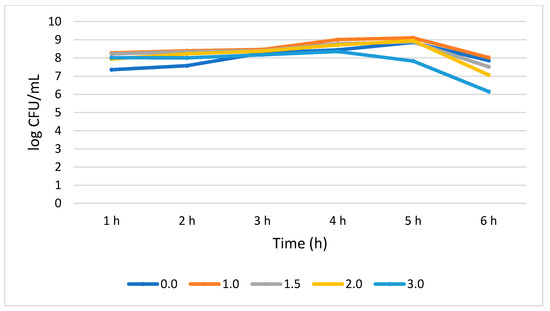
Figure 4.
Bile tolerance of L. plantarum measured at different concentrations ranges from 0.0 (negative control) to 3.0%.
S. cerevisiae′s tolerance to bile salt was very similar to what was displayed in the case of L. plantarum. No statistically significant (p > 0.05) change in the growth rate was noticed from 1 to 6 h of treatment at different bile concentrations, rather an insignificant increase in growth rate was observed. The growth rate at 1.0, 1.5, and 3.0% bile concentrations was increased by 0.18, 0.82, and 0.06 log CFU/mL, respectively, from 1 to 6 h. However, at 2% bile concentration, the growth rate statistically significantly (p < 0.05) increased by 1.71 log CFU/mL from 1 to 2 h, and steadily increased (p > 0.05) by 0.27 log CFU/mL from 2 to 3 h. A sudden decrease of 0.71 log CFU/mL occurred at 4 h. Generally, a significant (p < 0.05) growth increase (1.85 log CFU/mL) was observed for S. cerevisiae when subjected for 6 h at 2% bile (Figure 5).
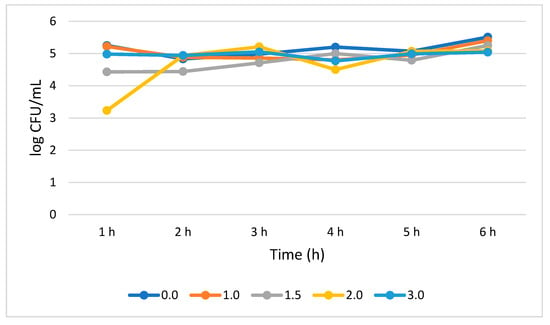
Figure 5.
Bile tolerance of S. cerevisiae measured at different concentration ranges from 0.0 (negative control) to 3.0%.
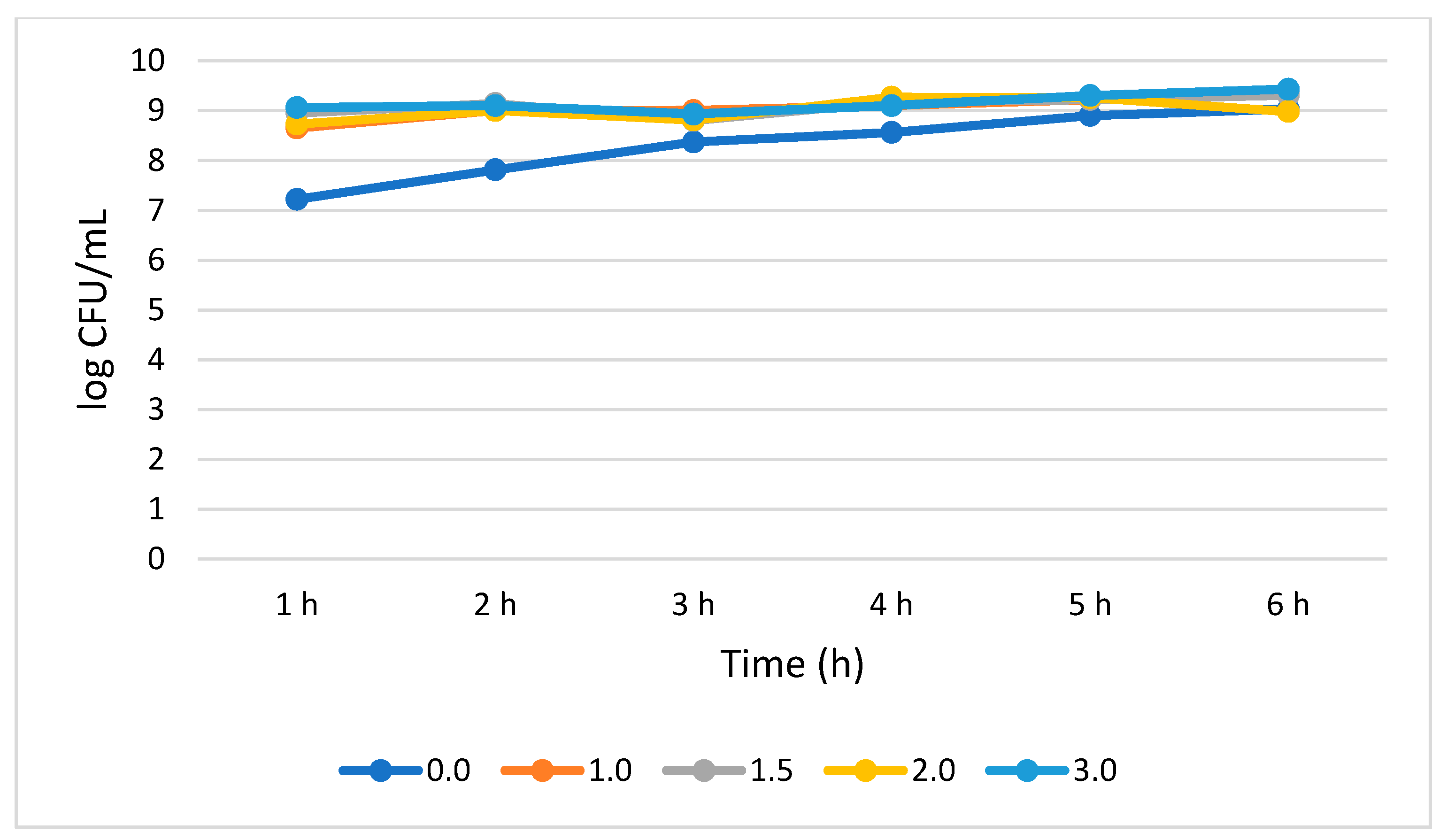
B. longum tolerated and survived all the bile concentration. S. cerevisiae showed no statistically significant (p > 0.05) growth increase rates across the subjected 6 h period. B. longum demonstrated a 0.72, 0.36, 