Abstract
Aggression is one of the major welfare concerns among group-housed sows, especially during feeding and regrouping. There are no simple solutions, but any attempt to reduce aggression should be considered. Therefore, the aim was to reduce aggression among group-housed gestating sows by feeding sows different dietary fiber using individual feeding places made from either short- or long-length partitions. Five blocks (n = 36 sows/block) of primiparous and multiparous sows were fed a dietary treatment of either 30% wheat middlings and 15% soybean hulls (MIDD-SY) or 30% distillers dried grains and 30% corn germ meal (DDGS-GM) and housed in pens (9 sows/pen) with individual feeding partitions that were either shoulder (short) or full-body (long) in length. Sow behavior, skin lesions, immune status, and performance were measured. Sow behavior, including aggression and lesion severity scores, were mainly affected by partition length. Aggressive encounters were greater and remained elevated among sows in pens with short partitions until 9 weeks post-grouping but were reduced among sows in pens with long partitions by 3 weeks. During feeding, sows in pens with short ones were more likely to be displaced than were those in pens with long ones. Percentages of time spent lying, standing, eating, and oral–nasal–facial behaviors were also differentially influenced by partition length. Dietary fiber differentially influenced immune status and productivity. For example, sows fed MIDD-SY had higher lymphocyte proliferation and increased neutrophils, while those fed DDGS-GM had deeper backfat and weaned heavier piglets. Overall, the length of the feeding partitions influenced the aggressive encounters, other behaviors, and lesion scores; in turn, the fibrous source differentially influenced several immune measures and sow productivity.
Keywords:
competitive feeding system; fiber; group pens; behavior; lesions; immune status; sows; well-being 1. Introduction
The housing of sows in group pens during gestation is perceived to be more welfare-friendly than individual housing because it allows them to exercise and interact socially [1]. However, sow aggression is a common problem associated with group housing, especially during feeding and regrouping of sows [2]. Aggression during feeding may be partly attributed to a hungry sow’s competitive nature to gain access to feed or other limited resources. Housing features, such as a feeding system, often exacerbate these encounters—whether a floor-feeding or electronic sow feeding system. A competitive feeding system provides little to no protection for the individual sow; aggression around feeding is often more intense. Smaller-bodied sows are often displaced from the feeder by larger sows, resulting in larger sows consuming other sows’ feed allotment [3]. Larger sows gain more weight and have better body condition scores; however, group-housed sows often have higher lesion scores and more variable conditions scores [4,5,6]. Feed restriction and feeding systems have been identified as possible factors associated with the development of aggression and oral–nasal–facial behaviors or stereotypes in dry sows.
Early attempts were made to develop diets or feeding strategies to reduce the aggression, feeding motivation, and oral–nasal–facial behaviors associated with restricted-fed sows. Feeding different fiber sources at different inclusion rates during gestation affected performance, productivity, and stereotypical behaviors [7,8,9]; this approach also reduced aggression and manipulative behaviors but improved satiety [9,10,11]. However, findings are variable [12,13], partly due to variation in the level and type of fiber fed, the feeding regimen, housing factors, and other sow traits (e.g., parity), which may independently or interactively affect aggressive encounters and other welfare outcomes [9]. Other strategies, including varying the group size and floor-space allowance [14] or adding individual feeding partitions [15], have also been used to minimize aggression among group-housed sows with variable outcomes. For example, floor-space allowance affected the lesion severity scores, whereas vulva lesions and oral–nasal–facial behaviors were reduced among sows fed a fiber gestation diet (15% wheat middlings and 30% soybean hulls) compared to those fed a control diet [9]. Moreover, both floor-space allowance and fibrous interactively affected aggression, such that sows housed in pens with increased floor-space allowance and fed a fiber diet had more bouts of aggression around the waterer than those in pens with less floor space [16]. Despite these attempts to reduce aggression, it continues to be a welfare concern among group-housed gestating sows. Therefore, the objective of this study was to determine the effects of feeding two different fiber-supplemented gestation diets to group-housed gestating sows kept in small pens with either short or long feeding partitions, this in an attempt to reduce aggression and enhance sow well-being in terms of behavior, immune status, skin lesions, and productivity.
2. Materials and Methods
2.1. Animals and Experimental Design
This study was conducted at the University of Illinois Swine Research Center (Urbana, IL, USA) from September 2013 to June 2015. The University of Illinois Institutional Animal Care and Use Committee approved all experimental procedures (protocol no. 13097; Urbana, IL, USA). A total of 180 crossbred primiparous (first-pregnancy gilts (parity 1); n = 47) and multiparous (parity categories 2, 3 and ≥4, n = 133) sows derived from the Genetiporc Fertilis-25 genetic line were assigned to a 2 × 2 factorial experiment. Thirty-six sows per block (n = 5), balanced for sow body weight and parity, were randomly allotted to one of two dietary fiber treatments and one of two feeding stall lengths at gestational D35 (n = 9 sows/diet–length–block combination). Dietary treatments consisted of fiber-supplemented diets composed of either 30% wheat middlings and 15% soybean hulls (MIDD-SY) or 30% distillers dried grains with solubles and 30% corn germ meal (DDGS-GM). The partitions used to make individual feeding places within the group-pens were either short (58.4 cm; (short); width = 48.3 cm) or long (203.2 cm (long); width = 57.2 cm) in length; with the short ones being long enough to protect the sow from head to shoulders, and the long ones extended from her head to rump. At D37, post-breeding groups of 9 sows were moved from individual gestation stalls to one of four experimental treatment pens: (1) MIDD-SY: short; (2) MIDD-SY: long; (3) DDGS-GM: short; and (4) DDSS-GM: long. All pens had a floor-space allowance of 1.7 m2/sow, which included the feeding stall space; thus, the available floor space outside of the stall space differs. Dietary treatments were offered to sows beginning on gestational D35 post-breeding, two days before moving into group pens to facilitate acceptance of the high-fiber diets without competition. Diets were added to the feeder in the same order each day at 06:30 h. Sows had ad libitum access to water via an individual nipple waterer fixed to each stall’s right side (9 nipples/pen). Sows remained in their assigned treatment pens until gestational D104, when they were moved to the farrowing facility. All sows were placed in individual farrowing crates in the farrowing room until the end of lactation. Piglets were weaned at 19 + 2 days of age.
