Abstract
Trade-offs between growth and reproduction in soybean require resource availability manipulations. Decapitation and reducing sink strength through deflowering can affect the source–sink ratio that affects plant growth, development, and yield. The current study assesses the effect of decapitation (Decap) and removal of the two lowest racemes (R2LR) and their combination on growth, flowering, and yield capacity of soybean through controlling the source–sink ratio and inducing the “stay-green” phenotype. Two field experiments were conducted during 2018 and 2019 in the Agronomy Farm located at Mansoura University, Egypt. Decapitation was done at the V4 stage (35 days after sowing, DAS), during which four nodes on the main stem had fully developed leaves beginning with the unifoliolate nodes, whereas R2LR was performed at the R2 stage (50 DAS), during which the plants had one open flower at one of the two uppermost nodes on the main stem. Results indicated that Decap, R2LR, and their combination significantly increased seed yield per plant through increasing plant growth and flowering and improving biochemical attributes. The combination between Decap and R2LR was generally more effective in positively modulating plant vegetative, reproductive, and physiological capacity than either Decap or R2LR alone. Moreover, the number of branches as well as pods/plant and IAA content responded positively to Decap, whereas net assimilation rate, seed growth rate, number of flowers/node, and cytokinins content responded positively to R2LR. Decap and R2LR were interpreted in light of their effects on inducing the “stay-green” phenotype and altering the source–sink ratio. Based on the findings, it can be concluded that concealing the apical dominance in conjunction with reducing the sink strength through guided raceme removal would be beneficial for the reproductive potential in soybean.
1. Introduction
Soybean (Glycine max L.) is an annual field crop belonging to the Fabaceae family and is considered as one of the most important cash crops all over the world [1]. Soybean is an important source of both protein and oil. Like other crops, soybean yield is often correlated with seed filling duration; consequently, a long filling period may be reflected in higher seed yield [2]. The length of the filling period is related to green leaf area duration [2,3]. In fact, prolongation of the green leaf area is similar to the “stay-green” trait, which may result from either the delay in the onset of leaf senescence, a reduced rate of senescence, or inhibition of chlorophyll breakdown [4]. It has been considered that delaying leaf senescence can enhance photosynthetic assimilating capacity and, consequently, seed yield [5]. For example, in soybean’s stay-green phenotype, photosynthetic activity during the grain-filling period was extended, and mean seed weight and seed yield per plant were increased [6].
In plants, there is a trade-off between activities of the axillary and terminal buds; an active shoot apex inhibits the outgrowth of axillary buds, a phenomenon termed as ‘apical dominance’, which allows plants to focus resources into the main axis of growth [7]. Removal of the shoot apex (decapitation, Decap) leads to the activation of dormant axillary buds, forming new branches. Another developmental manifestation of Decap is the realization of the “stay-green” phenomenon. For example, removing a shoot apex resulted in effects similar to the “stay-green” phenotype in soybean, where it increased chlorophylls and delayed pod maturation [8]. In addition, Decap maintained chloroplast integrity and retarded chlorophyll loss in other crops, such as tomato and tobacco [9]. Similarly, senescent leaves were induced to be regreened in Nicotiana rustica by decapitation [10]. It has been reported that Decap increased plant growth and yield in several legume species [11,12,13]. For example, Decap at the 5th leaf stage increased the branching, number of flowers and pod set, yield, and harvest index of cowpea [14]. However, it did not increase seed weight per plant in soybean and even reduced the seed yield [8]. Nevertheless, the effect of Decap on soybean flowering and reproductive processes is poorly investigated and less understood.
The two major processes that determine the yield are the production of assimilates by the leaves and the utilization of these assimilates in the developing seeds. Many soybean flowers are destined to be aborted due to competition for assimilates among reproductive organs [15]. So, reducing this competition by removing reproductive organs is an often-employed approach for controlling source–sink ratio, and consequently, the plant’s reproductive potential and yield [16]. Nevertheless, the previously reported approaches for reducing the sink strength often led to an enhanced flowering, but not yield [17,18]. So, there is a need to explore different sink-reduction approaches that can affect the source–sink ratio inductive to the yield, i.e., removing the two lowest racemes. The removal/damage of the reproductive organs often resulted in the “stay-green” phenotype in many legumes [4,19,20]. Hence, it is also necessary to study the “stay-green” effect of removing only the two lowest racemes in soybean to thoroughly interpret its effect on plant growth and reproductive capacity.
Apical dominance usually inhibits side branches. Removal of the apex releases the dormancy of the lateral buds and induces outgrowing shoots [21]. Auxin, a signaling molecule, plays a vital role in maintaining apical dominance [22]. It has been hypothesized that the level of auxin in the apical bud controls the inhibition of axillary buds by changing the cytokinin to auxin ratio [23]. Whereas auxin synthesized in the intact apex suppresses axillary bud outgrowth, cytokinin induced in the decapitated shoot enhances axillary bud outgrowth [21]. For example, Decap reduced auxin levels, which induce bud outgrowth through a transient increase in cytokinin [24]. In addition, the exogenous application of auxin to the stump of decapitated plants inhibits axillary bud outgrowth [25]. However, there is still a need to study the changes in auxin and cytokinin levels, their vegetative and reproductive implications, as well as the effects of these changes on the period during which leaves maintain their photosynthetic activity of soybean in response to reducing sink strength through racemes removal, and when this undertaking is joined with the removal of the apical dominance.