0.25, and 0.37 log CFU/mL growth increase at 1.0, 1.5, 2.0, and 3.0% bile concentrations, respectively, from 1 to 6 h. B. longum showed better survivability at 3.0% bile concentration as demonstrated by a total population of 9.43 log CFU/mL at 6 h. (Figure 6).
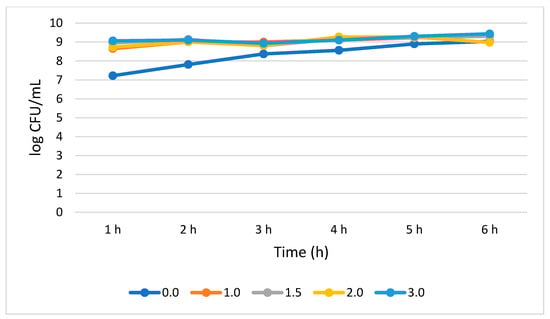
Figure 6.
Bile tolerance of B. longum measured at different concentrations ranges from 0.0 (negative control) to 3.0%.
3.3. Antimicrobial Effect of L. plantarum, B. longum, and S. cerevisiae
The spot test displayed antimicrobial activity for the L. plantarum and B. longum results (Table 1). L. plantarum and B. longum showed an inhibitory effect for E. coli O157:H7, S. typhimurium, and L. monocytogenes. However, S. cerevisiae showed no inhibitory effect for all three pathogens. L. plantarum depicted the largest ZOI for all three pathogens compared to B. longum. The ZOI recorded against E. coli O157:H7 was 31.2 and 19.8 mm for L. plantarum and B. longum, respectively. Similarly, L. plantarum showed a 29.7 and 15 mm ZOI against S. typhimurium and L. monocytogenes, respectively. B. longum showed a 15.5 and 11.4 mm ZOI against S. typhimurium and L. monocytogenes, respectively. Both the L. plantarum and B. longum demonstrated a maximum antimicrobial activity against E. coli O157:H7, followed by, S. typhimurium, and L. monocytogenes.

Table 1.
Antimicrobial activity of the probiotic strains determined by agar spot test.
