1. Introduction
The challenge for modern agriculture is to meet the requirements of the constantly growing number of people in the world with efficient food production [
1]. Due to the limited possibilities of extending agricultural production via new arable land, fertilization is an essential factor, supporting and intensifying agricultural production [
1,
2,
3].
Fertile soil is the key to sustainable plant production on an industrial scale but very few agricultural soils have sufficient nutrient content to meet crop requirements [
4]. Most of them require the regular use of fertilizers that supplement low-level, or missing, micronutrients and macronutrients in the soil [
1,
2,
4].
Currently, we have reached the point where increasing the fertilizer dose does not bring satisfactory yield-generating effects; on the contrary, it has a negative impact on the natural environment. The excess of nutrients supplied with the fertilizer evaporates into the atmosphere or is washed into the soil profile, creating a network of pollutants [
5].
Efficient use of ingredients in the fertilizer sector is the basis for a green economy to produce more food and energy while reducing environmental pollution [
6]. Product innovations that meet these requirements include both the chemical composition of fertilizers as well as their physical properties and the method of application [
1,
5].
Suspension fertilizers are an important tool for agriculture that meets the set environmental and agronomic criteria [
6,
7]. Their liquid formula and high concentration of fertilizing salts enable the more effective absorption of nutrients by plants [
8]. These fertilizers are part of the sustainable management of mineral components, offering the possibility of balancing their distribution and also creating the possibility of utilizing harmful waste [
6].
A novel feature of this article is to show the possibility of using hydrated waste, the management of which is often problematic, in the production of liquid suspension fertilizers. Previous works on this subject are very sparse and deal with the subject one-sidedly. A holistic view on this issue, taking into account the characteristics of these types of fertilizers, their impact on the environment, practical aspects of their production and use, and the variety of the waste that can be utilized in their production, allows us to see the opportunities for a wide range of recipients.
The article presents the characteristics of suspension fertilizers, taking into account their advantages and disadvantages, as well as the impact of their use on the natural environment and the economic aspects. The stability of suspension fertilizers is discussed in detail, as the main parameter determining their application. The possibilities of using various types of waste in their production are also discussed.
3. Benefits and Limitations Resulting from the Production and Use of Suspension Fertilizers
Suspension fertilizers combine the advantages of liquid and solid fertilizers but also have unique features, making them an attractive proposition on the fertilizer market.
The main advantage of these fertilizers is the high concentration of nutrients they offer while maintaining a liquid form. The concentration of fertilizer salts in the suspension is similar to the concentration of solid fertilizers and, therefore, is much higher than that achievable in fertilizers in the form of a solution [
8,
13,
14].
Raw materials used in the production of suspension fertilizers may be of lesser purity, as their complete solubility is not required [
13,
14,
16,
17]. It is also possible to use dusty materials and those that are sparingly soluble in water, such as finely ground rock phosphate [
18]. A useful feature of the suspension form of fertilizer is the possibility of using waste with a high water content for its production that contains valuable fertilizing ingredients [
8]. Waste in the form of suspension from the chemical and agri-food industries is not suitable for the production of other forms of fertilizers due to high humidity and pollution issues [
8].
Since the content of nutrients is not limited by their solubility, it is easy to obtain fertilizer suspensions with almost any proportions among the nutrients [
8,
17]. This creates great flexibility in the selection of fertilizer mixtures [
6]. Currently, almost all substitutes of classic solid fertilizers are produced in the form of suspension [
11].
Having any combination of components in the fertilizer suspension makes it possible to adjust the fertilizer composition to the specific needs of the cultivated plants [
6,
8]. At the same time, different variants of the ratio of individual fertilizer components enable their application according to soil fertility [
11].
An important advantage of this type of product is the possibility of introducing additional substances improving the condition of plants, together with the nutrients [
17]. Microelements in the desired concentrations and forms can be included in the structure of suspension fertilizers without any problems [
8,
11]. It is also possible to use fertilizer suspension as a carrier of plant protection products, vitamins, nitrification inhibitors, etc., regardless of their physical form [
8,
17]. Pesticides that are introduced into a suspension fertilizer may dissolve in it or be dispersed as fine, insoluble particles or immiscible liquids [
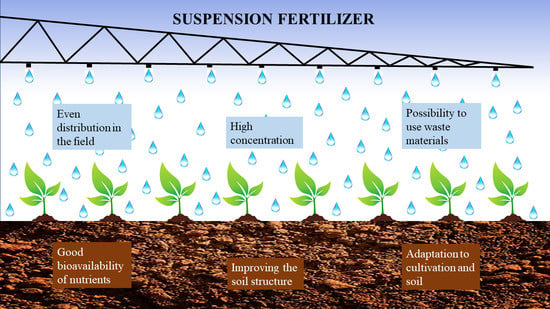
12]. The advantages of the agricultural application of suspension fertilizers are shown in
Figure 2.