Sows were artificially inseminated within 24 h after estrus onset and again 24 h later. Pregnancy was diagnosed ultrasonically by a trained technician on D29 ± 2 post-breeding using a VSS700 Ez Preg Checker (Veterinary Sales and Service Inc., Stuart, FL, USA). All sows were kept in individual gestation stalls (0.61 × 2.13 m) until they were moved to their assigned group treatment pens at D37 post-breeding. Sows were fed the same lactation diet, but the amount offered differed between pre- and post-farrowing. Pre-farrowing, all sows were fed 1.81 to 2.72 kg of a standard gestation diet, and then the feed was increased daily as consumption increased to ad libitum access. Seven sows were removed from the study due to extreme lesions/injuries or other health reasons independent of the experimental treatments.
2.2. Dietary Treatments
The MIDD-SY and DDGS-GM ingredients replaced a portion of the shelled corn and soybean meal and were formulated to meet or exceed the National Research Council (NRC) requirements for gestating sows (NRC, 2012; Table 1). Sows were fed 2.3 kg/day of MIDD-SY or 2.1 kg/day of DDGS-GM from gestational D35 to D90, and then from D91 to D104 were fed either 3.6 kg/day of the MIDD-SY or 3.4 kg/day of the DDGS-GM diet to adjust for the differences in energy between the two diets. The diets had a calculated composition (as fed basis) of 13.8% and 18.9% crude protein (CP), respectively. The difference between the two diets in percentage CP can be attributed to differences in the ingredients used to formulate the treatment diets.

Table 1.
Ingredient composition and nutrient levels of the gestational diets 1.
No control diet (low fiber) was used in this study because it was designed based on previous research findings by DeDecker [9]. Previously, we found a better sow performance and immune status among sows fed a diet that contained 30% soybean hulls and 15% wheat middlings compared with sows fed a corn–soybean-based diet with no additional fiber source [9,16]. We also found that fiber-fed sows drank water more often during feeding, which resulted in more aggressive bouts around the waterer [16]. The percentages of soybean hull and wheat middlings for this study were changed due to updated NRC requirements (NRC, 2012, Washington, DC, USA). The other fiber diet (DDGS-GM) was selected because it provides a high fiber level but contains more metabolizable energy (ME) than the diet based on soybean hulls and wheat middlings. Moreover, the ingredients for the DDGS-GM diet are more accessible and less expensive.
2.3. Behavior
Sow behavior was captured on 3 blocks of sows using EverFocus EQ120A/EN colored cameras (EverFocus Co., LTD., Duarte, CA, USA) fixed above each treatment pen. Data were acquired using the Geovision GVd1240 video capture combo card (Geovision, Inc., Irvine, CA, USA) and viewed using EZViewLog (Geovision, Inc., Irvine, CA, USA). Behavior was recorded for the first 48 h after grouping and then again for 24 h on a bi-weekly basis. Live behavioral observations were also registered during feeding at various time points, including first feeding post-grouping and then at 3-week intervals (3, 6, and 9 weeks post-grouping) until the sows were moved to the farrowing facility. Live postural and behavioral observations were taken using instantaneous sampling during the observational period, which included 30 min before feed delivery and then at 30 min increments post-feeding up to 120 min. Behaviors registered were eat, drink, sham-chewing, oral–nasal–facial (ONF), locomotion, stand, sit, and lay (Table 2). The frequencies and durations of each aggressive encounter during this period were also registered.

Table 2.
Description of the observed and registered behaviors between 06:30 and 09:00 h.
2.4. Blood Collection and Cell Isolation
Sows were nose-snared, and 15 mL of blood was collected via jugular venipuncture using syringes containing sodium heparin on gestational days 30, 70, 90, 104, and again at the end of lactation. Whole blood smears were made, fixed in methanol, stained with the Hema-3 staining system (Fisher Scientific, Houston, TX, USA), and then viewed under a light microscope to determine leukocyte differential counts. Total white blood cell counts (WBC) were made electronically using a Coulter Z1 particle counter (Beckman Coulter, Beckman, FL, USA).
Blood samples were centrifuged, and the buffy coat was diluted in RPMI medium (Gibco, Carlsbad, CA, USA), layered over Histopauqe-1077 (density: 1.077 g/mL; Sigma), and centrifuged at 700× g for 30 min at 25 °C. Lymphocytes were collected, washed twice and resuspended in RPMI, and counted.
2.5. Immune Measures and Interleukin-12 Assays and Cortisol
A mitogen-induced lymphocyte proliferation assay was performed using the CellTiter96 nonradioactive cell proliferation kit (Promega, Madison, WI, USA), as previously described by Sutherland [17]. Isolated porcine lymphocytes were used at a concentration of 5 × 106 cells/mL. Concanavalin A (ConA; Sigma Aldrich, Shanghai, China) and lipopolysaccharide (LPS; Sigma Aldrich, Shanghai, China) were used as mitogens (ConA: 0, 2, and 20 μg/mL; LPS: 0, 5, and 50 μg/mL) to stimulate T and B cells, respectively. Samples were run in triplicates. The plates were incubated, and then the reaction stopped. The plates were read using a microplate reader (Thermo Scientific Instruments, Richardson, TX, USA) at a wavelength of 600 nm. Results are expressed as a proliferation index: the optical density (550/690 nm) of the stimulated cells ÷ the optical density (550/690 nm) of the non-stimulated cells.
Total plasma cortisol and interleukin-12 (IL-12) were measured at gestational D30 (baseline) and D90 post-breeding. Cortisol was measured using a commercially available RIA kit (MP Biomedicals, Santa Ana, CA, USA). Briefly, 25 µL of sample or standard was added in duplicates to antibody-coated tubes and incubated with I-125-labeled cortisol. Tubes were placed in a water bath at 37 °C for 45 min, the liquid was aspirated, and then the samples were counted. The inter-assay and intra-assay coefficient of variation (CVs) were 8.3% and 9.1%, respectively, and sensitivity was 3 pg/mL Plasma IL-12 was measured using an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA) following manufacturer’s protocol. The inter-assay and intra-assay CVs were 4.9% and 7.7%, respectively, and sensitivity was 9 pg/mL.
2.6. Skin Lesions and Body Condition Scores
Skin lesion scores were assessed before the sows moved into their respective treatment pens, and then at 24 h post-mixing, every 3 days for the first 2 weeks post-grouping (Phase 1), and then bi-weekly after that until the end of the experimental period (Phase 2). Skin lesion scores were assessed and recorded as previously described by Salak-Johnson [5], with minor modifications as described by DeDecker [9]. The sow body regions scored were the head, ears, neck, breast, shoulders, side, back, udder, rear, vulva, front limbs, and hind limbs. Scores ranged from 0 to 7 for all body regions: score 0 = no lesions; 1 = dehairing/callus/balding; 2 = redness/swelling; 3 = swelling and callus/abscess; 4 = scabbed over scratch; 5 = marked wound/fresh scratch; 6 = severe wound/open wound; and 7 = severe swelling. Each body region was given a score, and all individual scores were added to calculate the total severity for each measurement day. The sow body condition score (BCS) was recorded at gestational days 30, 70, 90, and 104, and again at the end of lactation. Using the visual appraisal (sow’s rear aspect) method described by Coffey [18] and Salak-Johnson [5], the sow BCS (1 = emaciated to 5 = obese) was assessed by trained, experienced university personnel.