4. Discussion
Wang et al. [47], in agreement with our study, reported an increased L. plantarum cell viability with increased pH. According to their study, the survival rate of L. plantarum was 55.95% (18.80 × 107 cells/mL) and 18% (6.0 × 107 cells/mL) at pH 3.0 and 2.0, respectively. In this study, L. plantarum showed better survivability at a higher pH (3.5–6.0) than at a lower pH (2.5–3.0). At pH 3.5–6.0, L. plantarum survived after 5 h with an insignificant growth reduction of 0.06–0.44 log CFU/mL. On the other hand, at pH 2.5, the growth rate was significantly (p < 0.05) reduced by 4.32 log CFU/mL at 1 h. A similar study conducted by Anderson et al. [48] also reported a 6–7 log reduction of L. plantarum DSM 2648 and Lactiplantibacillus rhamnosus HN001 when challenged at pH 2.0 for 4 h, while no cell viability was observed at pH 4.0. However, in our study, no L. plantarum viable cells were detected after 2 h of incubation at pH 2.5. In contrast, Giri et al. [49] reported 5.8 log CFU/mL and 7.1 log CFU/mL viable L. plantarum L7 strain at pH 2 and 3, respectively. There has been numerous studies that reported the survival of L. plantarum at pH 2.0–2.5 for 2–6 h [50,51,52].
S. cerevisiae showed tremendous tolerance when challenged to a broad range of pH, ranging from 2.5–6.0. Similarly to our current study, the ability of S. cerevisiae to survive in a broad range of pH (up to pH 10) was also reported by Khisti et al. [53]. To be an effective probiotic, S. cerevisiae should be capable of withstanding a low pH. Our study demonstrated the successful growth of S. cerevisiae at pH 2.5 with a total population of 5.70 log CFU/mL at 5 h. Several studies have also reported the survival of S. cerevisiae at a low pH, for example, Van der Aa Kühle et al. [54] and Pennacchia et al. [55] reported the survival of S. cerevisiae at pH 2.5 for 4 and 2.5 h (6.52–7.66 log CFU/mL), respectively. However, a decrease in S. cerevisiae cell counts was observed by Moradi et al. [56] at pH 1.5 and 2.0.
In this study, B. longum demonstrated a sensitivity to a lower pH (2.5 and 3.0), but also showed stability at a higher pH (3.5 and above). A previous study showed that after 1 h exposure at pH 2.5, the growth of B. longum had reduced by 3.75 log CFU/mL [57], while our study showed a 5.71 log CFU/mL growth reduction. Similarly, there have been several studies in which B. longum showed reduced growth at low pH. For example, Ashraf and Smith [58] reported 40% growth reduction at pH 3.0 for 3 h, Ding and Shah [59] reported a reduced population of 3.29 log CFU/mL at pH 2.0 after 2 h. However, a wide range of acid tolerance by B. longum was observed by Vernazza et al. [44]. Although many studies reported the sensitivity of B. longum at a lower pH, its acid tolerance could be improved by temporary acid stress as evidenced by many studies [51,60].
Our results display that L. plantarum survived different concentrations of bile ranging from 1.0–3.0% without a significant growth reduction (0.25–1.86 log CFU/mL). The findings of this study agree with the outcomes of several studies that showed L. plantarum could survive in a wide range of bile concentrations (0.05–4.0) from 3 h to >24 h with up to 100% survival rate [49,61]. Jiang et al. [51] reported a 1.17 log CFU/mL reduction of L. plantarum at 0.45% bile, while our study showed a 0.25 log CFU/mL reduction at 1.0% bile. However, several studies have reported a significant reduction in viable cells with increasing bile concentrations. For example, Wang et al. [47] reported a significant decrease from 19.50 × 107 to 10.88 × 107 CFU/mL of L. plantarum B1 with an increase in bile concentration from 0.1% to 0.5%. Similarly, the viability of the L. plantarum DSM2648 was reduced by 2 log units when the bile concentration increased from 0.5% to 1% [48].
In the current study, the viable population decreased from 5.51 to 5.04 log CFU/mL upon the increase in bile concentration from 1.0% to 3.0%. Similarly, Khisti et al. [53], Van der Aa Kühle et al. [54], and Agarwal et al. [62] reported the survivability of S. cerevisiae at 1.2, 0.3, and 0.9% bile concentrations, respectively. Meanwhile, some other researchers also reported the tolerance of S. cerevisiae in bile concentration ranging from 0.5 to 1.0% [42,63].