The wide range of possibilities for enriching suspension fertilizers with additional ingredients allows for combining fertilization with other agrotechnical treatments. In addition to combining fertilization and plant protection, a variant including irrigation is also available [
8].
A common feature of liquid fertilizers, in both clear and suspension fertilizers, is the high homogeneity of the chemical composition [
8]. This results in significant functional benefits related to the even distribution of fertilizer on the field [
7,
8,
18]. There is no separation of components, as is the case with solid fertilization [
6,
7]. Precise dosing is particularly important in the case of microelements introduced into the fertilizer in small amounts [
6,
8].
The liquid form ensures the better availability and utilization of nutrients by plants in comparison with the equivalent doses of solid fertilizers [
6,
7]. Higher yields are also the result of a high, even distribution of nutrients and the adjustment of their composition to the individual needs of the cultivated plants [
6]. Moreover, suspension fertilizers give better yield results in those years with lower rainfall [
6].
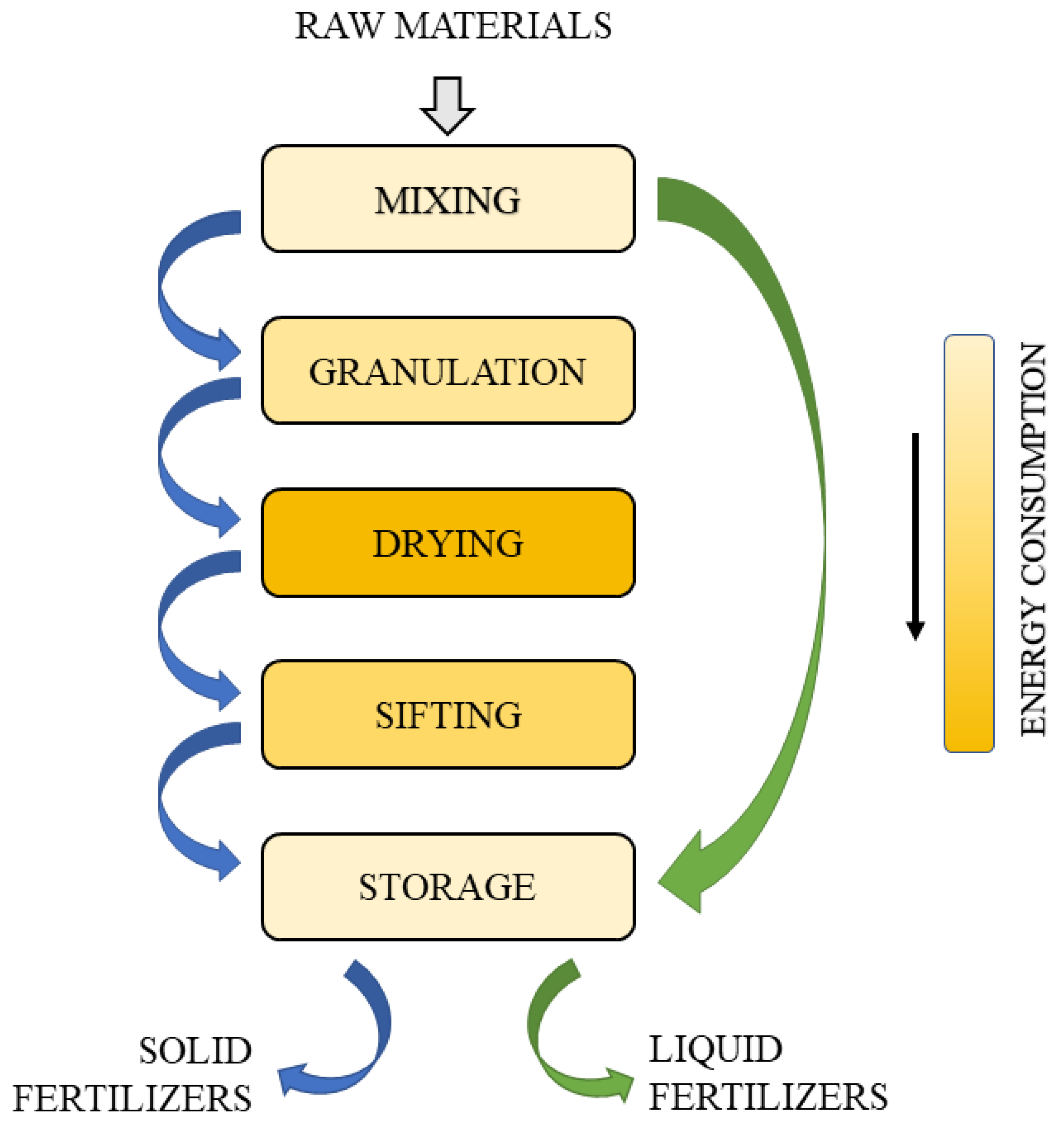
The production of granulated fertilizers at the stage of evaporation and granulation causes the emission of substances harmful to the environment into the atmosphere—mainly ammonia vapors, nitrogen oxides, sulfur oxides and a significant amount of dust from the finished products. In the production of liquid fertilizers, the technological line has been shortened at the most burdensome stages for the environment: granulation, drying, classification and packaging [
11]. Additionally, hermetic devices and pumps limit the emission of harmful dust and gases [
8].