2.7. Sow- and Litter-Related Traits
Multiple sows- and litter-related traits (n = 173 farrowings) were recorded. Sow body weight (BW) and backfat depth (BF) were taken on D30, D70 (BF excluded), D90, and D104, and at the end of lactation; at the same time, blood samples were taken. Sow BF was measured using a longitudinal imaging ultrasound scan (Aloka-500 V machine, Hitachi Aloka, Wallingford, CT, NE)—cranial to the last rib. Sow BW gains were calculated from D30 to D70, D70 to D90, D90 to D104, and total gain (D30 until D104), using sow body weight recorded on specific gestational weigh day. Total BW loss was calculated using body weight at gestational D30 and at the end of lactation (or at weaning). Litter-related traits included: total number of piglets born and born alive and numbers of females, males, stillborns, laid-on, euthanized, total mortality (no. stillborn + no. mummified + no. laid on + no. euthanized), and piglets weaned. The calculated piglet and litter traits included: mean piglet birth and weaning weight, mean weight gain from birth to weaning, litter weights at birth and weaning, and adjusted litter weights at birth and weaning.
2.8. Statistical Analysis
Data were analyzed using the MIXED model procedure of SAS (SAS Inst. Inc. Cary, NC, USA), with repeated measures utilizing a first-order autoregressive structure. A linear mixed-effects model was used to analyze the physiologic and performance traits. All traits, except the lesion scores and BCS, were tested for normality using Kolmogorov–Smirnov and Shapiro–Wilk tests. A natural logarithmic transformation was applied to the traits deviating from a normal distribution, which were physiology measures. The model included fixed effects of diet (MID-SOY or DDGS-GM), length (short or long), parity categories (1 and 2, 3 and ≥4), and all second- and third-order interactions between these factors. A random effect of the block was included. The physiologic measures model also included the gestational day that the blood samples were taken, whereas the behavior model included day post-mixing. The covariate of the corresponding blood measurement was at D30 of gestation (baseline measurement: D0, which is the start of the experiment) or the corresponding BW for the BCS and BF measurements. Lesion scores, BCS, and a few litter traits (number of stillborn, mummified, laid-on, and mortality), being ordinal variables, required analysis with PROC GLIMMIX (SAS Inst. Inc. Cary, NC, USA). All measurements were from a single sow; thus, the experimental unit was the sow for all traits [5,9]. Least square means were generated and separated statistically with pairwise t-tests (PDIFF option). Significance was set at p ≤ 0.05, whereas trends were discussed at p < 0.10.
3. Results
3.1. Behavior
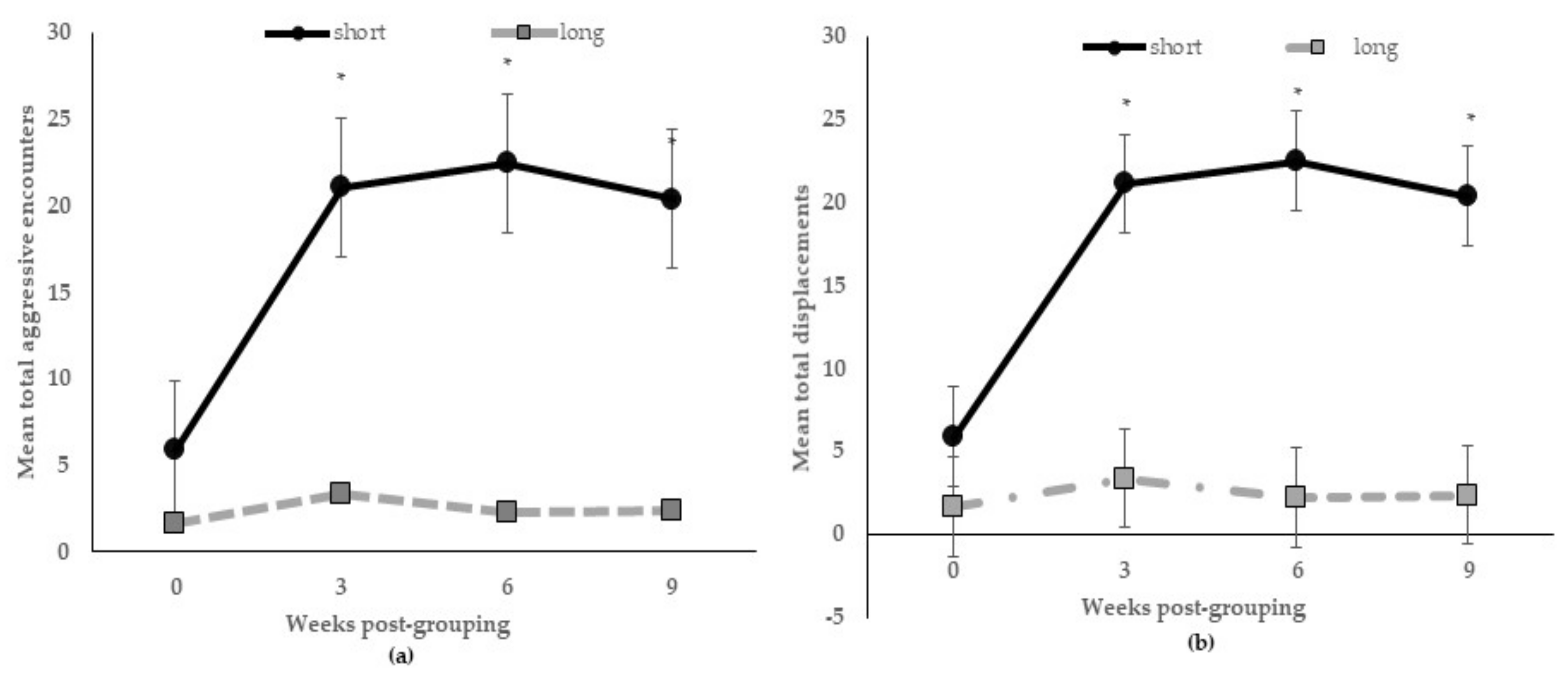
There were no interactive effects of diet by length on aggressive encounters at 24 h post-mixing or 3, 6, and 9 weeks post-grouping (p > 0.10), or other behaviors (p > 0.15) registered from 06:00 h to 08:30 h (included pre- and post-feeding time), but feeding stall partition length across weeks post-grouping occurred for aggressive encounters (p < 0.005) and displacements (p < 0.05). Figure 1a shows the total.