Bifidobacterium spp. are well known for their probiotic properties. Interestingly, this study showed an insignificant increase in Bifidobacterium growth ranging from 0.25 to 0.72 log CFU/mL when treated in 1.0–3.0% of bile for 6 h. Although we did not observe any significant growth reduction with increasing bile concentration, there have been several studies that reported a reduction of 30–80% when bile concentrations were increased from 0–3.0% [60,64]. Many researchers reported the tolerance of B. longum at varying concentrations of bile. Ashraf and Smith [58] reported a 1–2 log reduction of B. longum when treated in 2% bile salt for 12 h, while a sharp decrease of 5.4 log CFU/mL just after 5 min of exposure at 1% bile was observed in another study [57]. Supplementary materials are indicated in Table S1: Survival of L. plantarum, S. cerevisiae, and B. longum at different pH levels and Table S2: Survival of L. plantarum, S. cerevisiae, and B. longum at different bile concentrations.
Antimicrobial property is considered as an important characteristic of probiotics. A zone of inhibition (ZOI) with a diameter of 5 mm or larger was reflected as positive inhibition in our study. L. plantarum ZOI measured 15.0 mm, 31.2, and 29.7 for L. monocytogenes, E. coli O157: H7, and S. typhimurium, respectively. Indeed, previous studies have reported L. plantarum′s ability to inhibit L. monocytogenes and E. coli O157:H7 with a ZOI from 13.0 to 14.1 mm and from 10.0 to 16.5 mm, respectively [36,65]. A Polish study agrees with our study and reported a ZOI (18.27 ± 2.70 mm) for L. monocytogenes [38]. Similarly, there have been numerous studies which observed the positive inhibition by L. plantarum against significant pathogens [66,67]. It has been reported that many antimicrobial agents, for example, bacteriocins, organic acids, antimicrobial peptides, and hydrogen peroxide attribute the antimicrobial property of L. plantarum [27,68].
Numerous studies have shown that B. longum contains antagonistic property against major pathogens including both Gram-positive and Gram-negative bacteria [45]. Our study displayed the ZOI for B. longum in the range of 11.4–19.8 mm. B. longum has demonstrated strong inhibition against multidrug-resistant E. coli with a ZOI ranging from 11.77 to 23.10 mm [40] and against Staphylococcus aureus, E. coli O157: H7, and L. monocytogenes with ZOI of 11–17 mm [37]. However, Lahtinen et al. [41] reported 38 Bifidobacterium strains that showed no antimicrobial property against E. coli K-12 and S. enterica serovar Typhimurium ATCC 14028. It has been documented that bacteriocins or bacteriocin-like compounds produced by the members of the Bifidobacterium genus are well known for their antimicrobial properties against many pathogens [28,33,34].
In this study, S. cerevisiae showed no antagonistic effects (0 mm) against the tested pathogens. The inability of S. cerevisiae to inhibit the growth of E. coli O157:H7 and S. typhimurium as observed in the current study is in accordance with previous work by Srinivas et al. [42], where S. cerevisiae did not show any antagonistic activity towards E. coli O157:H7, S. typhimurium and Salmonella paratyphi. However, Khidhr and Zubaidy [69] reported a positive inhibition by S. cerevisiae var. boulardii against S. enterica with a 16 mm ZOI. Similarly, the antimicrobial property of S. cerevisiae against S. aureus and L. monocytogenes [39] and the reduction of the Salmonella load in chickens’ caecum [70] has also been reported.
5. Conclusions
S. cerevisiae showed better acid and bile tolerance in vitro than L. plantarum and B. longum. However, S. cerevisiae did not show any antimicrobial activity against E. coli O157:H7, S. typhimurium, and L. monocytogenes. L. plantarum and B. longum showed a strong inhibition against Gram-negative and Gram-positive bacteria. Our study suggests that S. cerevisiae, L. plantarum, and B. longum are potential probiotics which can be applied as an alternate for antibiotics in poultry production. Consumers′ awareness of antimicrobial-resistant foodborne pathogen in poultry products has driven the demand for chicken raised without antibiotics (RWA). Hence, L. plantarum, S. cerevisiae and B. longum can be an alternative to antibiotics in the control of bacterial infections in poultry.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0472/10/9/368/s1, Table S1: Survival of L. plantarum, S. cerevisiae, and B. longum at different pH levels as determined by viable cell count, Table S2: Survival of L. plantarum, S. cerevisiae, and B. longum at different bile concentrations as determined by viable cell count.