Figure 3 shows a comparison of the production stages of solid and liquid fertilizers.
Suspension fertilizers are convenient to use, transport, reload and store [
8,
18]. Their production and operation are not very laborious because they can be fully mechanized [
6,
13,
14]. As the fertilizer is liquid, it can be pumped through pipes and can be sprayed onto the soil through nozzles. Therefore, the amount of work associated with the application is reduced [
19]. These fertilizers do not require packaging, which reduces the consumption of non-biodegradable packaging, and do not contain ballast substances, e.g., anti-caking agents [
8].
The use of suspension fertilizers improves the soil structure, thanks to gelling clay substances. Before applying the fertilizer, these substances are designed to disperse the insoluble elements of the suspension system and, when applied to the soil, increase its absorbency [
8].
In the case of liquid fertilizers, the difficulties related to segregation and caking, which accompany the storage of solid fertilizers, are eliminated [
18]. Due to its liquid form, the explosion hazard associated with the use of solid ammonium nitrate was also excluded [
8,
19].
Despite numerous advantages, suspension fertilizers also have disadvantages that limit their use. Their main disadvantage is a short storage period, due to their limited stability over time. Therefore, these fertilizers are usually produced for local needs and their storage time is at most a few days [
14]. Suspension fertilizer stations must be located close to the fertilized farmland. The use of fertilizer suspensions is most advantageous in the case of large farms with non-dispersed arable land [
17]. In addition, suspension fertilizer can have a corrosive effect on storage tanks [
20].
4. The Impact of Suspension Fertilizers on the Natural Environment
Over many years, a simple correlation between the amount of fertilizer produced and the production of food has proven itself. It meant that the main goal of the fertilizer industry was to provide agriculture with a sufficiently large quantity of fertilizers. In order to meet these requirements, many new fertilizer factories were launched, and the production capacity was increased [
8]. This had a negative impact on the condition of the natural environment, due to the emission of toxic gases, sewage, dust and the produced waste [
8,
20].
Over-fertilization in the intensive agricultural production system is usually associated with the low efficiency of their use by arable crops. Excess amounts of nutrients, which the soil matrix cannot retain, are released into the atmosphere, e.g., N
2O, NO
x, NH
3, and N
2, and into groundwater and/or surface waters, e.g., NO
3, HPO
42−, H
2PO
4− [
21].
Elements such as nitrogen, phosphorus and potassium are the main factors determining plant production but, at the same time, they can be a potential source of hazards to environmental components such as water, soil, and the air. For this reason, one of the main pillars of sustainable fertilization is the effective use of nutrients [
6].
In terms of ecological fertilization, suspension fertilizers demonstrate a wide range of activities. Reducing the nuisance of fertilization for the environment can be achieved by using a liquid form of fertilizer [
10,
11]. It ensures better utilization of nutrients by plants, limiting the amount of leached nutrients into groundwater. On the other hand, the possibility of fertilization under the soil surface reduces the gaseous losses of nitrogen compounds [
6,
22].
Greater accuracy in the application of fertilizers in the field with the use of liquid fertilizers significantly improves the efficiency of fertilization [
10]. The excessive local concentration of a given nutrient causes its loss because the plant is unable to fully absorb it. Over time, nutrients are leached out of the root system, becoming inaccessible to the plant and accumulating in the environment.
Figure 4 shows the environmental benefits of using suspension fertilizers [
6,
10].
Using the suspension form of a particular fertilizer is also ecologically beneficial, due to the possibility of utilizing waste substances from other industries in its production [
20]. As a result, valuable components can be recovered from the waste stream, and the amount of waste deposited and the extraction of natural resources is reduced [
9,
20].
The popularization of suspension fertilizers in common use is a response to the current and possible future problems related to the growing amount of waste and the depletion of natural resources. Suspension fertilizers fit into the changing structure of farms, manifested by increased mechanization and the drive toward large-scale, undivided arable land.
5. Economic Effects of Using Suspension Fertilizers
The production and application of suspension fertilizers is justified from the economic point of view, which has a major impact on the demand for this form of fertilizers among farmers. These benefits are a consequence of the physical form of the fertilizer, which is the suspension.
The production of mineral fertilizers is one of the large-tonnage processes, where production costs strongly depend on the raw materials used [
23]. In the case of suspension fertilizers, it is possible to use raw materials of lower purity and limited solubility, which are usually cheaper [
11,
24]. In their production, for example, dusting materials and sparingly soluble salts of micronutrients can be used [
8,
24].
Agri-food production largely contributes to the exploitation of natural resources. Their scarcity leads to higher production costs in the sectors that use them, including the fertilizer sector [
4]. In this aspect, suspension fertilizers create additional opportunities to reduce costs by managing waste substances for their production [
8,
23].
