Infrared Thermography of the Mammary Gland in Sows with Regard to Health and Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Housing and Feeding
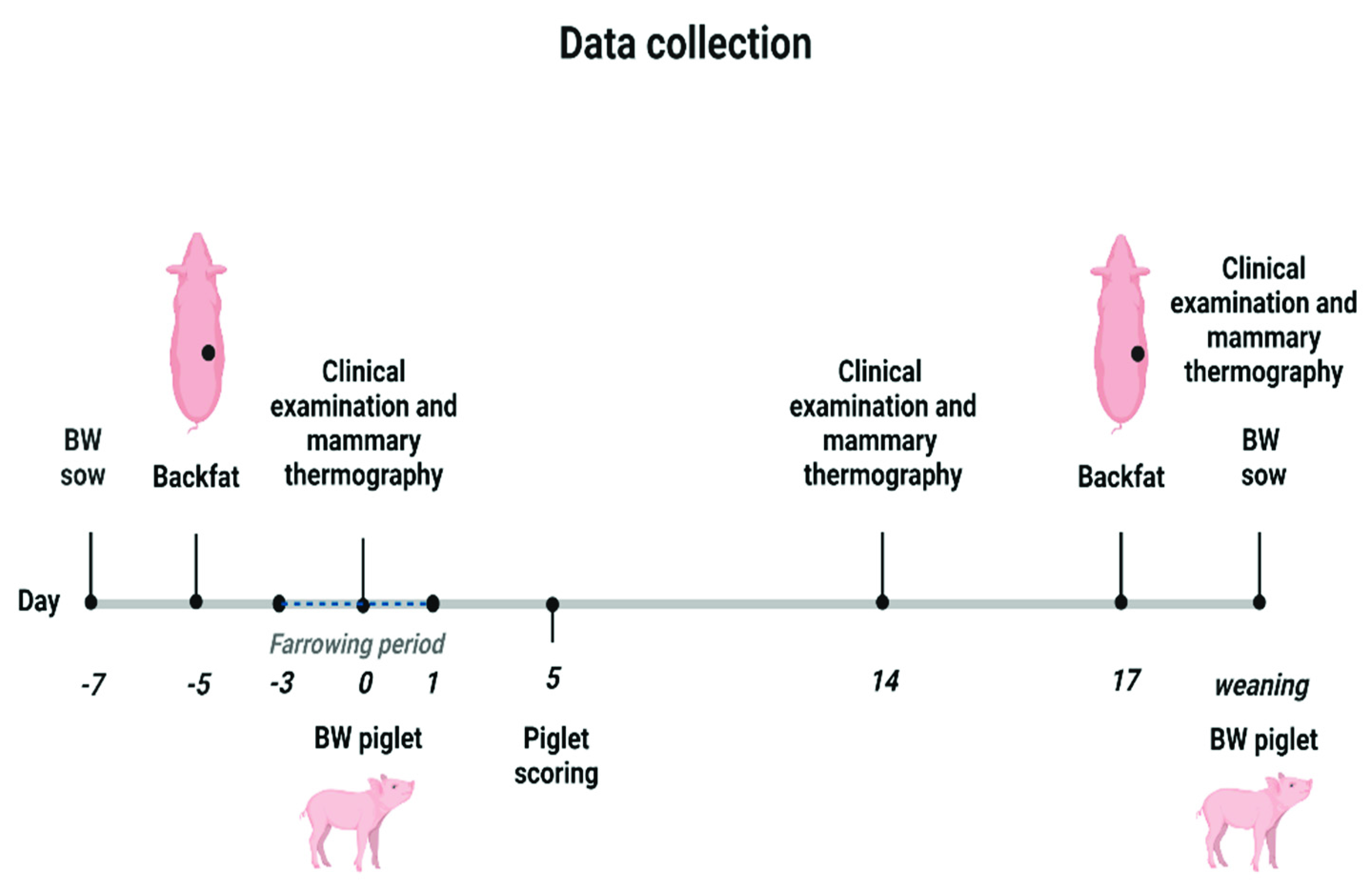
2.2. Design of the Study and Data Collection
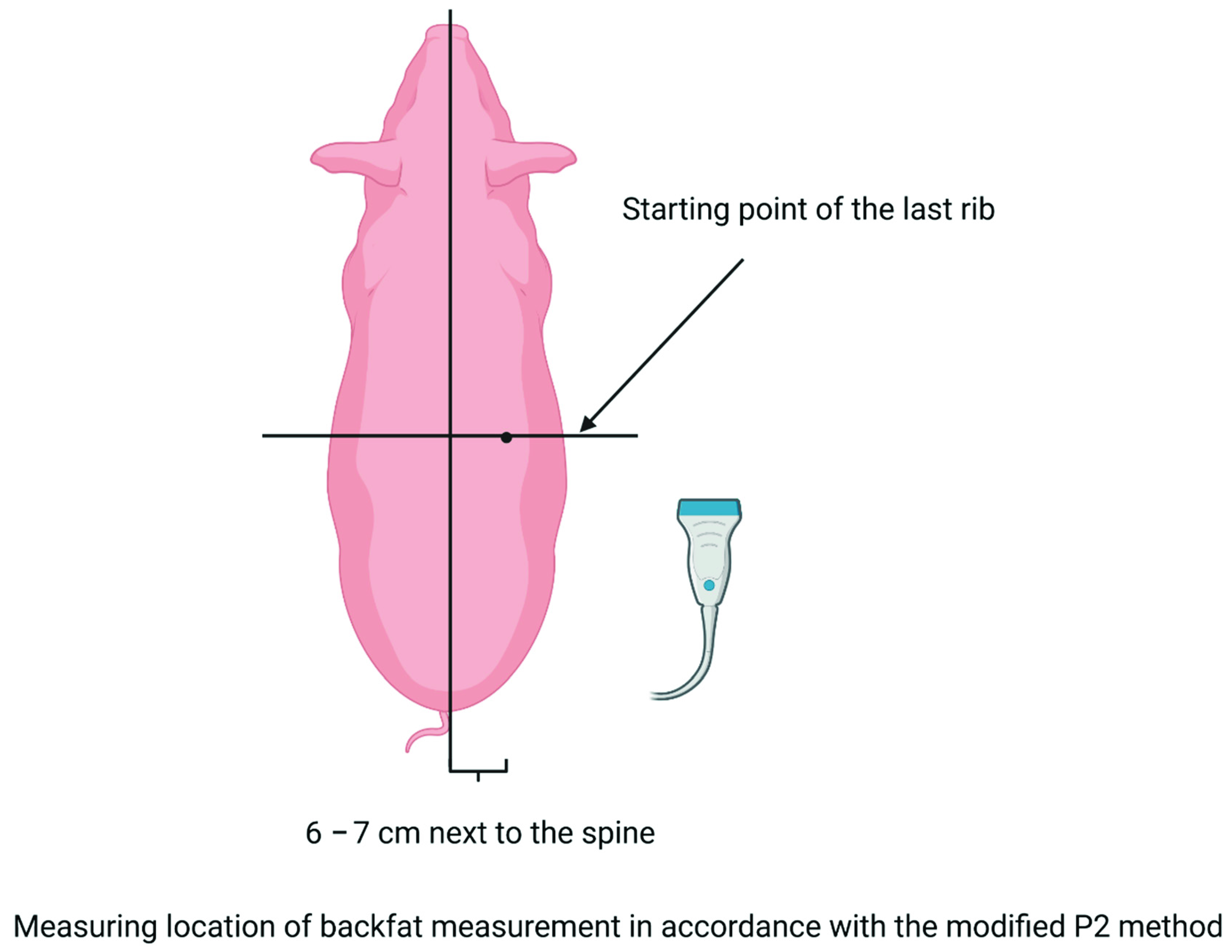
2.3. Measurements
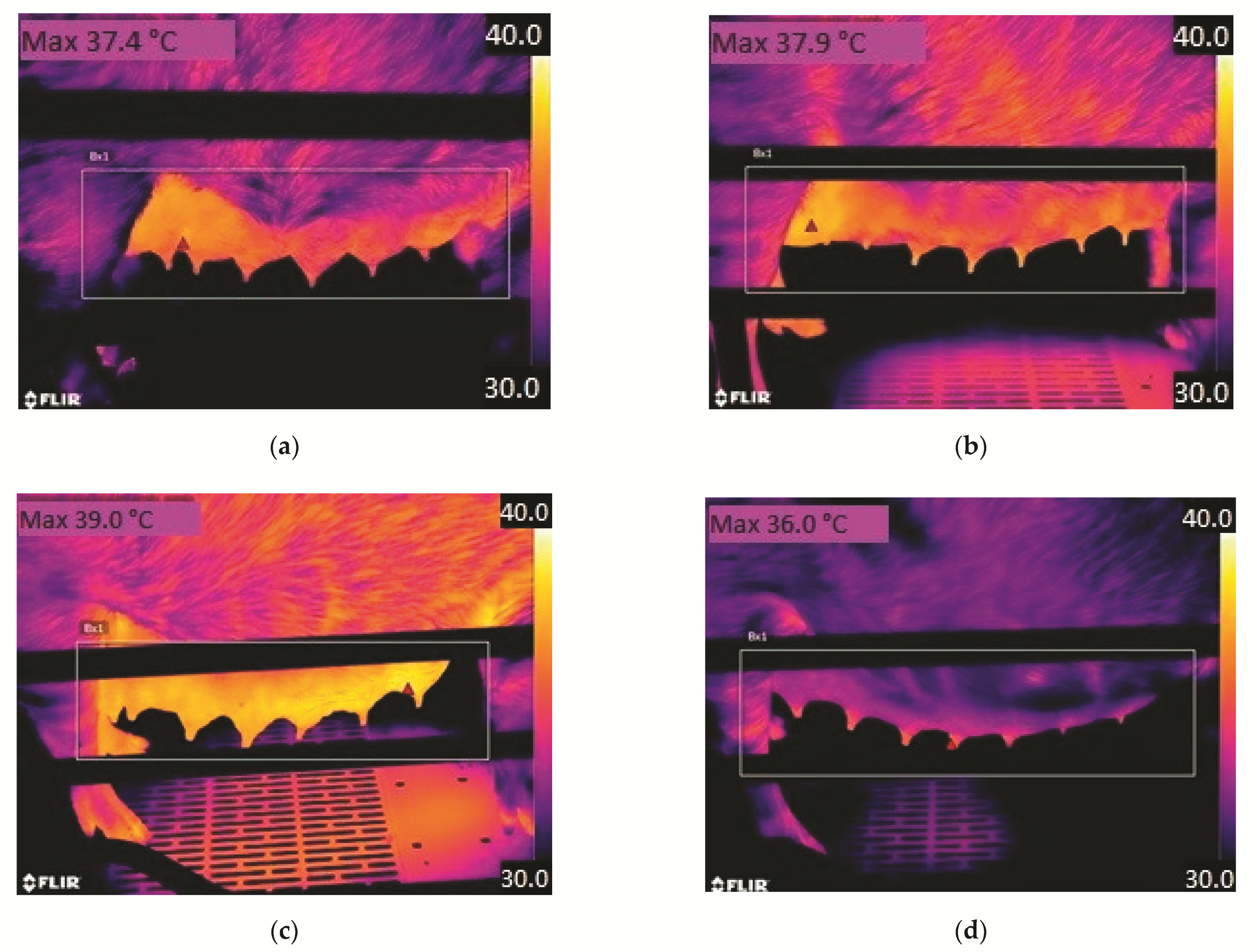
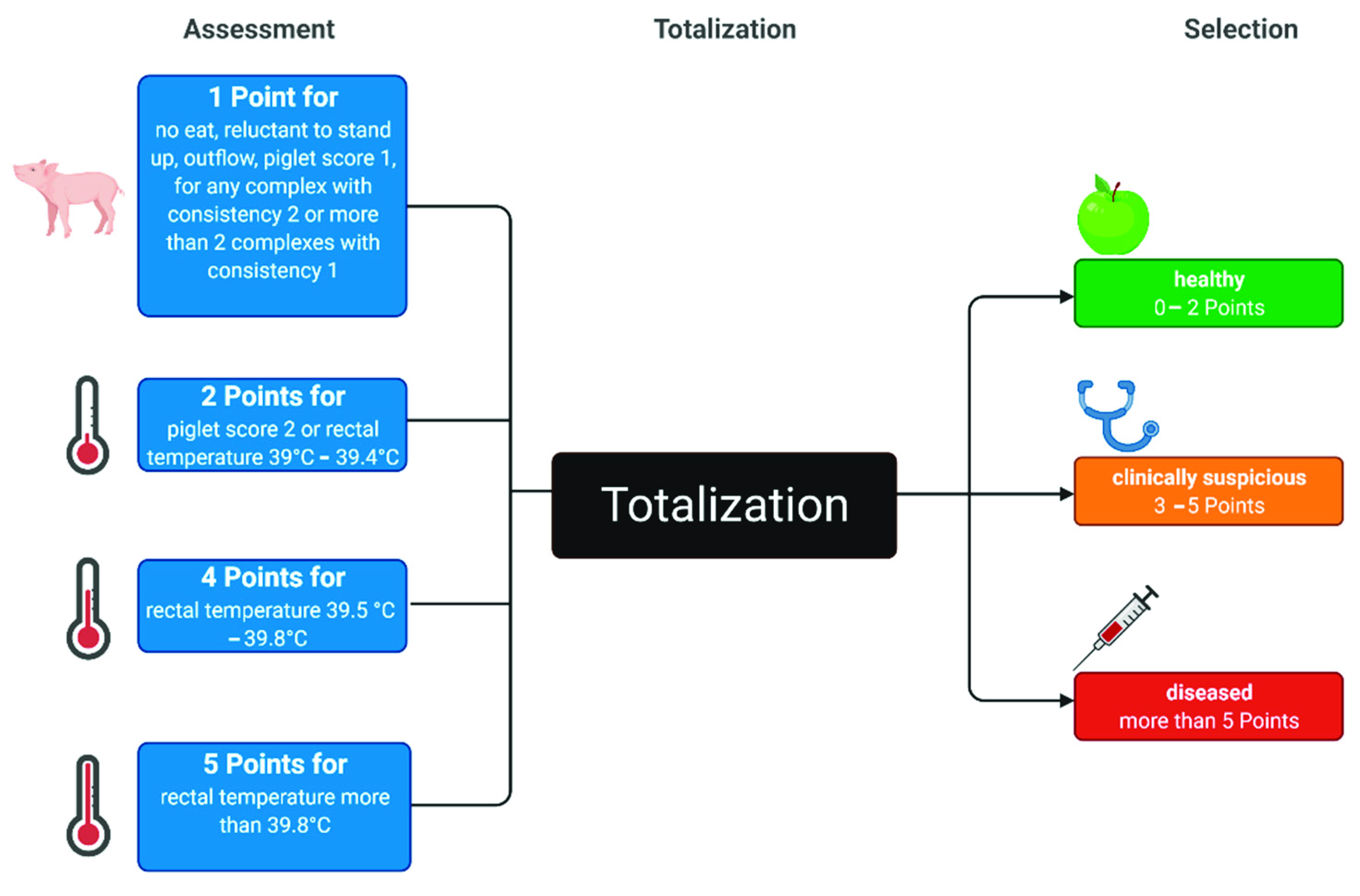
2.3.1. Clinical Examination and Mammary Thermography
2.3.2. Performance Parameters
2.4. Statistical Analysis
3. Results
3.1. Performance Parameters
3.2. Thermography-Associated Data
3.3. Correlation Analysis
4. Discussion
4.1. Performance Parameters
4.2. Infrared Thermography
4.3. Correlations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koketsu, Y.; Iida, R.; Piñeiro, C. A 10-year trend in piglet pre-weaning mortality in breeding herds associated with sow herd size and number of piglets born alive. Porcine Health Manag. 2021, 7, 4. [Google Scholar] [CrossRef]
- Rutherford, K.M.D.; Baxter, E.M.; D’Eath, R.B.; Turner, S.P.; Arnott, G.; Roehe, R.; Ask, B.; Sandøe, P.; Moustsen, V.A.; Thorup, F.; et al. The welfare implications of large litter size in the domestic pig I: Biological factors. Anim. Welf. 2013, 22, 199–218. [Google Scholar] [CrossRef]
- Jahresbericht 2008 der VzF GmbH. 2008, p. 9. Available online: https://www.vzf-gmbh.de/download (accessed on 26 June 2021).
- Jahresbericht 2020 der VzF GmbH. 2020, p. 10. Available online: https://www.vzf-gmbh.de/download (accessed on 3 July 2021).
- Blavi, L.; Solà-Oriol, D.; Llonch, P.; López-Vergé, S.; Martín-Orúe, S.M.; Pérez, J.F. Management and Feeding Strategies in Early Life to Increase Piglet Performance and Welfare around Weaning: A Review. Animals 2021, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Prunier, A.; Heinonen, M.; Quesnel, H. High physiological demands in intensively raised pigs: Impact on health and welfare. Animal 2010, 4, 886. [Google Scholar] [CrossRef] [Green Version]
