The Quality of Superior Seedless Bunches during Shelf Life as Determined by Growth on Different Rootstocks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Materials and Experimental Layout
2.2. Physical Properties of Bunches
2.3. Chemical Properties of Bunches
2.4. Cellular Metabolism Enzyme Activities
2.5. Estimation of Phenolic Compounds and Browning Enzyme Activities
2.6. Malondialdehyde (MDA) and Electrolyte Leakage (EL%)
2.7. Statistical Analysis
3. Results and Discussion
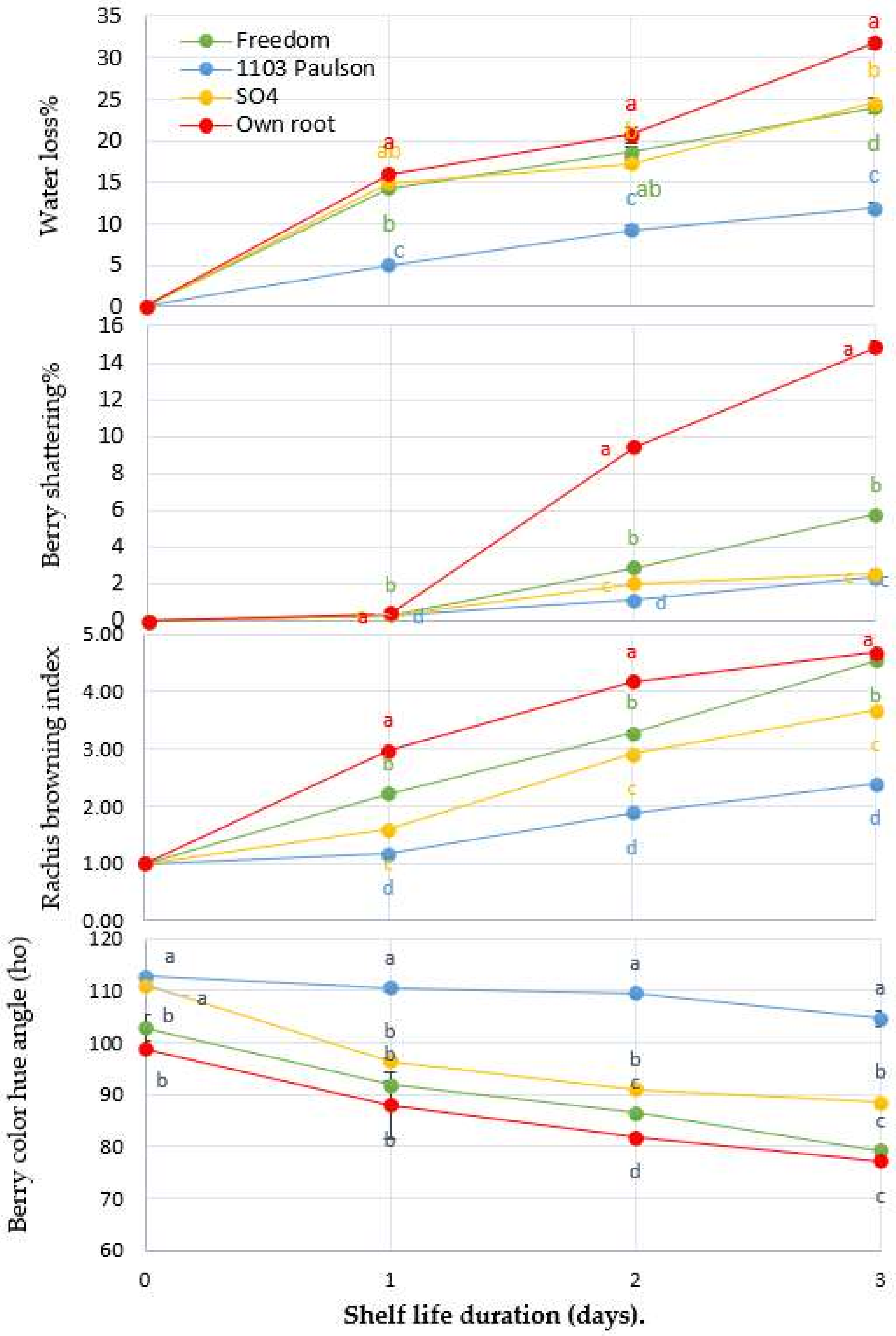
3.1. Effect of Rootstocks on Physical Attributes: Water Loss%, Rachis Browning Index (RB Index), and Berry Shatter % (BS), and Color (ho)
3.2. Berry Firmness, Separation Force, and Ascorbic Acid Content
3.3. Effect of Rootstocks: Soluble Solids Concentration (SSC%), Titratable Acidity (TA), and SSC/TA Ratio
3.4. Cellular Metabolism Enzyme Activities
3.5. Phenolic Compounds and Browning Enzyme Activities
3.6. Malondialdehyde (MDA) and Electrolyte Leakage % (EL%)
3.7. Multivariate Analysis of Rachis Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Banna, M.F.; Lo’ay, A.A. Evaluation berries shattering phenomena of ‘Flame seedless’ vines grafted on different rootstocks during shelf life. Sci. Hortic. Amst. 2019, 246, 51–56. [Google Scholar] [CrossRef]
- FAOSTAT. Source FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 19 August 2021).