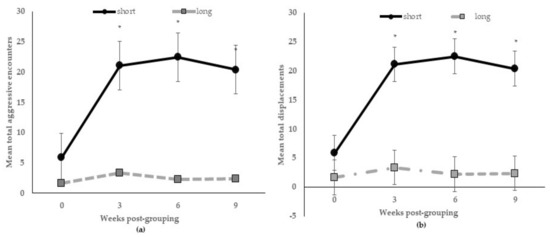
Figure 1.
Mean numbers of aggressive encounters (a) and displacements (b) registered during feeding at 0, 3, 6, and 9 weeks post-grouping for sows kept in group pens with short or long feeding stall partitions. * indicates that treatment groups at those weeks post-grouping are different at p < 0.005.
The number of aggressive encounters significantly increased from D1 until 3 weeks (gestational D60) post-grouping and remained elevated at 9 weeks (gestational D102) among sows in pens with the short feeding partitions; whereas, aggressive encounters declined sharply by 3 weeks post-grouping among sows in pens with long ones; also, sows in the pens with short partitions were more likely to displace others during feeding at all weeks post-grouping (Figure 1b).
Moreover, 24 h post-grouping, the total number of aggressive encounters were higher (p < 0.05) for sows in pens with short partitions than sows in pens with long ones (130.0 versus 77.5 ± 18.1, no./24 h, respectively); but, each bout lasted longer (p < 0.01) between sows in pens with long ones (7.23 versus 2.94 ± 0.4, s/bout). Shown in Table 3 are the main effects of diet and partition length treatments on behavior. The only behavior affected by both was eating; sows fed the DDGS-GM diet or in pens with short partitions spent less time eating. Percent sitting behavior was greater for sows fed the DDGS-GM diet than sows fed the MIDD-SY diet. The length of partition differentially affected the other behaviors; those sows in pens with short partitions spent more time lying and standing, while sows in pens with long ones spent more time engaged in oral–nasal–facial behaviors.

Table 3.
Mean percentages of sow behavior registered between 06:00 h (30 min before feeding) and 09:00 h (2 h post-feeding) at 30-min intervals starting at 24 h post-mixing, and then at 3-week intervals until gestational D104.
3.2. Immune Measures and Cortisol
The only interactive effect of diet by length occurred for the neutrophil-to-lymphocyte ratio with the highest ratio (p < 0.05) being among sows in the MIDD-SY: long (2.19 ± 0.36) treatment compared with sows in all other treatment pens (MIDD-SY: short = 1.19, DDGS-GM: short = 1.04, or DDGS-GM: long = 0.95 ± 0.36, respectively). All other immune measures were influenced by diet, not partition length (p > 0.15). Mean WBC counts (7.2 versus 6.8 ± 0.33 107/mL, p < 0.05), lymphocytes (45.0 versus 40.1 ± 0.96%, p < 0.01), and monocytes (3.73 versus 3.08 ± 0.87%, p < 0.05) were higher among sows fed the DDGS-GM diet; whereas, neutrophils (50.1 versus 45.1 ± 0.97%, p = 0.01) and the neutrophil–to–lymphocyte ratio (1.3 versus 1.0 ± 0.05, p < 0.005) were higher among sows fed the MIDD-SY diet. Both, the ConA- and LPS-induced lymphocyte proliferation indexes were greater for sows fed the MIDD-SY diet than sows fed the DDGS-GM diet (1.10 versus 0.99 ± 0.01, p = 0.02 and 1.14 versus 1.05 ± 0.01, p = 0.04, respectively). No treatment effects were found for plasma cortisol or IL-12 (p > 0.10).
Table 4 shows the effects of diet on the immune measures at various days throughout gestation and end of lactation. At gestational D90, the total WBC counts were significantly higher for sows fed MIDD-SY, but by D104 and at the end of lactation, those fed DDGS-GM had higher counts. Sows fed the MIDD-SY diet also had greater (p < 0.01) neutrophil numbers at gestational D70 and throughout gestation and lactation than those fed the DDGS-GM diet, which resulted in a higher neutrophil-to-lymphocyte ratio at D90 and end of lactation among the MIDD-SY-fed sows. Conversely, lymphocyte percentages were higher (p < 0.05) for sows fed the DDGS-GM diet at gestational D70, D90, and end of lactation (Table 4).

Table 4.
Effects of diet by gestational day on total white blood cell counts and other descriptive leukocyte measures and neutrophil–to–lymphocyte ratio for sows at days 30, 70, 90, 104, and end of lactation 1.