The technology of the production of suspension fertilizers requires lower operating and investment outlays [
8,
11]. Compared to the production of solid fertilizers, the construction of granulation and drying nodes is unnecessary, which account for about 60% of fertilizer production costs [
6,
11]. The liquid form ensures fewer losses related to storage, transport and application [
6]. Other benefits of using liquid fertilizer include the elimination of the cost of water evaporation [
6,
8,
19].
Savings are also generated by reducing the number of passes in the field when fertilization is combined with other agrochemical treatments. This is a significant saving, due to rising fuel and labor prices. A high concentration of suspension fertilizer reduces transport costs [
7].
Since no waste substances are generated in the production process, the costs of waste disposal or storage are eliminated [
9,
16].
6. The Required Parameters for Suspension Fertilizers
The primary role of fertilizers is to increase the quality and quantity of the crop. In order to achieve the assumed yield-forming effects, each form of fertilizer has individual requirements resulting from its physical form.
Obtaining the desired parameters of suspension fertilizers requires an appropriate method of their production, the use of appropriate raw materials and semi-finished products, as well as the availability of specialized equipment [
8].
Due to the large use of liquid suspension fertilizers in the United States, many procedures are the result of operating experience in this field by the American Tennessee Valley Authority [
8].
In the case of suspension fertilizers, it is important to maintain the homogeneity of composition in the entire volume of the fertilizer, from its production to the moment of application [
8]. A stable structure during storage determines the possibility of trading these fertilizers [
8,
13,
14]. In suspensions with low gel strength, the suspended particles easily agglomerate into larger particles and sink faster. If the falling particles form a fluffy sludge, they are easily re-dispersed by agitation or recirculation. If, on the other hand, the formed sediment is rubbery, it is impossible to restore the gel structure. To remedy this, it is necessary to use more stabilizing agents, improve the quality of the dispersion, reduce the size of the suspended particles, quickly cool the mixture to produce smaller crystals, or select the appropriate ingredients to create crystals of the appropriate shape. Long storage time influences the growth of crystals that, due to their weight, are more and more difficult to keep suspended in the gel structure. This is the result of slow cooling and cyclic temperature changes. This effect can be minimized by periodic stirring or bubbling. The separation of a clear liquid layer on the surface or in the middle of the suspension, called the supernatant, is an undesirable phenomenon. This is due to shrinkage of the gel structure, called syneresis. It may cause the excessive growth of crystals at the interface, which may result in the formation of deposits capable of cementing the bottom of the reservoir. A blend with such a defect can be easily homogenized by mixing and recirculation. The formation of a supernatant layer indicates improper preparation of the suspension, in which the gel structure is physically unstable until syneresis occurs. In order to improve the parameters of such a suspension, it is necessary to use a larger amount of stabilizing agent at the preparation stage or to better disperse it [
12]. Crystals can also be formed as a result of vibrations generated in the transport process. The number of deposited crystals should not exceed 2% of the product volume [
8]. For the suspension to be allowed for use, the formed crystals should pass through a 0.246 mm (60 mesh) sieve [
8,
12]. However, if the crystals present in the suspension can be crushed by recirculation of the suspension or bubbling gas, the fertilizer is still fit for use [
25].
For application reasons, an important parameter of suspension fertilizers is the possibility of free, gravity outflow. This value, determined by the pourability index according to American standards, should be 98% at 27 °C [
8].
The desired rheological properties are a high apparent viscosity at low shear forces and low apparent viscosity at high shear forces [
8,
12,
25]. Preferred viscosity values are not more than 1000 mPa·s at 27 °C and 1500 mPa·s at 30 °C [
8,
25]. Suspensions exhibiting this viscosity are stable under pumping and gravity flow, i.e., low shear conditions. On the other hand, low viscosity at high shear rates facilitates the formation of drops during the application of the fertilizer suspension [
8,
25]. Therefore, this parameter is necessary to determine the technical parameters of equipment intended for the application of suspension fertilizers [
13,
14]. The permissible range of density for suspension fertilizers should be in the range of 1200–1400 g/cm
3, while the fluidity determined with the use of the outflow cup should be characterized by the outflow time within the range, at 10–15 s [
26].
For suspension fertilizers based on monoammonium phosphate, additional criteria were established due to the impurities introduced with it into the fertilizer. For such a fertilizer to be successfully stored for a long time, the quantity of water-insoluble solids, the total amount of cationic impurities (Fe
2O
3, Al
2O
3, CaO and MgO) and the ratio of these cations to fluorine are important. Monoammonium phosphate products with a low cationic to fluorine ratio, low water-insoluble solids and low R
2O
3 (Fe
2O
3 + Al
2O
3) levels result in a higher-quality suspension that is stored longer than products with a higher cation to fluorine ratio and high solids insoluble in water and R
2O
3 [
25].