Only one approach is usually used to enhance legumes yield: either decapitation [13] or manipulation of the source–sink ratio through removing the reproductive organs [16,26]. So, assessing the combined effect of the two approaches is still needed to understand possible mechanisms regulating the soybean yield. Abnormal senescence and the stay-green effect are caused by both genetic and environmental factors, including organ damage [4]. There is ample evidence that removing reproductive organs causes the “stay-green” phenotype in soybean and other legumes. It has been reported that the removal of both flowers and pods delays senescence [19,27]. Delayed senescence usually results in extending the duration of leaf greenness and lengthening the seed-filling period, leading to higher seed yield [2]. In cowpea, the stay-green genotypes, which are characterized by the maintenance of the non-senescent leaf area, were of higher yield than normally senescing genotypes [28].
The current study was conducted to assess the combined effect of decapitation and removal of the two lowest racemes on the growth, flowering, and yield capacity of soybean through their effects on altering the source–sink ratio and inducing the “stay-green” phenotype. We hypothesize that both decapitation and the removal of the two lowest racemes in soybean could affect photo-assimilates allocation between the source and sink, which might promote the plant’s reproductive potential. We expect the two treatments would affect the cytokinin to auxin ratio, which delays leaf senescence but induces axillary buds’ outgrowth and affects the number of developed flowers and fruits, as well as seed set.
2. Materials and Methods
2.1. Experimental Site, Plant Material and Agronomic Practices
Two field experiments were conducted during the 2018 and 2019 growing seasons at the Agronomy Farm of Mansoura University, Egypt, from May to November. The meteorological data and the soil properties of the study area were described in our previous studies [29,30]. The experimental plots comprised four ridges of 3.5 m in length; the spacing between ridges was 70 cm and between hills was 25 cm.
Soybean seeds (Giza 111) were obtained from Sakha Agricultural Research Station, Kafr El-Sheikh, Egypt. Giza 111 cultivar is characterized by a high number of branches, high seed yield, and lower pod splitting [31,32,33]. The seeds were surface-sterilized with 1% sodium hypochlorite for 5 min, then washed with sterilized water. Thereafter, the seeds were sown (3 per hill) manually on the shoulder of the ridges. After three weeks, the plants were thinned to two healthy plants per hill. The fertilizers were applied at 180, 361, and 120 kg ha−1 nitrogen (N), phosphorus (P), and potassium (K) using urea (46% N), calcium superphosphate (15.5% P2O5), and potassium sulfate (48% K2O), respectively, as sources.
2.2. Decapitation and Raceme Removal Treatments
The applied treatments were the removal of the apical bud (decapitation, Decap), removal of the two lowest racemes (R2LR), and their combination. These treatments were compared with intact plants as control. Decapitation was done at the V4 stage (35 days after sowing, DAS), during which four nodes on the main stem had fully developed leaves beginning with the unifoliolate nodes, whereas R2LR was performed at the R2 stage (50 DAS), during which the plants had one open flower at one of the two uppermost nodes on the main stem. All treatments were replicated four times, with the consideration of the entire ridge as a replicate.
2.3. Recorded Data and Analyses
Growth data were recorded at the of R3 stage (65 DAS) in 5 randomly selected plants from each replicate (ridge). Another sample was taken 10 days later to estimate biomass and leaf area. Data of the two samples were used to calculate net assimilation rate (NAR) according to Radford [34]’s formulae as follows: NAR = [(W2 − W1) (loge A2 − loge A1)]/[(A2 − A1) (t2 − t1)]; where W1, A1 and W2, A2 are dry weight and leaf area at time 1 and time 2, respectively. Seed growth rate (SGR) was estimated during the period spanning from the beginning of R5 stage to the end of R6 stage (between 75 and 90 DAS) for the developing seeds in the pods at the 1st node from the base of the 3rd raceme from plant base of at least 7 plants from each replicate. Seed growth rate was calculated based on the equation of Guffy et al. [35] as: SGR = (SDW2 − SDW1)/(t2 − t1); where SDW1 and SDW2 are seed dry weight at t1 and t2, respectively, and t1 and t2 are the times at which SDW1 and SDW2 were estimated, respectively.
Flowering capacity, represented mainly by the number of flowering nodes and number of flowers per node, was determined at the beginning of the R5 stage (75 DAS). Biochemical constituents, i.e., total chlorophylls, carotenoids, cytokinins, IAA, and relative water content (RWC) as well as membrane stability index (MSI) were determined at the beginning of the R5 stage (75 DAS). For the determination of total chlorophylls and carotenoids, 0.5 g leaf tissue was grounded in 80% acetone, and leaf pigments were extracted and determined according to Lichtenthaler [36].
The concentrations of indoleacetic acid (IAA) and cytokinins were determined according to Rauf and Sadaqat [37]. Leaf samples were stirred and extracted using 80% cold methanol, to which 40 mg L−1 butylhydroxytoluene was added as an antioxidant for 60 hr at 4 °C. The methanolic extracts were filtered through both 0.45 mm and 0.2 mm pore-size filters, and then passed through a Sep-Pack C18 cartridge eluted with 80% methanol. The eluates were reduced to the aqueous phase by a rotary evaporator, made to 500 µL with diluted formic acid (0.2%), and then purified by high-performance liquid chromatography (HPLC) using an octadecyl reversed-phase column. The elution solvent was methanol in formic acid at pH 3.0. The fractions were collected and reduced to dryness before being methylated with ethereal diazomethane. Cytokinins and IAA quantification were carried out using the enzyme-linked immunosorbent assay method. The level of each hormone per sample was measured 5 times, and the values were corrected for recovery.
Relative water content (RWC) was determined by the method of Gonzalez and Gonzalez-Vilar [38] as follows:
where FW = fresh weight, DW = dry weight, TW = turgid weight. For determination of turgid weight, leaf disks were submerged for 8 h in distilled water, thereafter they were blotted dry gently and weighed.