3.3. Sow- and Litter-Related Measures
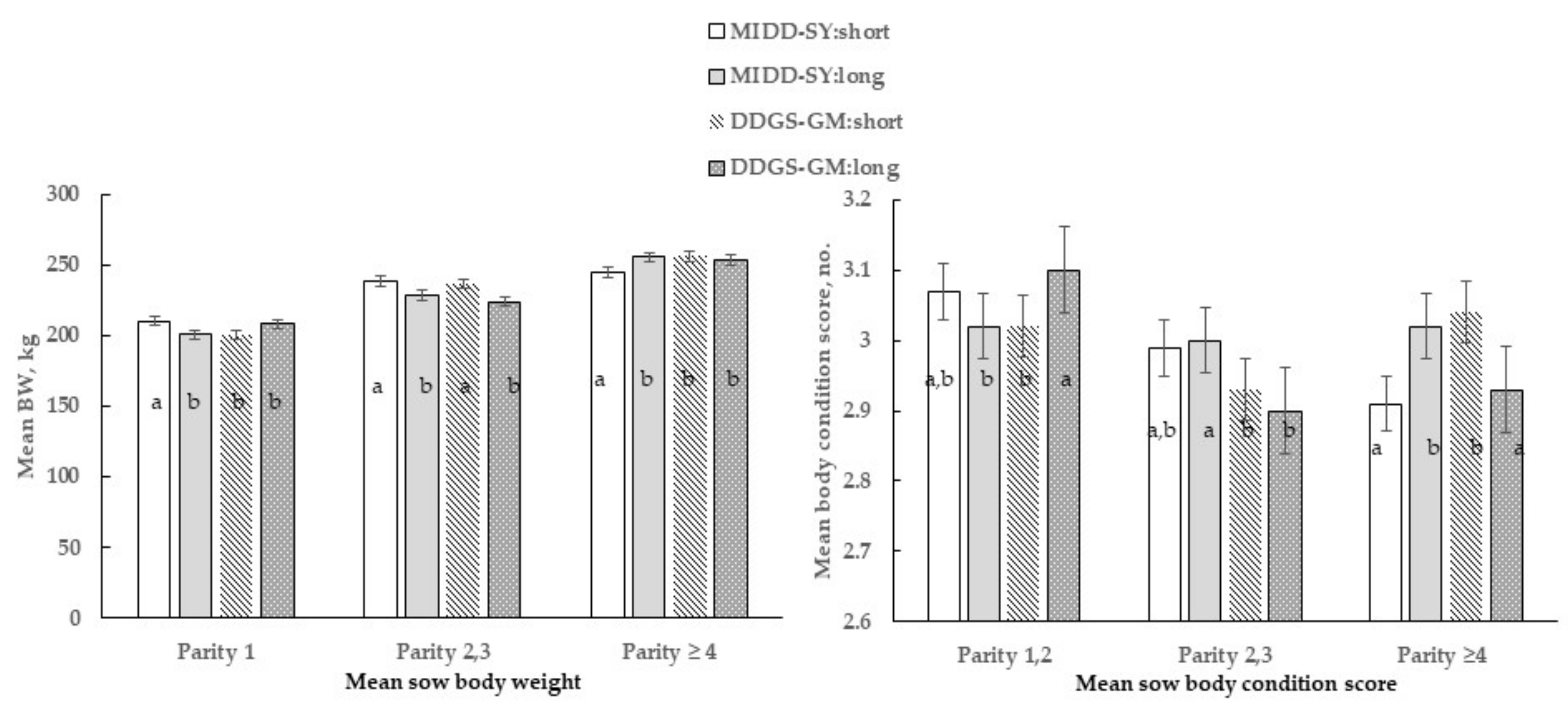
The only 3-way interactive effect of parity by diet by length occurred for mean body weight (p = 0.01) and BCS (p < 0.01), as shown in Figure 2. Parity categories 1 and 2, 3 sows in the MIDD-SY: short treatment were heavier than the sows in pens with long partitions (MIDD-SY: long). However, parity ≥ 4 sows in the MIDD-SY: long treatment were heavier (p < 0.005) than contemporaries in pens with short ones (MIDD-SY: short). Parity 1 sows in the DDGS-GM: long treatment was heavier than contemporaries in pens with short partitions (DDGS-GM: short). Conversely, the Parity 1 sows in the DDGS-GM: short treatment had higher BCS (p < 0.05) than contemporaries in the DDGS-GM: long or in MID-SOY: short treatments and sows in all other parity categories and treatments.
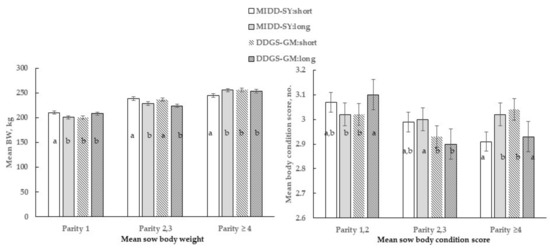
Figure 2.
Three-way interactive effect of parity by diet by length on mean sow body weight (BW) and mean body condition scores for group pen sows. Within parity bars with different letters differ at p < 0.05.
Overall, parity category ≥ 4 sows were the heaviest, parity category 2,3 sows intermediate, and parity 1 sows the lightest, regardless of treatment. Only during Phase 2, the lesion scores were most severe among (p < 0.05) sows in the DDGS-GM: long (28.0 ± 1.1, No.) treatment compared with sows in all other treatments (DDGS-GM: short = 20.2, MIDD-SY: short = 20.0, or MIDD-SY: long = 24.4 ± 1.1, No.; diet by length). Overall, the total mean skin lesion scores and scores across various body regions were most severe (p < 0.05) among sows in pens with long partitions than those with short ones (Table 5).

Table 5.
The main effect of length of partition on mean skin lesion scores 1.
Finally, diet (p < 0.01) affected sow body weight gains from gestational D70 to D104, D90 to D104, and total gain and weight loss from gestational D104 through the end of lactation, but not length (p > 0.20). Sows fed MIDD-SY gained more weight (p < 0.01) from gestational D70 to D104 and D90 to D104 and lost more (p < 0.01) weight from gestational D104 through the end of lactation compared to those fed DDGS-GM (Table 6). Those fed DDGS-GM gained the most (p < 0.01) BW overall, tended to be the heaviest (p = 0.09), and had the deepest mean BF depth (p = 0.05) at the end of lactation, and overall (p < 0.01) compared with sows fed the MIDD-SY diet (Table 6). The number of piglets weaned was similar (p > 0.10) among sows, regardless of diet, but sows fed DDGS-GM weaned heavier piglets than sows fed MIDD-SY (7.2 versus 6.7 ± 0.2 kg; p = 0.02) and also tended to have less stillborn piglets (1.29 versus 1.89 ± 0.2; p = 0.08).

Table 6.
Main effects of fibrous diet source on mean body weight, gain, and loss, backfat depth for gestating sows kept in group pens (9 sows/pen) 1.
4. Discussion
4.1. Aggression and Lesion Scores
Most often, sows kept in group pens experience acute stress associated with social conflict or aggression related to competition for food among hungry sows, especially becoming intense when a competitive feeding system (minimal protection) is used [19]. Sow aggression, plasma cortisol, and skin lesion scores are reduced when full-body length partitions are used [15,20], and feeding sows fibrous diets can also reduce aggression [20,21], stereotypes [10,22], and other oral [9,13] behaviors, and improve satiety [23]. We found that the aggressive encounters, displacements, and other behaviors during the observational period were primarily altered by the feeding partition length, not the type of fibrous diet fed to group-housed gestating sows. Specifically, sows in pens with the shoulder-length (short) partitions had more bouts of aggression and were more likely to displace one another during feeding across the entire gestational period. Despite more frequent aggressive encounters and displacements among sows in these pens, the group’s increased aggressive encounters did not result in compromised sow well-being. Over time, the sows housed in pens with the shorter partitions may have adapted to acts of aggression during feeding by eating faster (shorter bouts of eating) and maintaining a more stable hierarchy, as indicated by a less-stressed profile (i.e., lower neutrophil-to-lymphocyte ratios).