Author Contributions
Conceptualization, J.I. and A.K.-N.; methodology, J.I., A.K.-N., S.N.N.; validation, J.I.; formal analysis, J.I. and A.K.-N.; investigation, J.I. and A.K.-N.; resources, A.K.-N.; writing—original draft preparation, J.I.; writing—review and editing, J.I., A.K.-N., S.N.N., A.I.M., M.N.; visualization, J.I. and A.K.-N.; supervision, A.K.-N.; project administration, A.K.-N.; funding acquisition, A.K.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by United States Department of Agriculture/National Institute of Food and Agriculture. Grant No. Accession No: 1014615; Project: TENX-1813-FS.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Kumar, D.; Pornsukarom, S.; Thakur, S. Antibiotic Usage in Poultry Production and Antimicrobial-Resistant Salmonella in Poultry. In Food Safety in Poultry Meat Production; Springer: Heidelberg/Berlin, Germany, 2019; pp. 47–66. [Google Scholar]
- Agyare, C.; Boamah, V.E.; Zumbi, C.N.; Osei, F.B. Antibiotic use in poultry production and its effects on bacterial resistance. In Antimicrobial Resistance-A Global Threat; Intech Open: London, UK, 2018. [Google Scholar]
- Topp, E. Agriculture and Agri-Food Canada′s research program on antimicrobial resistance. Can. Commun. Dis. Rep. 2017, 43, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, Y.; Shen, J.; Zhang, Q.; Wu, C. Tracking Campylobacter contamination along a broiler chicken production chain from the farm level to retail in China. Int. J. Food Microbiol. 2014, 181, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ishola, O.; Mosugu, J.; Adesokan, H. Prevalence and antibiotic susceptibility profiles of Listeria monocytogenes contamination of chicken flocks and meat in Oyo State, south-western Nigeria: Public health implications. J. Prev. Med. Hyg. 2016, 57, E157–E163. [Google Scholar] [PubMed]
- Mukerji, S.; O’Dea, M.; Barton, M.; Kirkwood, R.; Lee, T.; Abraham, S. Development and transmission of antimicrobial resistance among Gram-negative bacteria in animals and their public health impact. Essays Biochem. 2017, 61, 23–35. [Google Scholar] [PubMed]
- Wang, H.; Ren, L.; Yu, X.; Hu, J.; Chen, Y.; He, G.; Jiang, Q. Antibiotic residues in meat, milk and aquatic products in Shanghai and human exposure assessment. Food Control 2017, 80, 217–225. [Google Scholar] [CrossRef]
- Jammoul, A.; El Darra, N. Evaluation of antibiotics residues in chicken meat samples in Lebanon. Antibiotics 2019, 8, 69. [Google Scholar] [CrossRef]
- Marazuela, M.; Bogialli, S. A review of novel strategies of sample preparation for the determination of antibacterial residues in foodstuffs using liquid chromatography-based analytical methods. Anal. Chim. Acta. 2009, 645, 5–17. [Google Scholar] [CrossRef]
- Ban on Antibiotics as Growth Promoters in Animal Feed Enters into Effect. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_05_1687 (accessed on 15 July 2020).
- U.S. Bans Antibiotics Use for Enhancing Growth in Livestock. Available online: https://www.accessscience.com/content/u-s-bans-antibiotics-use-for-enhancing-growth-in-livestock/BR0125171 (accessed on 20 June 2020).
- Laxminarayan, R.; Van Boeckel, T.; Teillant, A. The Economic Costs of Withdrawing Antimicrobial Growth Promoters from the Livestock Sector. OECD Food; Agriculture and Fisheries Papers, No. 78; OECD Publishing: Paris, France, 2015; Volume 78. [Google Scholar]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Hargis, B.M.; Tellez, G. The Use of Probiotics in Poultry Production for the Control of Bacterial Infections and Aflatoxins. In Prebiotics and Probiotics-Potential Benefits in Human Nutrition and Health; IntechOpen: London, UK, 2019. [Google Scholar]
- OECD-FAO. OECD-FAO Agricultural Outlook 2018–2027; OECD Publishing: Paris, France, 2018. [Google Scholar]