Research by the Tennessee Valley Authority has shown that the addition of fluoride ions can improve the physical properties of monoammonium phosphate fertilizers containing metallic impurities. In granular ammonium phosphate, compounds that form a sludge and gel have been identified, after the use of ammonia in the production of suspension fertilizers. The main cause of the gelation or solidification of suspension products made of monoammonium phosphate, where all phosphorus present is in the form of orthophosphate, are iron, aluminum and ammonium phosphates derived from monoammonium phosphate, which, when converted to suspension fertilizer, forms an iron-aluminum-phosphate-water gel. This gel traps and retains a large volume of water, causing the suspension to become highly viscous or to solidify during production and/or storage [
27]. The total amount of cationic impurities, expressed as the percentage by weight of Fe
2O
3, Al
2O
3, CaO and MgO, in granular monoammonium phosphate, in relation to the amount of fluorine, should be at a maximum of 3, and the solids content should be no more than 17% [
25,
27].
It was confirmed in related studies that the addition of fluorine prevents the precipitation of the metal-phosphate-H
2O compounds contained in ammonium phosphate. Fluoride precipitates magnesium, calcium, and aluminum as fluoride instead of phosphate compounds, which greatly increases the amount of the solution phase, improves the solubility of nitrogen and phosphate in water and prevents the formation of sludge or gels during storage. The maximum amount of fluorine addition, in the form of 23% fluorosilicic acid, is only 0.7% by weight, which is decomposed in suspension into ammonium fluoride, silica gel and ammonium fluorosilicate. When fluorosilicic acid is added after the ammonification step, or in a reaction step where the pH is at least 6, the silica gel hydrates, resulting in an increase in viscosity. Adding it before the ammonification step releases fluorine as NH
4F, and the heat of the reaction dehydrates the silica gel, preventing the slurry from gelling. The addition of fluorine in the form of fluorosilicic acid also significantly reduces the content of P
2O
5, which is insoluble in citrate from monoammonium phosphate [
27].
The given recommendations for suspension fertilizers are relatively easy to meet without the use of specialized equipment and with good-quality raw materials.
7. Stability of Fertilizer Suspensions
Stability is the basic factor determining the quality of the suspension. The components of the fertilizer suspension can be kept in a dispersed form only in the presence of a thickener [
15]. A high concentration of nutrients in suspension fertilizers is possible, due to the introduction of a substance keeping them in suspension in a regular manner [
7]. The main factor ensuring the normative properties of the suspension fertilizer is the dispersion gelling agent [
8]. It plays a stabilizing role, giving the liquid the features of a non-Newtonian system where sedimentation is inhibited. The addition of this factor is also accompanied by an increase in the viscosity of the liquid [
8,
11].
For suspension fertilizers, high gel strength values are highly desirable, affecting the stabilization of suspensions during transport and storage. Moreover, dispersing agents introduced into liquid fertilizers must have an appropriate chemical composition, especially a limited content of heavy metals, and should not interfere with the availability of nutrients for plants [
11].
Clay minerals work best in this role [
8,
13]. They have a large specific surface, which results in excellent sorption properties [
28]. Their addition means that, apart from their swelling and ion-exchange action in the fertilizer, after introducing them into the soil they will improve its structure [
11,
29]. This influences the increase of water sorption in the soil and the accumulation of nutrients, which significantly improves the development of vegetation [
14,
28].
Clay minerals consist of crystals with a well-defined structure that is mainly layered, with a length of less than 2 µm and a thickness of less than 10 nm [
8,
28]. The silicate layers are connected by weak interlayer bonds in which the weakly bound cations can be exchanged for other cations and water [
8]. The interlayer water loosens the inter-package structure, causing swelling and the formation of crystal agglomerates, forming a needle-like gel network structure [
8,
11]. Taking into account the crystal structure, clay minerals can be divided into kaolin, with a uniform chemical composition, and smectite, which differ significantly from each other [
28].
Minerals from the smectite group are layered silicates with a block structure. There are exchangeable cations between the packages, most often Mg
2+ and Ca
2+, as well as Na
+. The type of dominant cations in exchange positions affects the properties of the clay material [
30]. A common property of smectite-rich rocks is the ability to absorb cations and to swell, disperse with water, and form thixotropic suspensions [
7,
30].
In industry, clay minerals in the amount of 0.5–3% are most often used to stabilize fertilizer suspensions [
8]. These are usually minerals from the smectite group, such as attapulgites and bentonites [
8,
10,
13]. For this purpose, organic compounds can also be used, e.g., sorbitol, starch, protein hydrolysates and keratin hydrolysates 1 [
7,
8,
31].
In the United States, attapulgite clay is generally used in the production of fertilizer suspensions [
11]. It perfectly meets the requirements for the production and use of fertilizer suspensions; however, it is only available in limited areas in the USA. The main component of this mineral is clusters of hydrated threadlike crystals of magnesium aluminum silicate [
11,
32]. In the water environment, these agglomerates break down during dispersion, forming a gel with a needle-like mesh. This structure increases the viscosity of the medium and inhibits the sedimentation process of solid fertilizer particles [
11,
19].