- Theil, P.K.; Lauridsen, C.; Quesnel, H. Neonatal piglet survival: Impact of sow nutrition around parturition on fetal glycogen deposition and production and composition of colostrum and transient milk. Animal 2014, 8, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Devillers, N.; Farmer, C.; Le Dividich, J.; Prunier, A. Variability of colostrum yield and colostrum intake in pigs. Animal 2007, 1, 1033–1041. [Google Scholar] [CrossRef] [Green Version]
- Farmer, C.; Maes, D.; Peltoniemi, O. Mammary system. In Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Hoboke, NJ, USA, 2019; pp. 313–338. [Google Scholar]
- Hole, C.V.; Ayuso, M.; Aerts, P.; Prims, S.; Van Cruchten, S.; Van Ginneken, C. Glucose and glycogen levels in piglets that differ in birth weight and vitality. Heliyon 2019, 5, e02510. [Google Scholar] [CrossRef] [Green Version]
- Quesnel, H.; Brossard, L.; Valancogne, A.; Quiniou, N. Influence of some sow characteristics on within-litter variation of piglet birth weight. Animal 2008, 2, 1842–1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiniou, N.; Dagorn, J.; Gaudré, D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 2002, 78, 63–70. [Google Scholar] [CrossRef]
- Wolf, J.; Žáková, E.; Groeneveld, E. Within-litter variation of birth weight in hyperprolific Czech Large White sows and its relation to litter size traits, stillborn piglets and losses until weaning. Livest. Sci. 2008, 115, 195–205. [Google Scholar] [CrossRef]
- Hojgaard, C.K.; Bruun, T.S.; Theil, P.K. Impact of milk and nutrient intake of piglets and sow milk composition on piglet growth and body composition at weaning. J. Anim. Sci. 2020, 98, skaa060. [Google Scholar] [CrossRef]