MSI was determined according to Premchandra et al. [39]. Leaf discs (0.1 g) were thoroughly washed in distilled water and then placed in 10 ml of distilled water at 40 °C for 30 min. At the end of this period, their electrical conductivity was recorded (C1). Subsequently, the same sample was placed in a boiling water bath (100 °C) for 10 min, and their electrical conductivity was also recorded (C2). MSI was calculated as:
Yield and its components, as well as seeds’ protein and oil percentage, were determined after seed ripening at the end of the R8 stage (120 DAS). Yield and its components were estimated in five randomly selected plants from each replicate, whereas biochemical analyses were analyzed in three randomly selected plants from each replicate. Plants assigned for yield determination were harvested by hand, air-dried for 2 weeks, and pods on the main stems and branches were counted. After threshing by hand, the numbers of 3- or more-seeded pods and seed yield per plant were recorded. Seed yield was adjusted to 130 g kg−1 moisture content. The pod set percentage was calculated as the number of pods divided by the number of flowers. Protein and oil contents in the seeds were determined according to the standard methods of AOAC [40]. Nitrogen was estimated by the micro Kjeldhal method and multiplied with a factor of 6.25 to estimate crude protein percentage. The soxhlet extraction method was used to estimate oil percentage.
2.4. Statistical Analysis
Data of the two experimental seasons were subjected to analysis of variance (ANOVA) using SAS Software, version 8.2 (SAS Institute, 2001) [41]. The differences among the mean values were determined using Duncan’s multiple range tests (DMRT) with significance at P ≤ 0.05.
3. Results
3.1. Effect of Decapitation and Raceme Removal on Growth and Photoassimilation Capacity
Decapitation (Decap) and its combination with removal of the two lowest racemes (R2LR) increased the number of branches (Figure 1A) and leaves (Figure 1B), and thereby increased the plant fresh (Figure 1C) and dry (Figure 1D) weight. However, the increase recorded in plant fresh weight in response to the combined treatment did not reach the significance level. The enhancing effect of the Decap treatment only on both the number of branches and leaves was higher compared with that of its combination with R2LR. The number of branches and leaves as well as the plant fresh and dry weights were not significantly affected by the treatment of R2LR only.
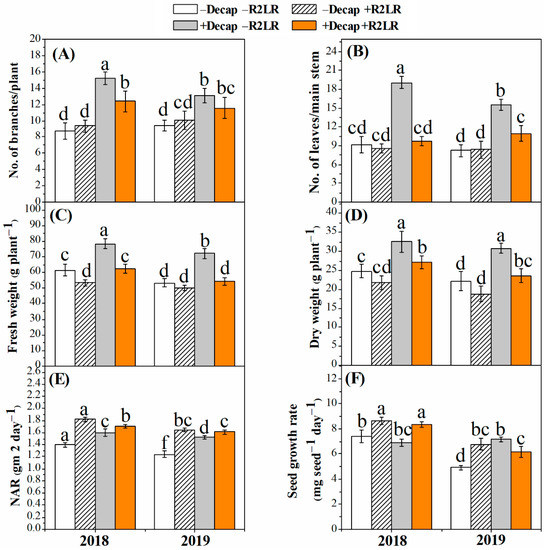
Figure 1.
Effect of decapitation and raceme removal treatments on the number of branches (A), number of leaves (B), fresh weight (FW, C), dry weight (DW, D), net assimilation rate (NAR, E), and seed growth rate (SGR, F) of soybean plants. −R2LR and +R2LR: no removal and removal of the two lowest racemes, respectively; −Decap and +Decap: no decapitation and decapitation, respectively. Different letters above the bars indicate significant differences between the treatments, according to Duncan’s multiple range test at P ≤ 0.05. Bars represent the means ± SD (n = 4).
Net assimilation rate (NAR) was significantly increased in response to all adopted treatments (Figure 1E). The highest increase in NAR in both experimental seasons was obtained due to the treatment of R2LR only, followed by its combination with Decap. The percent increases in NAR were 30.0, 22.1% and 32.2, 29.8% in response to R2LR and R2LR+Decap during the growing seasons of 2018 and 2019, respectively. Likewise, all treatments, generally, increased seed growth rate (Figure 1F). However, the Decap only treatment did not significantly affect seed growth rate (SGR) during the first growing season. The percent increases in SGR were 16.4, 12.8% and 37.8, 25.4% in response to R2LR and R2LR+Decap during the growing seasons of 2018 and 2019, respectively (Figure 1F).
3.2. Effect of Decapitation and Raceme Removal on Flowering Potential
The number of flowering nodes as well as the number of flowers per node were increased in response to R2LR, Decap, and their combination (Figure 2). However, the increase recorded in the number of flowering nodes (Figure 2B) in response to Decap was not significant during both experimental seasons. In addition, though Decap increased number of flowers per node during both experimental seasons, the increase was significant only during the second season (Figure 2C). The treatment in which only the two lowest racemes were removed increased number of flowers per node by 98.3 and 87.0%, during the first and second season, respectively. The corresponding increases in response to the combined treatment (R2LR+Decap) were 49.6 and 50.6% in 2018 and 2019, respectively. The total number of nodes on the main stem was either unaffected (the first experimental season) or reduced (the second experimental season) (Figure 1A).
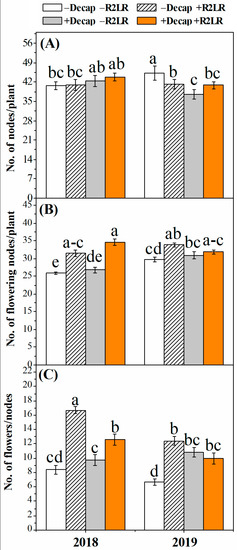
Figure 2.