- Alagawany, M.; El-Hack, M.E.A.; Farag, M.R.; Sachan, S.; Karthik, K.; Dhama, K. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ. Sci. Pollut. Res. Int. 2018, 25, 10611–10618. [Google Scholar] [CrossRef]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Zhong, H.; Li, N.; Xu, H.; Zhu, Q.; Liu, Y. Effect of probiotics on the meat flavour and gut microbiota of chicken. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Popova, T. Effect of probiotics in poultry for improving meat quality. Curr. Opin. Food Sci. 2017, 14, 72–77. [Google Scholar] [CrossRef]
- Kumar, S.; Chen, C.; Indugu, N.; Werlang, G.O.; Singh, M.; Kim, W.K.; Thippareddi, H. Effect of antibiotic withdrawal in feed on chicken gut microbial dynamics, immunity, growth performance and prevalence of foodborne pathogens. PLoS ONE 2018, 13, e0192450. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An overview of beneficial effects. In Lactic Acid Bacteria: Genetics, Metabolism and Applications; Springer: Heidelberg/Berlin, Germany, 2002; pp. 279–289. [Google Scholar]
- Salamoura, C.; Kontogianni, A.; Katsipi, D.; Kandylis, P.; Varzakas, T. Probiotic fermented milks made of cow′s milk, goat′s milk and their mixture. J. Biotechnol. 2014, 185, 125. [Google Scholar] [CrossRef]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Mountzouris, K.; Tsirtsikos, P.; Kalamara, E.; Nitsch, S.; Schatzmayr, G.; Fegeros, K. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 2007, 86, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S. The role of probiotics in the poultry industry. Int. J. Mol. Sci. 2009, 10, 3531–3546. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Higgins, S.; Vicente, J.; Wolfenden, A.; Tellez, G.; Hargis, B. Temporal effects of lactic acid bacteria probiotic culture on Salmonella in neonatal broilers. Poult. Sci. 2007, 86, 1662–1666. [Google Scholar] [CrossRef]
- Haben Fesseha, M. Probiotics and Its Potential Role in Poultry Production: A Review. Vet. Med. 2019, 4, 69–76. [Google Scholar]
- Cortés-Zavaleta, O.; López-Malo, A.; Hernández-Mendoza, A.; García, H. Antifungal activity of lactobacilli and its relationship with 3-phenyllactic acid production. Int. J. Food Microbiol. 2014, 173, 30–35. [Google Scholar] [CrossRef]
- Drider, D.; Bendali, F.; Naghmouchi, K.; Chikindas, M.L. Bacteriocins: Not only antibacterial agents. Probiotics Antimicro. Prot. 2016, 8, 177–182. [Google Scholar] [CrossRef]
- Saxelin, M.; Tynkkynen, S.; Mattila-Sandholm, T.; de Vos, W.M. Probiotic and other functional microbes: From markets to mechanisms. Curr. Opin. Biotechnol. 2005, 16, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Ratsep, M.; Naaber, P.; Koljalg, S.; Smidt, I.; Shkut, E.; Sepp, E. Effect of L. plantarum strains on clinical isolates of Clostridium difficile in vitro. J. Probiotics Health 2014, 2, 1–5. [Google Scholar] [CrossRef]
- Millette, M.; Luquet, F.; Lacroix, M. In vitro growth control of selected pathogens by Lactobacillus acidophilus-and Lactobacillus casei-fermented milk. Lett. Appl. Microbiol. 2007, 44, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, Y.; Yu, L.; Wang, J.; Huang, M.; Zhu, N. Effects of L. plantarum on intestinal integrity and immune responses of egg-laying chickens infected with Clostridium perfringens under the free-range or the specific pathogen free environment. BMC Vet. Res. 2020, 16, 47. [Google Scholar] [CrossRef]
- Cheikhyoussef, A.; Pogori, N.; Chen, W.; Zhang, H. Antimicrobial proteinaceous compounds obtained from bifidobacteria: From production to their application. Int. J. Food Microbiol. 2008, 125, 215–222. [Google Scholar] [CrossRef]
- Noordiana, N.; Fatimah, A.; Mun, A. Antibacterial agents produced by lactic acid bacteria isolated from Threadfin Salmon and Grass Shrimp. Int. Food Res. J. 2013, 20, 117–124. [Google Scholar]
- Zinedine, A.; Faid, M. Isolation and characterization of strains of Bifidobacteria with probiotic proprieties in vitro. World J. Dairy Food Sci. 2007, 2, 28–34. [Google Scholar]
- Arena, M.P.; Silvain, A.; Normanno, G.; Grieco, F.; Drider, D.; Spano, G.; Fiocco, D. Use of L. plantarum strains as a bio-control strategy against food-borne pathogenic microorganisms. Front Microbiol. 2016, 7, 464. [Google Scholar] [CrossRef]