- Devillers, N.; Le Dividich, J.; Prunier, A. Influence of colostrum intake on piglet survival and immunity. Animal 2011, 5, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Kobek-Kjeldager, C.; Moustsen, V.; Theil, P.; Pedersen, L. Effect of litter size, milk replacer and housing on production results of hyper-prolific sows. Animal 2020, 14, 824–833. [Google Scholar] [CrossRef]
- Moreira, L.; Menegat, M.; Barros, G.; Bernardi, M.; Wentz, I.; Bortolozzo, F. Effects of colostrum, and protein and energy supplementation on survival and performance of low-birth-weight piglets. Livest. Sci. 2017, 202, 188–193. [Google Scholar] [CrossRef]
- Quesnel, H.; Farmer, C.; Theil, P.K. Colostrum and milk production. In The Gestating and Lactating Sow; Farmer, C., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; pp. 173–192. [Google Scholar]
- Kemper, N. Update on postpartum dysgalactia syndrome in sows. J. Anim. Sci. 2020, 98, S117–S125. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Jacobsen, S.; Andersen, P.H.; Bækbo, P.; Cerón, J.J.; Dahl, J.; Escribano, D.; Theil, P.K.; Jacobson, M. Hormonal and metabolic indicators before and after farrowing in sows affected with postpartum dysgalactia syndrome. BMC Vet. Res. 2018, 14, 334. [Google Scholar] [CrossRef] [Green Version]
- Angjelovski, B.; Radeski, M.; Djadjovski, I.; Mitrov, D.; Bojkovski, J.; Adamov, N.; Dovenski, T. Prevalence and clinical signs of postpartum dysgalactia syndrome at the first day after farrowing in farmed sows in the Republic of Macedonia. Maced. Vet. Rev. 2019, 42, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, G.A.; Vanderhaeghe, C.; Janssens, G.P.; Dewulf, J.; Maes, D.G. Risk factors associated with postpartum dysgalactia syndrome in sows. Vet. J. 2010, 184, 167–171. [Google Scholar] [CrossRef]
- Mirko, C.; Bilkei, G. Acute phase proteins, serum cortisol and preweaning litter performance in sows suffering from periparturient disease. Acta Vet. Scand. 2004, 54, 153–161. [Google Scholar]