Bentonite is also an aluminosilicate with a layered structure, with the ability to form a gel, especially after activation with Na
2CO
3 solution [
11]. Bentonites are formed as a result of the weathering of silicate-aluminum rocks and subsequent sedimentation in the aquatic environment [
7].
The effect on gel strength was also noted with the addition of chemical agents, such as tetrasodium diphosphate and fluorosilicate acid. The increase in gel viscosity and strength in suspensions containing polyphosphates is caused by the retraction of condensed phosphates to orthophosphates. Orthophosphates are less soluble compared to polyphosphates, so the number of suspended particles in the suspension increases [
12].
To prevent the potential degradation of the gel, the gelling agent is pretreated. As a result, it is possible to crush them to the maximum, activate them with alkali metal salt solutions and introduce them to production directly in the form of a gel structure [
8].
Despite the use of the correct gelling agent, there may be disadvantageous consequences changing the properties of the system. In the fertilizer suspension, which is a multicomponent and multiphase system, this may take place under the influence of an agent stabilizing harmful chemical reactions and physical phenomena. Often, freshly prepared suspensions have a greater gel strength than suspensions stored for longer (more than 24 h). This is due to air bubbles that are drawn in at the stage of preparing the suspensions. The strength of the gel decreases as the air bubbles leave the system. Gel strength is also dependent on how the suspension has been handled in earlier steps. The strength of the gel in the suspension immediately after mixing is lower than it is in the same suspension when left for several hours. One very important aspect in the case of agricultural suspensions is the ability to quickly recover gel strength after the breakdown of the gel structure, due to mixing and pumping [
8,
12].
Temperature may also have a negative impact on suspension stability [
10]. Due to temperature changes during storage, large beam-shaped crystals may crystallize out of a saturated solution. Their presence in the suspension makes it difficult to pump the product and also causes the overgrowing of tanks and spray nozzles, disrupting the fertilization process [
8,
10].
One of the methods of preventing this phenomenon is the addition of small amounts of a substance modifying the shape of the crystals. Such a modifier may, for example, be H
2SiF
6, introduced into the fertilizer in the amount of 0.3–0.5%. It prevents the flocculation process that promotes sedimentation and the syneresis process. Syneresis is a phenomenon caused by the shrinkage of the gel, as a result of which, a layer of clear liquid appears over the gel. The presence of this layer promotes the nucleation and growth of large crystals. In order to eliminate this undesirable effect, fertilizer producers increase the amount of the added gelling agent and have also perfected the method of its activation [
8]. A practical dispersing treatment is, for example, the addition of tetrasodium pyrophosphate, which improves the dispersion [
12].
However, it is mainly necessary to ensure the correct pH of the solution. In the case of fertilizers based on ammonium phosphate, this results in the precipitation of fine (NH
4)
2HPO
4 crystals in place of large NH
4H
2PO
4 beam crystals [
8].
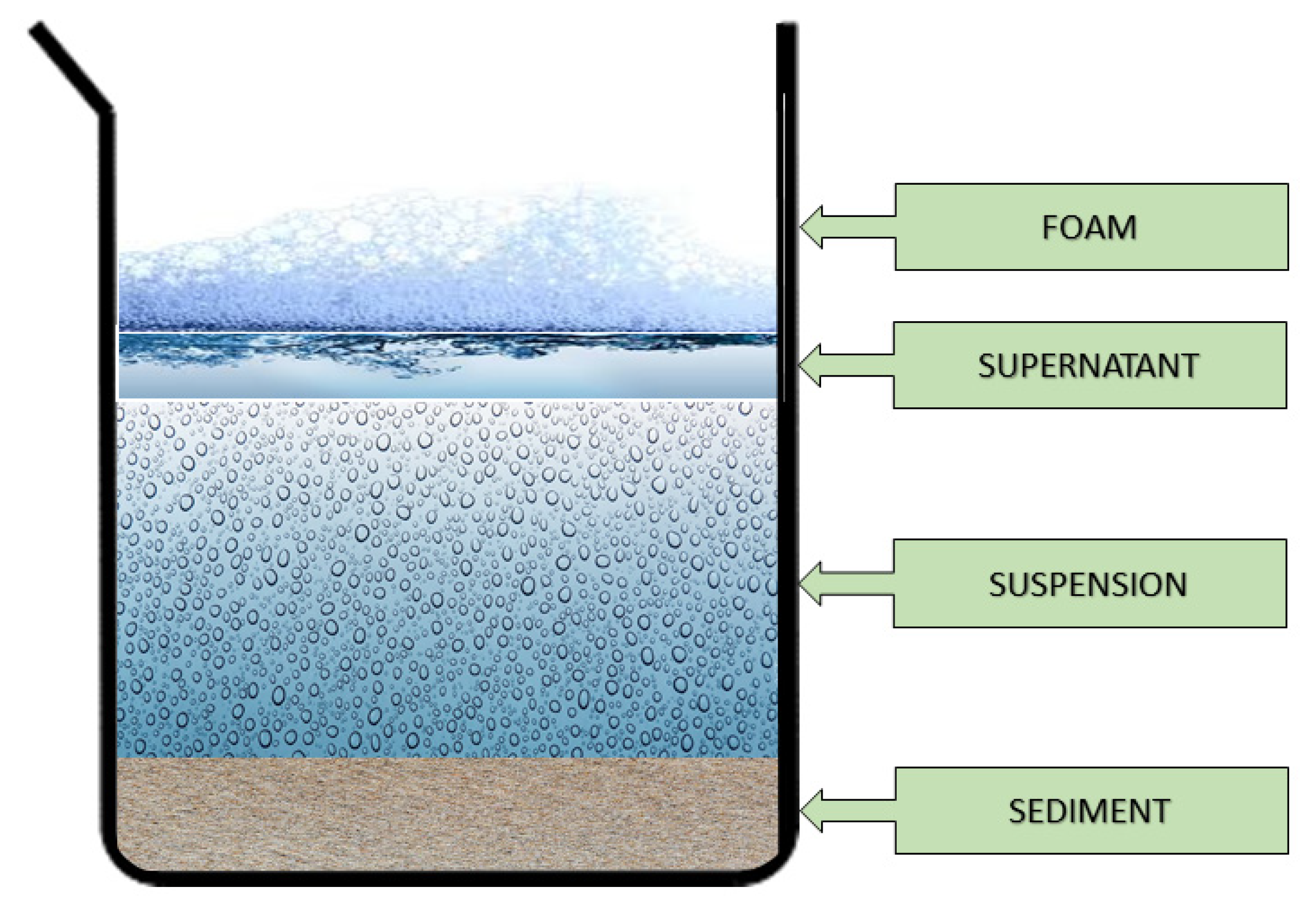
The production and longer storage of suspension fertilizers may be accompanied by the process of stratification. As a result, layers appear in the product, as shown in