Effect of decapitation and raceme removal treatments on the number of nodes (A), number of flowering nodes (B) and number of flowers/node (C) of soybean plants. −R2LR and +R2LR: no removal and removal of the two lowest racemes, respectively; −Decap and +Decap: no decapitation and decapitation, respectively. Different letters above the bars indicate significant differences between the treatments, according to Duncan’s multiple range test at P ≤ 0.05. Bars represent the means ± SD (n = 4).
3.3. Effect of Decapitation and Raceme Removal on Seed Yield Potential and Seeds’ Quality
Removal of the two lowest racemes, decapitation, and their combination increased pod set, number of pods per plant, number of three- or more-seeded pods per plant, and seed yield per plant during both experimental seasons (Figure 3). Pod set percentage was increased by 18.8, 9.9, 24.9% and 28.9, 17.4, 32.5% in response to R2LR, Decap, and R2LR+Decap during the first and second season, respectively (Figure 3A). The corresponding increases in the number of pods produced per plant were 14.5, 45.7, 85.2% and 11.7, 51.6, 77.5%, respectively (Figure 3B). As the number of pods containing three or more seeds was also increased in response to R2LR, Decap, and their combination during both experimental seasons (Figure 3C), seed yield per plant was considerably increased (114.9, 171.3, 129.1% and 61.6, 145.1, 136.6%, respectively) (Figure 3D). The combined treatment was the most effective for inducing pod set, number of pods per plant, and number of three- or more-seeded pods per plant during both experimental seasons.
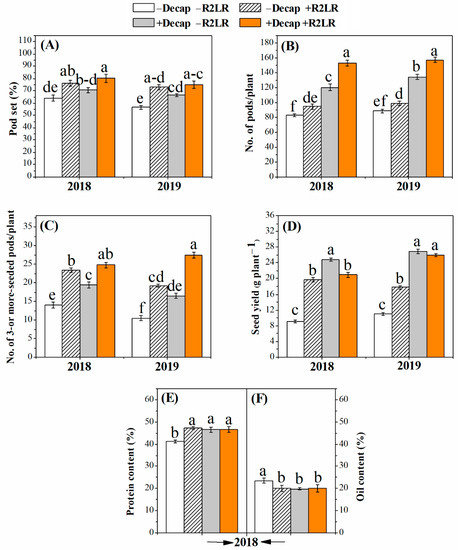
Figure 3.
Effect of decapitation and raceme removal treatments on pod set(%, A) number of pods per plant (B), number of 3- or more-seeded pods per plant (C) seed yield per plant (D), protein content (%, E) and oil content (%, F) of soybean plants. −R2LR and +R2LR: no removal and removal of the two lowest racemes, respectively; −Decap and +Decap: no decapitation and decapitation, respectively. Different letters above the bars indicate significant differences between the treatments, according to Duncan’s multiple range test at P ≤ 0.05. Bars represent the means ± SD (n = 4).
The quality of the seeds, as assessed by their contents from protein and oil, was affected by the adopted source–sink manipulating treatments (Figure 3E,F). Removal of the two lowest racemes, decapitation, and their combination increased protein percentage (Figure 3E), but decreased oil percentage (Figure 3F) in the seeds. Compared with the control, seeds’ protein percentage was increased by 14.5, 12.4, and 12.6% in response to R2LR, Decap, and R2LR+Decap, respectively. The decrease in oil percentage was 15.1, 15.5, and 14.7%, respectively (Figure 3F).
3.4. Effect of Decapitation and Raceme Removal on Photosynthetic Pigments, Relative Water Content and Membrane Stability Index
All adopted treatments increased total chlorophylls and total carotenoids concentrations, and the treatment in which removal of the two lowest racemes and decapitation were combined was the most effective in this respect (Figure 4). Total chlorophylls concentration was increased by 68.7, 87.5% and 17.4, 31.7% in response to R2LR and R2LR+Decap during the first and second season, respectively (Figure 4A). The corresponding increases in total carotenoids concentration were 70.5, 82.3% and 18.1, 31.8%, respectively (Figure 4B). Relative water content (RWC) was increased in response to the individual source–sink manipulation treatments, either R2LR alone or Decap alone, during the first experimental season, whereas it was not significantly affected during the second season (Figure 4C). In addition, the treatments that enhanced the feature of membrane stability index were the removal of the two lowest racemes and its combination with decapitation, but only during the first season (Figure 4D).
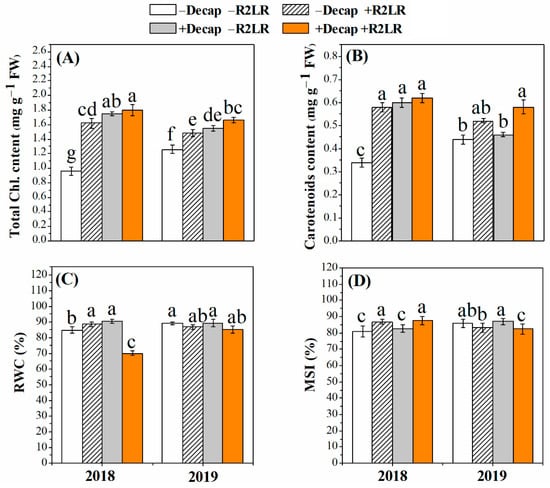
Figure 4.
Effect of decapitation and raceme removal treatments on chlorophylls content (A), carotenoids content (B), Relative water content (RWC, C), and membrane stability index (MSI, D) of soybean plants. −R2LR and +R2LR: no removal and removal of the two lowest racemes, respectively; −Decap and +Decap: no decapitation and decapitation, respectively. Different letters above the bars indicate significant differences between the treatments, according to Duncan’s multiple range test at P ≤ 0.05. Bars represent the means ± SD (n = 4).