- Sărăndan, H.; Sărăndan, R.; Petroman, I.; Rada, O.; Balint, A.; Faur, B. Growth rate and mortality in suckling piglets and their correlation to the sows’milk yield. Sci. Pap. Anim. Sci. Biotechnol. 2009, 42, 277–282. [Google Scholar]
- Kaiser, M.; Jacobson, M.; Andersen, P.H.; Bækbo, P.; Cerón, J.J.; Dahl, J.; Escribano, D.; Jacobsen, S. Inflammatory markers before and after farrowing in healthy sows and in sows affected with postpartum dysgalactia syndrome. BMC Vet. Res. 2018, 14, 83. [Google Scholar]
- Schmidt, M.; Hoffmann, G.; Ammon, C.; Schön, P.; Manteuffel, C.; Amon, T. Anwendung der Infrarotthermografie bei ferkelführenden Sauen. Landtechnik 2013, 68, 228–231. [Google Scholar]
- Martineau, G.-P.; Farmer, C.; Peltoniemi, O.; Ramirez, A.; Schwartz, K.; Stevenson, G. Mammary system. In Diseases of Swine, 10th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Eds.; Wiley Blackwell: Chichester, UK, 2012; Volume 10, pp. 270–293. [Google Scholar]
- Gerjets, I.; Kemper, N. Coliform mastitis in sows: A review. J. Swine Health Prod. 2009, 17, 97–105. [Google Scholar]
- Hirsch, A.; Philipp, H.; Kleemann, R. Investigation on the efficacy of meloxicam in sows with mastitis–metritis–agalactia syndrome. J. Vet. Pharmacol. Ther. 2003, 26, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Gerjets, I.; Traulsen, I.; Reiners, K.; Kemper, N. Assessing individual sow risk factors for coliform mastitis: A case–control study. Prev. Vet. Med. 2011, 100, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, F. Vergleichende infrarotthermographische und bakteriologische Untersuchungen am gesunden sowie durch Mastitis veränderten Gesäuge beim Schwein. Ph.D. Thesis, Tierärztliche Hochschule Hannover, Hannover, Germany, 2016. [Google Scholar]
- Traulsen, I.; Naunin, K.; Muller, K.; Krieter, J. Untersuchungen zum Einsatz der Infrarotthermographie zur Messung der Körpertemperatur bei Sauen. Zuchtungskunde 2010, 82, 437–446. [Google Scholar]