- EL KHOLY, M.; EL SHINAWY, S.; Meshref, A.; Korny, A. Screening of antagonistic activity of probiotic bacteria against some food-borne pathogens. J. Food Biosci. Sci. Technol. 2014, 4, 1–14. [Google Scholar]
- Ołdak, A.; Zielińska, D.; Rzepkowska, A.; Kołożyn-Krajewska, D. Comparison of antibacterial activity of L. plantarum strains isolated from two different kinds of regional cheeses from Poland: Oscypek and Korycinski Cheese. Biomed Res. Int. 2017, 2, 1–10. [Google Scholar] [CrossRef]
- Sim, K.Y.; Yee, C.F.; Anton, A. Probiotic potential and antimicrobial activities of micro-organisms isolated from an indigenous fish sauce. Borneo Sci. 2016, 31. [Google Scholar]
- Abdelhamid, A.G.; Esaam, A.; Hazaa, M.M. Cell free preparations of probiotics exerted antibacterial and antibiofilm activities against multidrug resistant E. coli. Saudi Pharm. J. 2018, 26, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, S.J.; Jalonen, L.; Ouwehand, A.C.; Salminen, S.J. Specific Bifidobacterium strains isolated from elderly subjects inhibit growth of Staphylococcus aureus. Int. J. Food Microbiol. 2007, 117, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, B.; Rani, G.S.; Kumar, B.K.; Chandrasekhar, B.; Krishna, K.V.; Devi, T.A.; Bhima, B. Evaluating the probiotic and therapeutic potentials of S. cerevisiae strain (OBS2) isolated from fermented nectar of toddy palm. AMB Express 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Ho, Y.; Abdullah, N.; Jalaludin, S. Acid and bile tolerance of Lactobacillus isolated from chicken intestine. Lett. Appl. Microbiol. 1998, 27, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Vernazza, C.L.; Gibson, G.R.; Rastall, R.A. Carbohydrate preference, acid tolerance and bile tolerance in five strains of Bifidobacterium. J. Appl. Microbiol. 2006, 100, 846–853. [Google Scholar] [CrossRef]
- Tejero-Sariñena, S.; Barlow, J.; Costabile, A.; Gibson, G.R.; Rowland, I. Antipathogenic activity of probiotics against S. typhimurium and Clostridium difficile in anaerobic batch culture systems: Is it due to synergies in probiotic mixtures or the specificity of single strains? Anaerobe 2013, 24, 60–65. [Google Scholar] [CrossRef]
- Kizerwetter-Swida, M.; Binek, M. Selection of potentially probiotic Lactobacillus strains towards their inhibitory activity against poultry enteropathogenic bacteria. Pol. J. Microbiol. 2005, 54, 287–294. [Google Scholar]
- Wang, S.; Peng, Q.; Jia, H.; Zeng, X.; Zhu, J.; Hou, C.; Liu, X.; Yang, F.; Qiao, S. Prevention of E. coli infection in broiler chickens with L. plantarum B1. Poult. Sci. 2017, 96, 2576–2586. [Google Scholar] [CrossRef]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum DSM 2648 is a potential probiotic that enhances intestinal barrier function. FEMS Microbiol. Lett. 2010, 309, 184–192. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Saha, S.; Sukumaran, V.; Park, S.C. Use of a potential probiotic, L. plantarum L7, for the preparation of a rice-based fermented beverage. Front Microbiol. 2018, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Shin, D.; Chang, S.Y.; Bogere, P.; Park, M.R.; Ryu, S.; Lee, W.J.; Yun, B.; Lee, H.K.; Kim, Y. Comparative genome analysis and evaluation of probiotic characteristics of L. plantarum strain jdfm lp11. Korean J. Food Sci. Anim. 2018, 38, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, F.; Wan, C.; Xiong, Y.; Shah, N.P.; Wei, H.; Tao, X. Evaluation of probiotic properties of L. plantarum WLPL04 isolated from human breast milk. J. Dairy Sci. 2016, 99, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Tao, X.; Wan, C.; Li, S.; Xu, H.; Xu, F.; Shah, N.P.; Wei, H. In vitro probiotic characteristics of L. plantarum ZDY 2013 and its modulatory effect on gut microbiota of mice. J. Dairy Sci. 2015, 98, 5850–5861. [Google Scholar] [CrossRef] [PubMed]
- Khisti, U.V.; Kathade, S.A.; Aswani, M.A.; Anand, P.K.; Bipinraj, N.K. Isolation and Identification of S. cerevisiae from Caterpillar Frass and Their Probiotic Characterization. Biosci. Biotechnol. Res. Asia 2019, 16, 179–186. [Google Scholar] [CrossRef]