Interestingly, sows in pens with the longer feeding partitions had greater total lesion scores. The ability of sows to displace each other from the feeding places was more difficult among sows in pens with the longer partitions; thus, displacement and aggression diminished over time, resulting in longer bouts of eating among these sows. It is plausible that the reduced floor space available outside of the feeding places made it more difficult for sows to move, turn, and leave the feeding stall, resulting in higher skin lesion scores possibly due to contact with the pen gating. These findings imply that when individual feeding stalls are used, not only should the length of the partition be considered, but the amount of floor space afforded, either total or outside the feeding place, should be taken into account because both can affect aggression, skin lesions, and other behaviors [19,24]. Here, all pens had the same total floor space (1.7 m2/sow), which included the feeding space area created by the partitions, resulting in the open-pen area being different between treatments. Thus, the floor space afforded to sows outside of the feeding places was reduced in pens with long partitions. Skin lesions are often a proxy for aggressive interactions when sows are group-housed, but these sows had the most severe scores. It is plausible that the reduced space outside of the feeding stalls may have unwittingly hindered the sows’ ability to freely leave or retreat to or from these feeding spaces, not aggression. Based on these results, sows in either pen environment managed to cope with the pen environment’s constraints by eliciting the appropriate biological response without compromising sow productivity and immune status, but neither the pen-feeding partition nor environment was optimal.
4.2. Immune Status and Stress
The fibrous diet source differentially influenced the immune status of group-housed gestating sows. For example, the immunological profile among sows fed MIDD-SY was indicative of physiological stress as neutrophils, and the neutrophil-to-lymphocyte ratios were elevated, especially at gestational D90 and D104, and still evident at the end of lactation. Conversely, these sows had a more stimulated adaptive immune response, as evidenced by a greater lymphocyte proliferation response to both ConA and LPS, indicating both T- and B-cells were more metabolically active. Moreover, sows in the MIDD-SY: long treatment had the highest neutrophil-to-lymphocyte ratio than sows in all other treatments. Neutrophilia is a classical response in animals experiencing stress, which results in a high neutrophil–to–lymphocyte ratio due to increased neutrophils and decreased lymphocytes [25,26]. Often, the stress-induced reduction in circulating lymphocytes is not due to the destruction of cells but rather to glucocorticoid-induced alteration in lymphocyte “trafficking” or redistribution of cells from the blood to other immune compartments [27]. The shift in leukocyte populations among these sows was not primarily driven by cortisol, which does not necessarily imply that these sows were not experiencing more stress due to the restricted usable floor space. It has been reported that the neutrophil-to-lymphocyte ratio still rises even if cortisol is not elevated [5]; thus, it is likely that sows were experiencing stress due to the restricted open floor space in the pens with the full body-length partitions. The restricted floor space outside of the feeding places may have hindered the sows’ ability to make postural changes with ease and contributed to the higher skin lesion scores. It is also plausible that the leukocyte shift in the periphery, resulting in more neutrophils and fewer lymphocytes, is reflective of immune cells trafficking to the tissue to help mitigate the skin lesions, especially among those fed the MIDD-SY diet and housed in these pens. It is likely that feeding dietary fiber and fitting small group pens with full-body-length individual feeding partitions may reduce aggression during feeding. However, the type of fibrous diet fed differentially affects the animals’ immune status and other well-being measures; therefore, the optimal treatment combination was not identified for improving the well-being among all sows within the group.
4.3. Other Behaviors and Productivity
Surprisingly, the dietary fiber treatment did not influence aggression, postural behaviors, or oral–nasal–facial behaviors during the 2.5 h feeding period, but did affect the sow- and litter-related traits. This is most likely due to the short observational period (included pre- and post-feeding). Previously, researchers reported that feeding gestating sows fibrous diets could affect productivity and performance, but the outcomes were inconsistent. Holt [28] reported that sows gained less body weight, lost more backfat depth, and farrowed fewer piglets when fed a highly fibrous diet; whereas, DeDecker [9] found that sows fed a fibrous diet (similar to the one fed within) gained more body weight from gestational D30 to D90 compared to sows fed the control diet. Nevertheless, when floor-space treatments were taken into account, the sows fed the fiber diet and kept in areas with less floor space (1.7 m2) farrowed more live piglets and produced heavier litters than sows that had a 2.3 m2 floor space [9]. However, we found that feeding sows the DDGS-GM diet during gestation using individual feeding places resulted in sows having a heavier and deeper backfat depth at the end of lactation and weaning heavier piglets than sows fed the MIDD-SY diet, despite being housed in pens at the same floor-space allowance and group size as those sows in the DeDecker study. Conversely, Li [29] found a negative impact on sow- and litter-related traits when sows were fed 40% distillers dried grains with solubles. One possible explanation may be the differences in percentages in fiber type between DeDecker, Li, and the current study. However, the differences found within may be partly due to the energy difference between the MIDD-SY and DDGS-GM diets fed to these sows.
Sows were fed more of the MIDD-SY than DDGS-GM diets in an attempt to equalize the ME. It is plausible that different welfare outcomes may be partly attributed to the energetic tradeoff in dietary energy density since the corn germ meal provides more energy. It has been shown that sow BW and BF depth and gain are improved as the energy level increases, with the greatest improvement achieved at the highest energy level [30]. Feed intake during lactation was not measured, but observationally, the animal care staff reported that sows fed DDGS-GM tended to consume more feed during lactation. Both diets are mostly insoluble, which results in a feeling of fullness by prolonging chewing activity and saliva production [31]; however, it is plausible that the distillers dried grains with solubles was not as filling or not as bulky per se. These sows had shorter eating bouts and gained less body weight during gestation and were allotted less fed to achieve similar metabolizable energy levels between the two diets. However, the physicochemical properties are different between the diets, which may have affected the feed intake during lactation. It is plausible that sows fed the DDGS-GM diet may have differentially partitioned energy where sows diverted more energy into productivity during gestation. During lactation, they compensated and consumed more energy during lactation. Sun [32] found that including konjac flour in gestation diets affected lactation intake and sows’ reproductive performance and tended to improve the piglets’ performance, but the mechanism is unknown.