- Vicente-Perez, R.; Avendano-Reyes, L.; Mejia-Vazquez, A.; Álvarez-Valenzuela, F.D.; Correa-Calderon, A.; Mellado, M.; Meza-Herrera, C.A.; Guerra-Liera, J.E.; Robinson, P.; Macias-Cruz, U. Prediction of rectal temperature using non-invasive physiologic variable measurements in hair pregnant ewes subjected to natural conditions of heat stress. J. Therm. Biol. 2016, 55, 1–6. [Google Scholar] [CrossRef]
- Knížková, I.; Kunc, P.; Gürdil, G.A.K.; Pinar, Y.; Selvi, K. Applications of infrared thermography in animal production. J. Fac. Agric. 2007, 22, 329–336. [Google Scholar]
- Soerensen, D.D.; Pedersen, L.J. Infrared skin temperature measurements for monitoring health in pigs: A review. Acta Vet. Scand. 2015, 57, 5. [Google Scholar] [CrossRef] [Green Version]
- Roberto, J.V.B.; De Souza, B.B. Use of infrared thermography in veterinary medicine and animal production. J. Anim. Behav. Biometeorol. 2020, 2, 73–84. [Google Scholar] [CrossRef]
- Hemsworth, P.; Mellor, D.J.; Cronin, G.M.; Tilbrook, A.J. Scientific assessment of animal welfare. N. Z. Vet. J. 2015, 63, 24–30. [Google Scholar] [CrossRef]
- Sathiyabarathi, M.; Jeyakumar, S.; Manimaran, A.; Jayaprakash, G.; Pushpadass, H.A.; Sivaram, M.; Ramesha, K.; Das, D.; Kataktalware, M.A.; Prakash, M.A. Infrared thermography: A potential noninvasive tool to monitor udder health status in dairy cows. Vet. World 2016, 9, 1075. [Google Scholar] [CrossRef] [Green Version]
- Sathiyabarathi, M.; Jeyakumar, S.; Manimaran, A.; Pushpadass, H.A.; Sivaram, M.; Ramesha, K.; Das, D.; Kataktalware, M.A.; Jayaprakash, G.; Patbandha, T.K. Investigation of body and udder skin surface temperature differentials as an early indicator of mastitis in Holstein Friesian crossbred cows using digital infrared thermography technique. Vet. World 2016, 9, 1386. [Google Scholar] [CrossRef] [Green Version]