Figure 5, in the order below: sedimentation, a cloudy layer of suspension, a layer of clear liquid (supernatant) and a layer of permanent foam. The reason for this may be errors made during the production of fertilizers; it may also be related to the absorption of air bubbles and carbon dioxide, the gradual desorption of air, and the coexistence of the emulsion phase formed during the emulsification of mutually insoluble liquids, which occurs when combining suspension fertilizers and plant protection products [
8,
12].
The stability of the system may be positively influenced by the chemical reactions taking place in it, eliminating the effects of reducing the viscosity of the system, which may be caused by air desorption or the biodegradation of organic components, in the case of mineral-organic fertilizers. Positive effects, in this aspect, are observed by using poly-phosphorus compounds or condensed phosphates in fertilizers as a source of phosphorus. Polyphosphoric anions have the ability to sequestrate Fe
3+ and Al
3+ ions and, successively, as the poly-phosphorus chain decays into ortho-phosphorus ions, they release aluminum and iron compounds in colloidal form. Thus, the viscosity of the system increases, inhibiting the sedimentation process [
8].
The use of phosphate raw material in the form of post-neutralization sludge also brings a number of benefits because, apart from the very good absorption of phosphorus it offers, it has a stabilizing effect on the fertilizer suspension. Replacing the phosphorus raw material in the fertilizer with at least 50% from post-neutralization sludge eliminates the need to use a stabilizing additive. The high stability of suspensions in systems with post-neutralization sludge is the result of a strong salt effect. This increases the solubility of individual fertilizing components so that the obtained fertilizer solution has a density close to that of the suspension it contains. As a consequence of increasing the density of the solution, the suspended particles are more easily kept in the fertilizer suspension and do not sediment. If the fertilizer is supplied with phosphorus that is 50% from post-neutralization sludge, it is not advisable to use additional stabilizers, as this would increase the viscosity of the fertilizer unnecessarily and make their application difficult. By replacing the phosphate raw material, with at least 20% from post-neutralization sludge, a better-quality product in terms of stability is obtained than in the case of using ammonium phosphate entirely [
16].
The stability of the suspensions is a key parameter determining the quality of this type of liquid fertilizer. To ensure it is at the appropriate level, it is necessary to use a stabilizing agent and appropriate techniques for preparing suspensions. Nevertheless, the occurrence of delamination can, in most cases, be readily corrected by remixing.
8. Suspension Fertilizers from Waste Materials
Most production processes are accompanied, as well as the main product, by the generation of significant amounts of waste [
23,
26]. They are generally irrelevant to the main production but often have valuable components from the point of view of other technologies. Industrial waste is used to a varying degree but still has great economic and pro-environmental potential [
9,
26].
A new perspective of using the suspension form of fertilizers is the possibility of utilizing environmentally harmful waste in their production cycle [
8,
20]. Many wastes contain chemical compounds that are valuable from the point of view of fertilization, but some of their properties preclude their economic processing [
4,
26].