4.4. Sow Parity
Finally, another factor that may affect aggression, injuries, and stress is how the group is managed to distribute the parities. Although sow pens were balanced for parity and body weight, primiparous (Parity 1) sows in the MIDD-SY: short or DDGS-GM: long treatments had better body weight gain. Interestingly, younger sows are less stressed in these mixed parity pens since they had the lowest neutrophil-to-lymphocyte ratio and greater IL-12 at gestational days 30 and 90 [33]. Conversely, parity category 2,3 sows kept in pens with short feeding partitions had better gain regardless of diet, while parity category ≥4 sows had better gain when fed the DDGS-GM diet. We hypothesize that the biological cost in terms of repartitioning energy may differ among sows of different parities due to other factors, including social rank and previous experience—but not limited to these factors. Parity is correlated with social rank; for the most part, higher parity or heavier-bodied sows are dominant [34] and more engaged in aggressive encounters [15,21]. Pacheco and Salak-Johnson [35] reported that dominant sows (a subpopulation of sows from this study) were most often the initiator of the aggressive act and more likely to displace others from the feeder, especially in pens with short-length partitions. Dominant sows (larger-bodied) gained the least amount of body weight throughout gestation when housed in the pens with full body-length partitions. However, in late gestation, they gained the most body weight and had greater plasma cortisol and neutrophil-to-lymphocyte ratios throughout gestation and lactation [36]. The higher parity sows’ biological cost may be greater in pens with longer-length partitions due to floor-space restriction; the higher parity sows had difficulty displacing sows from feeding places. It was more difficult for them to exit the feeding space. Early on, it may have cost these sows more energy, as evidenced by their lower body weight. However, over time, they adapted and conserved energy by being less active and engaged in aggressive encounters.
5. Conclusions
In summary, the pen environments evaluated here affected behavioral responses, especially aggression and bouts of eating, while the source of dietary fibers fed differentially affected the sow’s immune status. The observed behavioral patterns and immune status changes indicated that sows might have adapted; even when aggression and skin lesions were high and the immunological profile was indicative of stress, there were no negative consequences on health, performance, and productivity. These differential biological responses between treatments seemed to indicate a wide regulatory range of allostatic mechanisms that enabled the sows to adapt successfully. No one single change can improve every aspect of animal well-being; thus, different strategies need to be used to minimize group-housed gestating sows’ challenges. One size does not fit all. Restricted-fed sows are hungry, leading to competition for feed; even when dietary fiber is fed and pens are fitted with individual feeding places made from shoulder length partitions, aggression during feeding is evitable. Moreover, if pens are not designed properly, there will still be welfare tradeoffs. Overall, these results suggest that feeding dietary fiber to gestating sows housed in small group pens at a floor-space allowance of 1.7 m2/sow, with short or long feeding partitions, differentially affects their welfare metrics, including immune measures, behavior, and productivity. Moreover, feeding dietary fiber and/or using feeding stalls in pens of group-housed gestating sows can potentially mitigate the negative consequences of aggressive behaviors and stress. However, the length and fibrous source need to be optimized.
Author Contributions
Conceptualization, J.S.-J.; methodology, J.S.-J.; validation, J.S.-J., M.L., and E.P.; formal analysis, M.L., and E.P.; investigation, M.L., and E.P.; resources, J.S.-J.; writing—original draft preparation, M.L.; writing—review and editing, M.L. and J.S.-J.; supervision, J.S.-J.; project administration, J.S.-J.; funding acquisition, J.S.-J. All authors have read and agreed to the published version of the manuscript.
Funding
The National Pork Board funded this research, grant number 12–200.
Institutional Review Board Statement
The study was conducted according to the guidelines of the 4th Edition of the Guide for the Care and Use of Agricultural Animals in Research and Teaching, 2010 and approved by the University of Illinois Institutional Animal Care and Use Committee approved all experimental procedures (protocol no. 13097; Urbana, Illinois, USA).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge H. Stein for formulating the diets, and the farm staff at the Swine Farm at the University of Illinois for the care of these animals, especially G. Bressner.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Elmore, M.R.P.; Garner, J.P.; Johnson, A.K.; Kirden, R.D.; Richert, B.T.; Pajor, E.A. Getting around social status: Motivation and enrichment use of dominant and subordinate sows in a group setting. Appl. Anim. Behav. Sci. 2011, 133, 154–163. [Google Scholar] [CrossRef]
- Remience, V.; Wavreille, J.; Canart, B.; Meunier-Salaun, M.C.; Prunier, A.; Bartiaux-Thill, N.; Nicks, B.; Vandenheede, M. Effects of space allowance on the welfare of dry sows kept in dynamic groups and fed with an electronic sow feeder. Appl. Anim. Behav. Sci. 2008, 112, 284–296. [Google Scholar] [CrossRef]
- Gonyou, H.W. The Social Behaviour of Pigs. In Social Behaviour in Farm Animals; Keeling, L.J., Gonyou, H.W., Eds.; CABI International: Wallingford, UK, 2001; pp. 147–176. [Google Scholar]
- Kranendonk, G.; Van der Mheen, H.; Fillerup, M.; Hopster, H. Social rank of pregnant sows affects their body weight gain and behavior and performance of the offspring. J. Anim. Sci. 2007, 85, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Salak-Johnson, J.L.; Niekamp, S.R.; Rodriguez-Zas, S.L.; Ellis, M.; Curtis, S.E. Space allowance for dry, pregnant sows in pens: Body condition, skin lesions, and performance. J. Anim. Sci. 2007, 85, 1758–1769. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Flowers, W.L.; Saraiva, A.; Yeum, K.J.; Kim, S.W. Effect of social ranks and gestation housing systems on oxidative stress status, reproductive performance, and immune status of sows. J. Anim. Sci. 2013, 91, 5848–5858. [Google Scholar] [CrossRef] [PubMed]
- McGlone, J.J.; Fullwood, S.D. Behavior, reproduction, and immunity of crated pregnant gilts: Effects of high dietary fiber and rearing environment. J. Anim. Sci. 2001, 79, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, J.A.; Jongbloed, A.W.; Spoolder, H.A.M.; Verstegen, M.W.A. Effects of hindgut fermentation of non-starch polysaccharides on the stability of blood glucose and insulin levels and physical activity in empty sows. Livest. Prod. Sci. 2005, 96, 165–174. [Google Scholar] [CrossRef]