- Sathiyabarathi, M.; Jeyakumar, S.; Manimaran, A.; Pushpadass, H.A.; Kumaresan, A.; Lathwal, S.; Sivaram, M.; Das, D.; Ramesha, K.; Jayaprakash, G. Infrared thermography to monitor body and udder skin surface temperature differences in relation to subclinical and clinical mastitis condition in Karan Fries (Bos taurus × Bos indicus) crossbred cows. Indian J. Anim. Sci. 2018, 88, 694–699. [Google Scholar]
- Brown-Brandl, T.M.; Eigenberg, R.A.; Purswell, J.L. Using thermal imaging as a method of investigating thermal thresholds in finishing pigs. Biosyst. Eng. 2013, 114, 327–333. [Google Scholar] [CrossRef]
- de Diego, A.C.P.; Sánchez-Cordón, P.J.; Pedrera, M.; Martínez-López, B.; Gómez-Villamandos, J.C.; Sánchez-Vizcaíno, J.M. The use of infrared thermography as a non-invasive method for fever detection in sheep infected with bluetongue virus. Vet. J. 2013, 198, 182–186. [Google Scholar] [CrossRef]
- George, W.; Godfrey, R.; Ketring, R.; Vinson, M.; Willard, S. Relationship among eye and muzzle temperatures measured using digital infrared thermal imaging and vaginal and rectal temperatures in hair sheep and cattle. J. Anim. Sci. 2014, 92, 4949–4955. [Google Scholar] [CrossRef] [Green Version]
- Kuratorium für Technik und Bauwesen in der Landwirtschaft e.V. (KTBL). In Tierschutzindikatoren: Leitfaden für die Praxis-Schwein; KTBL: Darmstadt, Germany, 2016.
- Caldara, F.R.; dos Santos, L.S.; Machado, S.T.; Moi, M.; de Alencar Nääs, I.; Foppa, L.; Garcia, R.G.; dos Santos, R.d.K.S. Piglets’ surface temperature change at different weights at birth. Asian Australas. J. Anim. Sci. 2014, 27, 431. [Google Scholar] [CrossRef] [Green Version]
- Hesse, A.; Hesse, D. Sauen automatisch auf Kondition füttern. Bauförderung Landwirtsch. Fachinf. BFL Online. 2006, pp. 1–5. Available online: http://www.bfl-online.de/media/agrifair-kondi-he.pdf (accessed on 7 May 2021).