The disadvantages of the waste include low solubility, high moisture content, unstable composition and high fragmentation, resulting in excessive dusting [
20,
26]. These features make their use in the continuous production of fertilizers difficult, and sometimes impossible [
26]. Clear liquid fertilizers have the least possibilities of using cheaper alternatives in the form of waste, due to the requirement of high purity of raw materials. However, in the case of solid granular fertilizers, the need to evaporate a large amount of water makes the process unprofitable. Compounds contained in waste may deteriorate the properties of such fertilizer—critical humidity, compressive strength of granules, and storage time [
20].
Nevertheless, these factors are not a problem in the production of suspension fertilizers. In this case, the nutrients do not have to be completely dissolved, and high water content is not an obstacle [
20,
26]. An additional advantage is batch production, which allows the use of raw materials with a variable composition [
26].
From the agronomic point of view, the use of waste for the production of fertilizers is possible when they contain significant amounts of plant nutrients and when their composition contains a limited quantity of substances that are not only toxic to plants but also to those humans and animals consuming the crops. Moreover, the components contained in the waste material should not cause changes resulting in the deterioration of the properties of the fertilizer and negatively affect the production technology [
20,
23]. In economic terms, the cost of their processing should not reduce the market competitiveness of the fertilizer based on them [
23]. However, for safety reasons, their use should not generate toxic gases [
20].
Converting waste into valuable fertilizer has positive environmental effects. First of all, the extraction of natural resources is reduced, and the amount of landfilled waste is limited. At the same time, the raw material that is of waste origin is much cheaper, which allows manufacturers to reduce production costs and, as a result, offer cheap and effective suspension fertilizer [
9,
23].
Recovering valuable components from the waste stream is part of the circular economy strategy. It aims to increase resource efficiency and reduce the dependence on imported raw materials [
31]. This is especially true of the phosphorus raw material, the limited deposits of which, in the form of apatite and phosphorites, contain significant amounts of impurities [
4,
31].
Many effective attempts have been made to manage waste from various industries. One of them is the management of sludge from the production of extinguishing agents formed during the alkaline hydrolysis of keratin, which is rich in phosphorus, potassium and calcium. By supplementing the waste with nitrogen, magnesium and microelements, a multicomponent suspension fertilizer was obtained with the desired agronomic and technological properties. Due to the use of magnesium and micronutrients in the form of an acetate solution and polypeptides, a synergistic protection effect against plant pathogens was obtained. On the other hand, the presence of an organic form of nitrogen, which is less freely available to plants, prolongs the effect of the fertilizer [
8].
Work on suspension fertilizers using post-extraction peat sludge produced in the process of alkaline extraction of humus compounds that are used for the production of drugs and peat preparations was also successfully completed. Nitrogen compounds in the form of ammonium sulfate and urea, microelements, and bentonite for stabilization purposes were introduced into the waste to produce a fertilizer suspension. During the production process, nitrogen compounds contained in peat were also activated. As a result, a fertilizer with confirmed agricultural effects and good physical properties was obtained [
8].
Livestock waste, such as poultry farm manure, can also be used for fertilization. To reduce the population of pathogenic bacteria and fungi, acidic substances, such as phosphoric and sulfuric acid or urea phosphate, can be added to this type of waste. The specific detoxification of farm waste, increasing the concentration of nutrients and neutralization of the suspension with ammonia water to a pH in the range of 6.5–7, results in a satisfactory fertilizer [
8].
The possibility of processing filter sludge from the production of extractive phosphoric acid into suspension fertilizer was also tested. This waste is characterized by not only a high moisture content of nearly 50% but also a high P
2O
5 content of approx. 20%. In order to transform the waste into fertilizer, the content of nutrients was supplemented by adding ground phosphate rock, potassium salt, urea-ammonium nitrate solution, ammonium sulfate, urea and bentonite stabilizing the whole, in amounts depending on the desired composition of the final fertilizer. Additionally, in this case, many positively assessed fertilizer combinations were identified [
26].
The production of magnesium sulfate, consisting of the decomposition of burned magnesite with sulfuric acid, is accompanied by the release of a precipitate, the so-called post-magnesium mud. It is a valuable source of calcium, magnesium and sulfur, which can be successfully reused in the production of suspension fertilizers [
9].
The effective use of sodium sulfate solutions, generating by-products via the production of trimethyl-propylene, in the production of suspension fertilizers was also confirmed. The use of this waste does not reduce the total concentration of nutrients. The only condition for suitability here is adequate purity [
14].
An interesting proposal is the use of waste potassium sulfate from the production of biofuels. The suspension fertilizers produced using it as a basis have favorable rheological properties and a high content of nutrients. There is no need to apply an additional substance stabilizing the suspension and there is a great possibility of configuration with other raw materials [
26].
Industrial processes generate a great deal of waste, the hydrated form of which makes it difficult to process economically. On the other hand, the disposal of waste in the production cycle of suspension fertilizers is relatively simple and does not require expensive modernization of the installation. In this matter, it is important to coordinate the activities of fertilizer producers and industrial plants.