- DeDecker, A.E.; Hanson, A.R.; Walker, P.M.; Salak-Johnson, J.L. Space allowance and high fiber diet impact performance and behavior of group-kept gestating sows. J. Anim. Sci. 2014, 92, 1666–1674. [Google Scholar] [CrossRef]
- Zonderland, J.J.; de Leeuw, J.A.; Nolten, C.; Spoolder, H.A.M. Assessing long term behavioural effects of feeding motivation in group-housed sows; what, when and how to observe. Appl. Anim. Behav. Sci. 2004, 87, 15–30. [Google Scholar] [CrossRef]
- Guillemet, R.; Dourmad, J.Y.; Meunier-Salaun, M.C. Feeding behavior in primiparous lactating sows: Impact of a high-fiber diet during pregnancy. J. Anim. Sci. 2006, 84, 2474–2481. [Google Scholar] [CrossRef]
- Verdon, M.; Hansen, C.F.; Rault, J.-L.; Jongman, E.; Hansen, L.U.; Plush, K.; Hemsworth, P.H. Effects of group housing on sow welfare: A review. J. Anim. Sci. 2015, 93, 1999–2017. [Google Scholar] [CrossRef]
- Sapkota, A.; Marchant-Forde, J.N.; Richert, B.T.; Lay, D.C. Including dietary fiber and resistant starch to increase satiety and reduce aggression in gestating sows. J. Anim. Sci. 2016, 94, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Hemsworth, P.H.; Rice, M.; Nash, J.; Giri, K.; Butler, K.L.; Tilbrook, A.J.; Morrison, R.S. Effects of group size and floor space allowance on grouped sows: Aggression, stress, skin injuries, and reproductive performance. J. Anim. Sci. 2013, 91, 4953–4964. [Google Scholar] [CrossRef] [PubMed]
- Andersen, I.L.; Boe, K.E.; Kristiansen, A.L. The influence of different feeding arrangements and food type on competition at feeding in pregnant sows. Appl. Anim. Behav. Sci. 1999, 65, 91–104. [Google Scholar] [CrossRef]
- DeDecker, A.E. Effect of Alternative, Individual and Group Housing Systems, and Management Factors in Group Pens on the Well-Being of Gestating Sows. Ph.D. Thesis, University of Illinois, Urbana, IL, USA, 2011. [Google Scholar]
- Sutherland, M.A.; Niekamp, S.R.; Rodriguez-Zas, S.L.; Salak-Johnson, J.L. Impacts of chronic stress and social status on various physiological and performance measures in pigs of different breeds. J. Anim. Sci. 2006, 84, 588–596. [Google Scholar] [CrossRef]
- Coffey, R.D.; Parker, G.R.; Laurent, K.M. Assessing Sow Body Condition; University of Kentucky Cooperative Extension Service: Lexington, KY, USA, 1999. [Google Scholar]
- Bench, C.J.; Rioja-Lang, F.C.; Hayne, S.M.; Gonyou, H.W. Group gestation housing with individual feeding, I: How feeding regime, resource allocation, and genetic factors affect sow welfare. Livest. Sci. 2013, 152, 208–217. [Google Scholar] [CrossRef]
- Barnett, J.L.; Hemsworth, P.H.; Cronin, G.M.; Newman, E.A.; McCallum, T.H.; Chilton, D. Effects of pen size, partial stalls and method of feeding on welfare-related behavioral and physiological responses of group-housed pigs. Appl. Anim. Behav. Sci. 1992, 34, 207–220. [Google Scholar] [CrossRef]
- Strawford, M.L.; Li, Y.Z.; Gonyou, H.W. The effect of management strategies and parity on the behavior and physiology of gestating sows housed in an electronic sow feeding system. Can. J. Anim. Sci. 2008, 88, 559–567. [Google Scholar] [CrossRef]
- Van der Peet-Schwering, C.M.C.; Kemp, B.; Binnendijk, G.P.; den Hartog, L.A.; Spoolder, H.A.M.; Verstegen, M.W.A. Performance of sows fed high levels of nonstarch polysaccharides during gestation and lactation over three parities. J. Anim. Sci. 2003, 81, 2247–2258. [Google Scholar] [CrossRef]
- Meunier-Salaun, M.C.; Edwards, S.; Robert, S. Effect of fibre on the behaviour and health of the restricted fed sow. Anim. Feed Sci. Tech. 2001, 90, 1562–1573. [Google Scholar] [CrossRef]
- Barnett, J.L. Modifying the Design of Group Pens with Individual Feeding Places Affects the Welfare of Pigs. In Fifth International Livestock Environment Symposium; Bottcher, R.W., Hoff, S.J., Eds.; American Society of Agricultural Engineers: St. Joseph, MN, USA, 1997; pp. 613–618. [Google Scholar]
- Dhabhar, F.S.; Miller, A.H.; McEwen, B.S.; Spencer, R.L. Stress induced changes in blood leukocyte distribution—Role of adrenal steroid hormones. J. Immunol. 1996, 157, 1638–1644. [Google Scholar] [PubMed]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Dhabhar, F.S.; Miller, A.H.; Stein, M.; McEwen, B.S.; Spencer, R.L. Diurnal and acute stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain Behav. Immun. 1994, 8, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.P.; Johnston, L.J.; Baidoo, S.K.; Shurson, G.C. Effects of a high-fiber diet and frequent feeding on behavior, reproductive performance, and nutrient digestibility in gestating sows. J. Anim. Sci. 2006, 84, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Baidoo, S.K.; Li, Y.Z.; Shurson, G.C.; Johnston, L.J. Interactive effects of distillers dried grains with solubles and housing system on reproductive performance and longevity of sows over three reproductive cycles. J. Anim. Sci. 2014, 92, 1562–1573. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.; Cao, M.; Lin, Y.; Che, L.Q.; Duraipandiyan, V.; Al-Dhabi, N.A.; Fang, Z.F.; Xu, S.Y.; Feng, B.; et al. Moderately, increased energy intake during gestation improves body condition of primiparous sows, piglet growth, performance, and milk fat and protein output. Livest. Sci. 2016, 194, 23–30. [Google Scholar] [CrossRef]
- Souza da Silva, C.; van den Borne, J.J.G.C.; Gerrits, W.J.J.; Kemp, B.; Bolhuis, J.E. Effects of dietary fibers with different physicochemical properties on feeding motivation in adult female pigs. Physiol. Behav. 2012, 107, 218–230. [Google Scholar] [CrossRef]
- Sun, H.Q.; Zhou, Y.F.; Tan, C.Q.; Zheng, L.F.; Peng, J.; Jiang, S.W. Effects of konjac flour inclusion in gestation diets on the nutrient digestibility, lactation feed intake and reproductive performance of sows. Animal 2014, 8, 1089–1094. [Google Scholar] [CrossRef]
- Salak-Johnson, J.L.; DeDecker, A.E.; Horsman, M.J.; Rodriguez-Zas, S.L. Space allowance for gestating sows in pens: Behavior and immunity. J. Anim. Sci. 2012, 90, 3232–3242. [Google Scholar] [CrossRef]
- Lopez, M. The Effects of High Fiber Diets and Feeding Partitions on the Well-Being of Group-Kept Sows. Master’s Thesis, University of Illinois, Urbana, IL, USA, 2015. [Google Scholar]
- Pacheco, E.; Salak-Johnson, J.L. Social status affects welfare metrics of group-housed gestating sows. J. Vet. Res. Anim. Husb. 2016, 1, 103–110. [Google Scholar]
- Pacheco, E. Assessing the Well-Being of Gestating Submissive Sows in Group Pens Using Multiple Welfare Metrics. Master’s Thesis, University of Illinois, Urbana, IL, USA, 2015. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).