- Hesse, A. Entwicklung einer automatisierten Konditionsfütterung für Sauen unter besonderer Berücksichtigung der Tierleistung; Bundesforschungsanstalt für Landwirtschaft (FAL): Braunschweig, Germany, 2003. [Google Scholar]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Zaninelli, M.; Redaelli, V.; Luzi, F.; Bronzo, V.; Mitchell, M.; Dell’Orto, V.; Bontempo, V.; Cattaneo, D.; Savoini, G. First evaluation of infrared thermography as a tool for the monitoring of udder health status in farms of dairy cows. Sensors 2018, 18, 862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Xiao, D.; Yang, Q.; Wen, Z.; Lv, L. Application of Infrared Thermography in Livestock Monitoring. Trans. ASABE 2020, 63, 389–399. [Google Scholar] [CrossRef]
- Feng, Y.-Z.; Zhao, H.-T.; Jia, G.-F.; Ojukwu, C.; Tan, H.-Q. Establishment of validated models for non-invasive prediction of rectal temperature of sows using infrared thermography and chemometrics. Int. J. Biometeorol. 2019, 63, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; De, U.; Kent, Y.; Rungsung, S.; Krishnaswamy, N.; Deka, B. Influence of seasonal variation on post-farrowing dysgalactia syndrome (PFDS) and serum biochemistry profiles in the periparturient sow. Trop. Anim. Health Prod. 2021, 53, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-H.; Hsu, T.-Y.; Chen, W.-J.; Horng, Y.-B.; Cheng, Y.-H. The Effect of Bacillus licheniformis-Fermented Products and Postpartum Dysgalactia Syndrome on Litter Performance Traits, Milk Composition, and Fecal Microbiota in Sows. Animals 2020, 10, 2044. [Google Scholar] [CrossRef] [PubMed]
- Gourdine, J.-L.; Bidanel, J.P.; Noblet, J.; Renaudeau, D. Rectal temperature of lactating sows in a tropical humid climate according to breed, parity and season. Asian Australas. J. Anim. Sci. 2007, 20, 832–841. [Google Scholar] [CrossRef]
- Stiehler, T.; Heuwieser, W.; Pfuetzner, A.; Burfeind, O. The course of rectal and vaginal temperature in early postpartum sows. J. Swine Health Prod. 2015, 23, 72–83. [Google Scholar]
- Schmitt, O.; O’Driscoll, K. Use of infrared thermography to noninvasively assess neonatal piglet temperature. Transl. Anim. Sci. 2021, 5, txaa208. [Google Scholar] [CrossRef]
- Wendt, M.; Eickhoff, K.; Koch, R. Die Messung der Hauttemperatur als Methode zur Erkennung fieberhaft erkrankter Schweine. Deut Tierarztl Woch 1997, 104, 29–33. [Google Scholar]
| Item | Gestation Diet | Lactation Diet |
|---|---|---|
| Crude protein | 140 | 165 |
| Crude fat | 33 | 37 |
| Crude fiber | 75 | 50 |
| Crude ash | 54 | 55 |
| Calcium | 7.2 | 9 |
| Phosphorus | 5.2 | 6.5 |
| Natrium | 2.5 | 2.5 |
| Energy (MJ ME/kg) | 11.4 | 13 |
| Lysine | 7.0 | 10 |
| Methionine | 2.4 | 3.0 |
| Item | Score | Meaning |
|---|---|---|
| Formation/Regression | 0 | in lactation |
| 1 | poorly formed/in regression | |
| 2 | not formed/without milk production | |
| Redness | 0 | physiological skin color |
| 1 | moderate redness | |
| 2 | strong redness | |
| Consistency | 0 | loose |
| 1 | elastic | |
| 2 | solid | |
| Nodes | 0 | not available |
| 1 | Nodes present in skin/subcutis | |
| 2 | Nodes present in mammary parenchyma | |
| Painfulness | 0 | not painful |
| 1 | low grade painful | |
| 2 | high grade painful |
| Item | n | Healthy | n | Clinically Suspicious | n | Diseased |
|---|---|---|---|---|---|---|
| TBP | 289 | 17.3 a ± 3.30 | 288 | 17.5 a ± 3.29 | 121 | 16.4 b ± 3.71 |
| NS | 289 | 1.06 ± 1.59 | 288 | 0.8 ± 1.64 | 121 | 1.11 ± 2.44 |
| NBA | 289 | 16.3 a ± 3.43 | 288 | 16.7 a ± 3.30 | 121 | 15.3 b ± 3.97 |
| PWM | 289 | 2.09 ± 2.25 | 288 | 2.41 ± 2.2 | 121 | 1.98 ± 2.13 |
| NWP | 289 | 12.1 ± 1.92 | 288 | 12.1 ± 2.10 | 121 | 12.4 ± 2.29 |
| BFT1 | 289 | 16.7 ± 2.60 | 301 | 16.5 ± 2.30 | 122 | 16.4 ± 2.20 |