3.5. Effect of Decapitation and Raceme Removal on Auxins and Cytokinins Content
The adopted treatments for affecting the source–sink ratio noticeably induced the biosynthesis of both IAA and cytokinins, so significant increases were recorded in their concentrations in response to either R2LR, Decap or their combination (Figure 5). Decapitation was more inductive to IAA (Figure 5A), whereas R2LR was more inductive to cytokinins (Figure 5B). Data also indicated that the highest IAA concentration was recorded in plants with which R2LR and Decap were combined, whereas the highest cytokinins concentration was obtained in plants only treated with R2LR. The concentration of IAA was increased by 80.7, 199.8, and 208.4% in response to R2LR, Decap, and their combination, respectively (Figure 5A). The corresponding increases in cytokinins concentration were 227.1, 49.1, and 137.7%, respectively (Figure 5B).
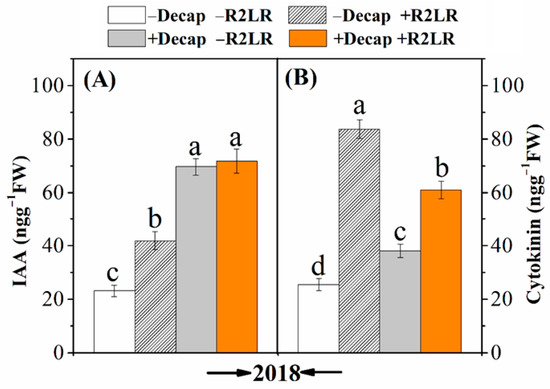
Figure 5.
Effect of decapitation and raceme removal treatments on IAA (auxins) (A) and cytokinins (B) contents of soybean leaves. −R2LR and +R2LR: no removal and removal of the two lowest racemes, respectively; −decap and +decap: no decapitation and decapitation, respectively. Different letters above the bars indicate significant differences between the treatments, according to Duncan’s multiple range test at P ≤ 0.05. Bars represent the means ± SD (n = 4).
4. Discussion
Results of the present study indicated that decapitation increased soybean’s seed yield per plant through increasing number of flowers per node (Figure 2) as well as pod set percentage, number of pods/plant, and number of 3- or more-seeded pods (Figure 3). Similar effects of Decap on pigeon peas, cowpea, and soybean yield were previously reported [12,14,42]. However, Amuti [8] did not find any consistent increase in seed yield with the application of Decap, though it increased the number of flowers, pod set, and number of 3- or more-seeded pods. Yield increase due to Decap may be mediated through increasing branching (Figure 1A), number of flowers per node (Figure 2C), and pod set percentage, as well as number of pods per plant (Figure 3A,B). In addition, the data indicated that Decap exerted physiological changes manifested by increasing cytokinins and IAA contents, which may have resulted in enhanced chlorophyll content (Figure 4A). The higher chlorophyll content in the leaves of Decap-treated plants, in addition to the higher number of remaining leaves, should increase the NAR (Figure 1E), which in turn, retard plant senescence, symptomatic of the “stay-green” phenotype. Besides, shoot apex removal yielded effects similar to the “stay-green” phenomenon where it increased chlorophylls and delayed pod maturation [8]. Similarly, chloroplast integrity was maintained in tomato and chlorophyll loss was retarded in both tomato and tobacco in response to Decap [9]. Moreover, senescent leaves were induced to be regreened in Nicotiana rustica by decapitation [10]. The observed retardation of senescence symptoms in response to Decap may also be due to the increases in cytokinins and IAA contents in decapitated plants (Figure 5). Earlier reports have reported cytokinins’ induction in response to Decap in chickpea, another legume crop [43,44].
Removal of the two lowest racemes (R2LR) increased the number of flowering nodes and flowers per node (Figure 2B,C) and seed yield per plant (Figure 3D), along with increasing NAR and SGR (Figure 1E,F), as well as the leaf’s photosynthetic pigments (Figure 4) and cytokinins content (Figure 5B). In line with these results, removing reproductive organs enhanced flowering in soybeans [45], pigeonpea [46], and peas [47]. In addition, deflowering-induced senescence delay was also reported in other crops [27,48]. In this context, Thomas and Howarth [4] ascribed the phenomenon of abnormal senescence and “stay-green” to both genetic and environmental factors, including organ damage. According to Guo and Gan [48], the removal of inflorescences can delay leaf senescence. Reproductive growth and the onset of leaf senescence are under correlative control in legumes [49]. Accordingly, the vegetative growth of plants was considerably extended with continuous removal of fruits [50].
The removal of the lower racemes enhanced the synthesis of cytokinins and pod set [51]. Besides, leaf senescence was delayed by increasing the endogenous level of cytokinins in tobacco plants [52]. In the present study, the enhancing effect of removing the two lowest racemes on flowering (Figure 2) and its retarding effect on the senescence of soybean leaves, as evidenced by chlorophyll retention and manifested by higher chlorophylls content (Figure 4A), could be attributed to the observed high level of cytokinins content (Figure 5B). Previous studies indicated that exogenous cytokinins application increased flower production, but decreased flower abortion [53] and retarded leaf senescence [54,55].
At the molecular level, Zhang et al. [19] reported that deflowering in soybean enhanced the activity of the flowering promoting gene Gm FT2a, but attenuated the flowering-inhibiting E1 gene. Cytokinins act to redirect the movement of assimilates into treated tissues, increasing sink strength and subsequent growth rates, hence preventing abscission of the developing flowers [53]. In soybean, exogenous application of cytokinin stimulated flower production and fruit set that increased total seed production [56]. In addition, senescence delay as a response to deflowering may be due to the attenuation of a senescence hormonal signal produced in reproductive organs and transported to leaves to activate their senescence program [57].