- Van der Aa Kühle, A.; Skovgaard, K.; Jespersen, L. In vitro screening of probiotic properties of S. cerevisiae var. boulardii and food-borne S. cerevisiae strains. Int. J. Food Microbiol. 2005, 101, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Pennacchia, C.; Blaiotta, G.; Pepe, O.; Villani, F. Isolation of S. cerevisiae strains from different food matrices and their preliminary selection for a potential use as probiotics. J. Appl. Microbiol. 2008, 105, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Moradi, R.; Nosrati, R.; Zare, H.; Tahmasebi, T.; Saderi, H.; Owlia, P. Screening and characterization of in-vitro probiotic criteria of Saccharomyces and Kluyveromyces strains. Iran. J. Microbiol. 2018, 10, 123–131. [Google Scholar]
- Ji, R.; Wu, J.; Zhang, J.; Wang, T.; Zhang, X.; Shao, L.; Chen, D.; Wang, J. Extending Viability of B. longum in Chitosan-Coated Alginate Microcapsules Using Emulsification and Internal Gelation Encapsulation Technology. Front Microbiol. 2019, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, R.; Smith, S. Commercial lactic acid bacteria and probiotic strains-tolerance to bile, pepsin and antibiotics. Int. Food Res. J. 2016, 23, 777–789. [Google Scholar]
- Ding, W.; Shah, N. Acid, bile, and heat tolerance of free and microencapsulated probiotic bacteria. J. Food Sci. 2007, 72, M446–M450. [Google Scholar] [CrossRef] [PubMed]
- Tahoun, A.; Masutani, H.; El-Sharkawy, H.; Gillespie, T.; Honda, R.P.; Kuwata, K.; Inagaki, M.; Yabe, T.; Nomura, I.; Suzuki, T. Capsular polysaccharide inhibits adhesion of B. longum 105-A to enterocyte-like Caco-2 cells and phagocytosis by macrophages. Gut Pathog. 2017, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Balasingham, K.; Valli, C.; Radhakrishnan, L.; Balasuramanyam, D. Probiotic characterization of lactic acid bacteria isolated from swine intestine. Vet. World 2017, 10, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Kamra, D.; Chaudhary, L.; Sahoo, A.; Pathak, N. Selection of S. cerevisiae strains for use as a microbial feed additive. Lett. Appl. Microbiol. 2000, 31, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Timson, D.J.; Annapure, U.S. Antioxidant properties and global metabolite screening of the probiotic yeast S. cerevisiae var. boulardii. J. Sci. Food Agric. 2017, 97, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Abdelazez, A.; Muhammad, Z.; Zhang, Q.-X.; Zhu, Z.-T.; Abdelmotaal, H.; Sami, R.; Meng, X.-C. Production of a Functional Frozen Yogurt Fortified with Bifidobacterium spp. Biomed Res. Int. 2017, 2017, 6438528. [Google Scholar] [CrossRef]
- Campana, R.; van Hemert, S.; Baffone, W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. 2017, 9, 12. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Kwok, L.-Y.; Chen, X.; Yu, H.; Yang, H.; Yang, J.; Xue, J.; Sun, T.; Zhang, H. Identification of potential probiotic Lactobacillus plantarum isolates with broad-spectrum antibacterialactivity. Dairy Sci. Technol. 2015, 95, 381–392. [Google Scholar] [CrossRef][Green Version]
- Yu, H.J.; Chen, Y.F.; Yang, H.J.; Yang, J.; Xue, J.G.; Li, C.K.; Kwok, L.Y.; Zhang, H.P.; Sun, T.S. Screening for Lactobacillus plantarum with potential inhibitory activity against enteric pathogens. Ann. Microbiol. 2015, 65, 1257–1265. [Google Scholar] [CrossRef]
- Al Kassaa, I.; Hamze, M.; Hober, D.; Chihib, N.-E.; Drider, D. Identification of vaginal lactobacilli with potential probiotic properties isolated from women in North Lebanon. Microb. Ecol. 2014, 67, 722–734. [Google Scholar] [CrossRef]
- Khidhr, K.O.; Zubaidy, Z.M.A. Isolation and Identification of S. cerevisiae var boulardii and its Uses as a Probiotic (in vitro). Rafidain J. Sci. 2014, 25, 1–11. [Google Scholar]
- Olatoye, I.; Okocha, R.; Olumide, P. Effects of commercial yeast probiotic (Antox R Supplement) on broiler chickens growth performance and Salmonella inhibition. J. Agric. Vet. Sci. 2014, 7, 46–50. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).