| BFT2 | 288 | 14.5 ± 2.17 | 301 | 14.1 ± 2.39 | 122 | 14.0 ± 2.23 |
| BFTD | 288 | 2.22 ± 1.61 | 301 | 2.33 ± 1.66 | 122 | 2.40 ± 1.73 |
| SW1 | 289 | 267 ± 31.6 | 301 | 262 ± 34.9 | 122 | 265 ± 29.8 |
| SW2 | 287 | 222 a ± 32.7 | 300 | 215 b ± 35.0 | 121 | 221 a ± 32.2 |
| SWD | 287 | 45.0 ± 16.9 | 300 | 46.6 ± 16.8 | 121 | 44.7 ± 15.8 |
| DWG | 226 | 194 a ± 32.1 | 235 | 191 a ± 33.1 | 97 | 183 b ± 38.2 |
| Item | n | Healthy | n | Clinically Suspicious | n | Diseased |
|---|---|---|---|---|---|---|
| RT0 | 314 | 38.8 a ± 0.29 | 313 | 39.2 b ± 0.24 | 127 | 39.9 c ± 0.43 |
| RT14 | 281 | 38.9 a ± 0.35 | 291 | 39.0 b ± 0.37 | 119 | 39.0 b ± 0.35 |
| RT21 | 273 | 38.5 a ± 0.41 | 287 | 38.7 b ± 0.43 | 115 | 38.7 b ± 0.48 |
| TH0 | 314 | 37.2 a ± 0.54 | 313 | 37.6 b ± 0.54 | 127 | 38.3 c ± 0.57 |
| TH14 | 279 | 37.6 a ± 0.60 | 291 | 37.7 b ± 0.60 | 118 | 37.8 b ± 0.69 |
| TH21 | 273 | 37.2 a ± 0.64 | 287 | 37.4 b ± 0.63 | 115 | 37.5 b ± 0.68 |
| p-Value (n) | Pearson Correlation Coefficient | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NBA | NWP | BFTD | SW2 | SWD | RT0 | RT14 | RT21 | TH0 | TH14 | TH21 | |
| NBA | 0.19 | 0.04 | −0.25 | 0.14 | 0.00 | 0.12 | 0.09 | 0.02 | 0.12 | 0.14 | |
| NWP | <0.0001 (698) | 0.01 | −0.13 | 0.07 | 0.03 | 0.13 | 0.15 | 0.06 | 0.13 | 0.13 | |
| BFTD | 0.3345 (682) | 0.7152 (682) | 0.00 | 0.00 | 0.07 | 0.07 | 0.04 | 0.04 | 0.06 | 0.06 | |
| SW2 | <0.0001 (680) | 0.0004 (680) | 0.9089 (708) | 0.31 | −0.1 | −0.38 | −0.38 | −0.05 | −0.31 | −0.36 | |
| SWD | 0.0004 (680) | 0.087 (680) | 0.8637 (711) | <0.0001 (708) | −0.05 | −0.02 | −0.02 | 0.18 | −0.07 | −0.09 | |
| RT0 | 0.9473 (697) | 0.4308 (697) | 0.0487 (710) | 0.0061 (707) | 0.1929 (707) | 0.15 | 0.21 | 0.68 | 0.16 | 0.19 | |
| RT14 | 0.0022 (656) | 0.0006 (656) | 0.0847 (672) | <0.0001 (672) | 0.6628 (672) | <0.0001 (690) | 0.57 | 0.13 | 0.56 | 0.48 | |
| RT21 | 0.0263 (643) | <0.0001 (643) | 0.3491 (664) | <0.0001 (662) | 0.6722 (662) | <0.0001 (674) | <0.0001 (667) | 0.2 | 0.44 | 0.69 | |
| TH0 | 0.6400 (698) | 0.1000 (698) | 0.2500 (711) | 0.1200 (708) | 0.1800 (708) | <0.0001 (754) | 0.0007 (691) | <0.0001 (675) | 0.39 | 0.34 | |
| TH14 | 0.0022 (656) | 0.0006 (656) | 0.0847 (672) | <0.0001 (670) | 0.0774 (670) | <0.0001 (688) | <0.0001 (688) | <0.0001 (665) | <0.0001 (688) | 0.75 | |
| TH21 | 0.0003 (643) | 0.0006 (643) | 0.12 (664) | <0.0001 (662) | 0.03 (662) | <0.0001 (674) | <0.0001 (667) | <0.0001 (675) | <0.0001 (675) | <0.0001 (665) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosengart, S.; Chuppava, B.; Schubert, D.C.; Trost, L.-S.; Henne, H.; Tetens, J.; Traulsen, I.; Deermann, A.; Visscher, C.; Wendt, M. Infrared Thermography of the Mammary Gland in Sows with Regard to Health and Performance. Agriculture 2021, 11, 1013. https://doi.org/10.3390/agriculture11101013
Rosengart S, Chuppava B, Schubert DC, Trost L-S, Henne H, Tetens J, Traulsen I, Deermann A, Visscher C, Wendt M. Infrared Thermography of the Mammary Gland in Sows with Regard to Health and Performance. Agriculture. 2021; 11(10):1013. https://doi.org/10.3390/agriculture11101013
Chicago/Turabian StyleRosengart, Stephan, Bussarakam Chuppava, Dana Carina Schubert, Lea-Sophie Trost, Hubert Henne, Jens Tetens, Imke Traulsen, Ansgar Deermann, Christian Visscher, and Michael Wendt. 2021. "Infrared Thermography of the Mammary Gland in Sows with Regard to Health and Performance" Agriculture 11, no. 10: 1013. https://doi.org/10.3390/agriculture11101013
APA StyleRosengart, S., Chuppava, B., Schubert, D. C., Trost, L.-S., Henne, H., Tetens, J., Traulsen, I., Deermann, A., Visscher, C., & Wendt, M. (2021). Infrared Thermography of the Mammary Gland in Sows with Regard to Health and Performance. Agriculture, 11(10), 1013. https://doi.org/10.3390/agriculture11101013