It has been proposed that the flower production and seed set are influenced by assimilates competition between source and sink organs [58]. For example, Brun and Betts [59] showed that a large proportion of soybean flowers are destined to be aborted due to deficiency of assimilates supply to flowers as a consequence of competition for assimilates among reproductive organs. Therefore, reducing the competition for assimilates by racemes removal, i.e., reducing sink strength, may have contributed to the reduction of flower abortion, through the effect of increasing source–sink ratio. Another possibility for the enhanced flowering in response to racemes removal recorded in the present study is the recommitment (reallocation) of available resources from vegetative to reproductive organs through the cytokinins effect (Figure 5B) that regulates flower production and fruit set [56].
In the present study, the removal of the two lowest racemes (R2LR) increased seed yield, which could be attributed to the R2LR-induced increase in seed growth rate (Figure 1F), number of flowering nodes, number of flowers per node (Figure 2), pod set, number of pods per plant and number of 3- or more-seeded pods/plant (Figure 3). So, R2LR increased the flowering and reproductive capacity of soybean. It has been reported that soybean seed yield is determined more by pod number than any other yield component [60]. As the number of developed flowers determines the number of pods, any factor that increases flower development or reduces abortion should increase the number of pods, and consequently, seed yield. Racemes removal induced effects having the potential to increase flower production and reduce their abortion. It has been argued that two factors determine the abortion percentage: availability of photoassimilates and certain hormones [61]. In addition to the developmental, reproductive, and physiological effects of R2LR, deflowering may have also altered the anatomical structure of the remaining racemes’ rachises via inducing the formation of their conductive treachery elements, rendering it with higher ability to provide the nutritional requirements of the developing pods as reported in mungbean [18].
5. Conclusions
Both decapitation and removing the two lowest racemes in soybean affected photoassimilates reallocation between the different reproductive organs. This process is coordinated through cytokinins and auxins. Decapitation stimulated the IAA accumulation, which enhanced axillary buds’ outgrowth, number of branches, and number of pods per plant. Likewise, removing the two lowest racemes enhanced cytokinins’ synthesis, which redirected the allocation of assimilates to modulate the source–sink ratio in a way inductive to yield, as indicated by increases in net assimilation rate and seed growth rate, coupled with reduced leaf senescence. Combining both treatments’ effects positively modulated plant growth, flowering, and biochemical features, leading to promotion of the plant’s reproductive potential. It seems that the “stay-green” effect is an integral part of the effects of both decapitation and removal of the two lowest racemes, through which senescence is delayed, chlorophyll is maintained, and photosynthetic capacity is preserved; thereby, the plant’s reproductive capacity is sustained. Consequently, concealing the apical dominance in conjunction with reducing the sink strength through guided raceme removal would benefit the reproductive potential in soybean.
Author Contributions
H.M.I. and M.S.S. designed the research. H.M.I. and M.S.S. participated in the measurements and data analysis. H.M.I. wrote the first draft of the manuscript. B.A., A.E.-K., M.A.E.-E., T.K., I.J., Z.U. and M.S.S. revised and edited the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
No external funding was received.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data supporting the findings of this study are included in this article.
Acknowledgments
The authors acknowledge Mansoura University, Egypt for supporting this work.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Ning, L.; Du, W.; Song, H.; Shao, H.; Qi, W.; Sheteiwy, M.S.A.; Yu, D. Identification of responsive miRNAs involved in combination stresses of phosphate starvation and salt stress in soybean root. Environ. Exp. Bot. 2019, 167, 103823. [Google Scholar] [CrossRef]
- Ellis, R.H.; Asumadu, H.; Qi, A.; Summwerfield, R.J. Effects of photoperiod and maturity genes on plant growth, partitioning, radiation use efficiency and yield in soybean [Glycine max (L.) Merr.] ‘Clark’. Ann. Bot. 2000, 85, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Luquez, V.M.; Guiamét, J.J. Effects of the ‘stay green’ genotype GGd1d1d2d2 on leaf gas exchange, dry matter accumulation and seed yield in soybean [Glycine max (L.) Merr.]. Ann. Bot. 2001, 87, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Thomas, H.; Howarth, C.J. Five ways to stay green. J. Exp. Bot. 2000, 51, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregersen, P.L.; Culetic, A.; Boschian, L.; Krupinska, K. Plant senescence and crop productivity. Plant Mol. Biol. 2013, 82, 603–622. [Google Scholar] [CrossRef]
- Guiamét, J.J.; Teeri, J.A.; Nooden, L.D. Effects of nuclear and cytoplasmic genes altering chlorophyll loss on gas exchange during monocarpic senescence in soybean. Plant Cell Physiol. 1990, 31, 1123–1130. [Google Scholar]
- Müller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef] [Green Version]
- Amuti, K. Effect of removal of flower buds, open flowers, young pods and shoot apex on growth and pod set in soybean. J. Exp. Bot. 1983, 34, 719–725. [Google Scholar] [CrossRef]
- Colbert, K.A.; Beever, J.F. Effect of debudding on root cytokinin export and leaf senescence in tomato and tobacco. J. Exp. Bot. 1981, 32, 121–127. [Google Scholar] [CrossRef]
- Zavaleta-Mancera, H.A.; Franklin, K.A.; Ougham, H.J.; Thomas, H.; Scott, I.M. Regreening of senescent Nicotiana leaves. I. Reappearance of NADPH-protochlorophyllide oxidoreductase and light-harvesting chlorophyll a/b-binding protein. J. Exp. Bot. 1999, 50, 1677–1682. [Google Scholar]
- Davis, M.P. Alteration of Apical Dominance in Soybeans [Glycine max (L.) Merr.] with Foliar Applications of Benzoic Acid Derivatives or Terminal Bud Removal and the Effects of Salicylic, Acetylsalicylic and Thiosalicylic Acids on Nitrate Reduction in Soybean Leaves. Ph.D. Thesis, University of Nebraska, Lincoln, NE, USA, 1982. [Google Scholar]
- Tayo, T.O. Growth, development and yield of Pigeon pea [Cajanus cajan (L.) Millsp.] in the lowland tropics 3. Effect of early loss of apical dominance. J. Agric. Sci. 1982, 98, 79–84. [Google Scholar] [CrossRef]
- Khan, E.A.; Hussain, I.; Ahmad, H.B.S.; Hussain, I. Influence of nipping and foliar application of nutrients on growth and yield of chickpea in rain-fed condition. Legum. Res. 2018, 41, 740–744. [Google Scholar] [CrossRef] [Green Version]
- Argall, J.E.; Stewart, K.A. Effects of decapitation and benzyladenine on growth and yield of Cowpea [Vigna unguiculate (L.) Walp.]. Ann. Bot. 1984, 54, 439–444. [Google Scholar] [CrossRef]
- Kokubun, M.; Shimada, S.; Takahashi, M. Flower abortion caused by preanthesis water deficit is not attributed to impairment of pollen in soybean. Crop Sci. 2001, 41, 1517–1521. [Google Scholar] [CrossRef]
- Kokubun, M.; Nonokawa, K.; Kaihattttsu, A.; Yashima, Y. Mechanisms controlling flower abortion in soybean. Tohoku J. Agric. Res. 2009, 60, 23–26. [Google Scholar]
- Chakraborty, R.; Chowdhury, T.E.; Sumon, M.D.J.I.; Mostofa, M.; Rahman, M.L. Contribution of flower removal on the performance of Mungbean. Eur. J. Exp. Biol. 2015, 5, 66–71. [Google Scholar]
- Mondal, M.M.A.; Fakir, M.S.A.; Prodhan, A.K.M.A.; Ismail, M.R.; Ashrafuzzaman, M. Deflowering effect on vasculature and yield attributes in raceme of mung bean [Vigna radiata (L.) Wilczek]. Aust. J. Crop Sci. 2011, 5, 1339–1344. [Google Scholar]
- Zhang, X.; Wang, M.; Wu, T.; Wu, C.; Jiang, B.; Guo, C.; Han, T. Physiological and molecular studies of stay green caused by pod removal and seed injury in soybean. Crop J. 2016, 4, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Egli, D.B.; Bruening, W.P. Depodding causes green-stem syndrome in soybean. Crop Manag. 2006, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Shimizu-Sato, S.; Tanaka, M.; Mori, H. Auxin–cytokinin interactions in the control of shoot branching. Plant Mol. Biol. 2009, 69, 429. [Google Scholar] [CrossRef] [Green Version]
- Ongaro, V.; Leyser, O. Hormonal control of shoot branching. J. Exp. Bot. 2008, 59, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Guan, S.C.; Wen, C.; Li, P.; Gao, Z.; Chen, X. Auxin and cytokinin coordinate the dormancy and outgrowth of axillary bud in strawberry runner. BMC Plant Biol. 2019, 19, 528. [Google Scholar] [CrossRef]
- Li, C.J.; Guevara, E.; Herrera, J.; Bangerth, F. Effect of apex excision and replacement by 1-naphthylacetic acid on cytokinin concentration and apical dominance in pea plants. Physiol. Plant. 1995, 94, 465–469. [Google Scholar] [CrossRef]
- Cline, M.G. Exogenous auxin effects on lateral bud outgrowth in decapitated shoots. Ann. Bot. 1996, 78, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Huff, A.; Dybing, C.D. Factors affecting shedding of flowers in soybean (Glycine max (L.) Merrill). J. Exp. Bot. 1980, 31, 751–762. [Google Scholar] [CrossRef]
- Miceli, F.; Crafts-Brandner, S.J.; Egli, D.B. Physical restriction of pod growth alters development of soybean plants. Crop Sci. 1995, 35, 1080–1085. [Google Scholar] [CrossRef]
- Gwathmey, C.O.; Hall, A.E.; Madore, M. Adaptive attributes of Cowpea genotypes with delayed monocarpic leaf senescence. Crop Sci. 1992, 32, 765–772. [Google Scholar] [CrossRef]
- Gao, C.; El-Sawah, A.M.; Ali, D.F.I.; Hamoud, Y.A.; Shaghaleh, H.; Sheteiwy, M.S. The integration of bio and organic fertilizers improve plant growth, grain yield, quality and metabolism of hybrid maize (Zea mays L.). Agronomy 2020, 10, 319. [Google Scholar] [CrossRef] [Green Version]
- El-Sawah, A.M.; El-Keblawy, A.; Ali, D.F.I.; Ibrahim, H.M.; El-Sheikh, M.A.; Sharma, A.; Hamoud, Y.A.; Shaghaleh, H.; Brestic, M.; Skalicky, M.; et al. Arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria enhance soil key enzymes, plant growth, seed yield, and qualitative attributes of guar. Agriculture 2021, 11, 194. [Google Scholar] [CrossRef]
- Metwally, A.A.; Safina, S.A.; Abdel-Wahab, E.I.; Abdel-Wahab, S.I.; Abdel-Wahab, T.I. Screening thirty soybean genotypes under solid and intercropping plantings in Egypt. J. Crop Sci. Biotech. 2021, 24, 203–220. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M.; et al. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. BMC Plant Biol. 2021, 21, 195. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Elgawad, H.A.; Xiong, Y.; Macovei, A.; Brestic, M.; Skalicky, M.; Shaghaleh, H.; Hamoud, Y.A.; El-Sawah, A.M. Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress and improve seed yield and quality of soybean plant. Physiol. Plant. 2021, 172, 2153–2169. [Google Scholar] [CrossRef] [PubMed]
- Radford, P.G. Growth Analysis Formulae—Their Use and Abuse. Crop Sci. 1967, 3, 171–175. [Google Scholar] [CrossRef]
- Guffy, R.D.; Hesketh, J.D.; Nelson, R.L.; Bernard, R.L. Seed growth rate, growth duration, and yield in soybean. Biotronics 1991, 20, 19–30. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Rauf, S.; Sadaqat, H.A. Effects of varied water regimes on root length, dry matter partitioning and endogenous plant growth regulators in sunflower (Helianthus annuus L.). J. Plant Interact. 2007, 2, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, L.; Gonzalez-Vilar, M. Determination of relative water content. In Handbook of Plant Ecophysiology Techniques; Reigosa, M.J., Ed.; Kluwer Academic: Dordrecht, The Netherlands, 2001; pp. 207–212. [Google Scholar]
- Premchandra, G.S.; Saneoka, H.; Ogata, S. Cell membrane stability, an indicator of drought tolerance as affected by applied nitrogen in soybean. J. Agric. Sci. Camb. 1990, 115, 63–66. [Google Scholar] [CrossRef]
- A.O.A.C. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Statistical Analysis Software, Version 8.2; SAS Institute: Cary, NC, USA, 2001.
- Thakur, S.; Lakpale, R. Impact of nipping on soybean (Glycine max) plant architecture, nodulation and yield. Indian J. Agron. 2014, 59, 477–480. [Google Scholar]
- Turnbull, G.N.C.; Raymond, A.A.M.; Dodd, C.I.; Morris, E.S. Rapid increases in Cytokinin concentration in lateral buds of chickpea (Cicer arietinum L.) during release of apical dominance. Planta 1997, 202, 271–276. [Google Scholar] [CrossRef]
- Mader, J.C.; Emery, R.J.N.; Turnbull, C.G.N. Spatial and temporal changes in multiple hormone groups during lateral bud release shortly following apex decapitation of chickpea (Cicer arietinum) seedlings. Physiol. Plant. 2003, 119, 295–308. [Google Scholar] [CrossRef]
- Lindoo, S.J.; Noodén, L.D. The interrelation of fruit development and leaf senescence in ‘Anoka’ soybeans. Bot. Gaz. 1976, 137, 218–223. [Google Scholar] [CrossRef]
- Pandey, R.K. Growth, development and yield physiology of pigeonpea. In Proc. International Workshop on Pigeonpea; International Crops Research Institute for the Semi-Arid Tropics: Patancheru, India, 1980; Volume 2, pp. 15–19. [Google Scholar]
- Parvez, M.A.; Muhammad, F.; Ahmad, M. Effect of depodding on the growth and yield of Peas (Pisum sativum L.). Pak. J. Biol. Sci. 2000, 8, 1281–1282. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Gan, S.S. Translational researches on leaf senescence for enhancing plant productivity and quality. J. Exp. Bot. 2014, 65, 3901–3913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noodén, L.D.; Penney, J.P. Correlative controls of senescence and plant death in Arabidopsis thaliana (Brassicaceae). J. Exp. Bot. 2001, 52, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Lindoo, S.J.; Noodén, L.D. Studies on the behavior of the senescence signal in anoka soybeans. Plant Physiol. 1977, 59, 1136–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokubun, M.; Honda, I. Intra-raceme variation in pod-set probability is associated with cytokinin content in soybeans. Plant Prod. Sci. 2000, 3, 354–359. [Google Scholar] [CrossRef]
- Smart, C.M.; Scofield, S.R.; Bevan, M.W.; Dyer, T.A. Delayed leaf senescence in tobacco plants transformed with tmr, a gene for cytokinin production in Agrobacterium. Plant Cell 1991, 3, 647–656. [Google Scholar] [CrossRef]
- Van Staden, J. Cytokinins and senescence. Senescence Aging Plants 1988, 281–328. [Google Scholar]
- Kim, H.J.; Ryu, H.; Hong, S.H.; Woo, H.; Lim, P.O.; Lee, I.C.; Sheen, J.; Nam, H.G.; Hwang, I. Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 814–819. [Google Scholar] [CrossRef] [Green Version]
- Reese, R.N.; Dybing, C.D.; White, C.A.; Page, S.M.; Larson, J.E. Expression of vegetative storage protein (VSP-b) in soybean raceme tissues in response to flower set. J. Exp. Bot. 1995, 46, 957–964. [Google Scholar] [CrossRef]
- Nagel, L.; Brewster, R.; Riedell, W.E.; Reese, R.N. Cytokinin regulation of flower and pod set in soybeans (Glycine max (L.) Merr.). Ann. Bot. 2001, 88, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Hayati, R.; Egli, D.B.; Crafts-Brandner, S.J. Carbon and nitrogen supply during seed filling and leaf senescence in soybean. Crop Sci. 1995, 35, 1063–1069. [Google Scholar] [CrossRef]
- Turc, O.; Tardieu, F. Drought affects abortion of reproductive organs by exacerbating developmentally driven processes via expansive growth and hydraulics. J. Exp. Bot. 2018, 69, 3245–3254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brun, W.A.; Betts, K.J. Source/sink relations of abscising and nonabscising soybean flowers. Plant Physiol. 1984, 75, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Kokubun, M. Design and evaluation of soybean ideotypes. Bull. Tohoku Natl. Agric. Exp. 1988, 77, 77–142. [Google Scholar]
- Yashima, Y.; Kaihatsu, A.; Nakajima, T.; Kokubun, M. Effect of source/sink ratio and Cytokinin application on pod set in soybean. Plant Prod. Sci. 2005, 8, 139–144. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).