- El-Hady, E.S.; Shaltout, A.D.; Desouky, I.M.; Haggag, L.F. Characteristics of some grape cultivars as affected by some grape rootstocks. Middle East J. Agric. Res. 2014, 3, 609–617. [Google Scholar]
- Kelany, A.E.; Abdel-Wahab, S.M.; Abdel-Hafeez, A.A.; Emam, I.A. Effect of pre-harvest treatments on cluster quality of flame seedless table grape cultivar during cold storage. J. Hortic. Sci. Ornam. Plants 2011, 3, 11–21. [Google Scholar]
- Koepke, T.; Dhingra, A. Rootstocks scion somatogenetic interactions in perennial composite plants. Plant Cell Rep. 2013, 32, 1321–1337. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Doaa, M.H. The potential of vine rootstocks impacts on ‘Flame Seedless’ bunches behavior under cold storage and antioxidant enzyme activity performance. Sci. Hortic. 2020, 260, 108844. [Google Scholar] [CrossRef]
- Jin, Z.-X.; Sun, T.-Y.; Sun, H.; Yue, Q.-Y.; Yao, Y.-X. Modifications of ‘Summer Black’ grape berry quality as affected by the different rootstocks. Sci. Hortic. 2016, 210, 130–137. [Google Scholar] [CrossRef]
- Gao-Takai, M.; Katayama-Ikegami, A.; Nakano, S.; Matsuda, K.; Motosugi, H. Vegetative Growth and Fruit Quality of ‘Ruby Roman’ Grapevines Grafted on Two Species of Rootstock and Their Tetraploids. Hortic. J. 2017, 86, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Gijon, M.D.; Gimenez, C.; Perez-Lopez, D.; Guerrero, J.; Couceiro, J.F.; Moriana, A. Rootstock influences the response of pistachio (Pistacia vera L. cv. Kerman) to water stress and rehydration. Sci. Hortic. Amst. 2010, 125, 666–671. [Google Scholar] [CrossRef]
- Koundouras, S.; Tsialtas, I.T.; Zioziou, E.; Nikolaou, N. Rootstock effects on the adaptive strategies of grapevine (Vitis vinifera L. cv. Cabernet-Sauvignon) under contrasting water status: Leaf physiological and structural responses. R. Agric. Ecosyst. Environ. 2008, 128, 86–96. [Google Scholar] [CrossRef]
- Bica, D.; Gay, G.; Morando, A.; Soave, E.; Bravdo, B.A. Effect of rootstock and Vitis vinifera genotype on photosynthetic parameters. Acta Hortic. 2000, 526, 373–379. [Google Scholar] [CrossRef]
- Paranychianakis, N.V.; Aggelides, S.; Angelakis, A.N. Influence of rootstock, irrigation level and recycled water on growth and yield of Soultanina grapevines. Agric. Water Manag. 2004, 69, 13–27. [Google Scholar] [CrossRef]
- Main, G.; Morris, J.; Striegler, K. Rootstock effects on Chardonel productivity, fruit, and wine composition. Am. J. Enol. Vitic. 2002, 53, 37–40. [Google Scholar]
- Gonçalves, B.; Moutinho-Pereira, J.; Santos, A.; Silva, A.P.; Bacelar, E.; Correia, C.; Rosa, E. Scion–rootstock interaction affects the physiology and fruit quality of sweet cherry. Tree Physiol. 2006, 126, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Zhiyuan, Y. Study on the rootstocks for Fujiminori grape variety. South China 2003, 32, 57–58. [Google Scholar]
- Smith, J.P. Investigations Into the Mechanisms Underlying Grapevine Rootstock Effects on Scion Growth and Yield. Ph.D. Thesis, Charles Sturt University, Wagga Wagga, Australia, 2004. [Google Scholar]
- Pulko, B.; Vršic, S.; Valdhuber, J. Influence of various rootstocks on the yield and grape composition of sauvignon blanc. Czech J. Food Sci. 2012, 30, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Kubota, N.; Li, X.G.; Yasui, K. Effects of rootstocks on sugar, organic acid, amino acid, and anthocyanin contents in berries of potted ‘Fujiminori’ grapes. J. Jpn. Soc. Hortic. Sci. 1993, 62, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Wolpert, J.; Walker, A.; Weber, E.; Bettiga, L.; Smith, R.; Verdegaal, P. Rootstocks and Phylloxera; Viticultural Notes; University of California Cooperative Extension: Napa County, CA, USA, 1994; Number 6. [Google Scholar]
- Artés-Hernández, F.; Tomás-Barberán, F.A.; Artés, F. Modified atmosphere packaging preserves quality of SO2-free ‘Superior seedless’ table grapes. Postharvest Biol. Technol. 2006, 39, 146–154. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Corzo, P.; Palou, L.; Mitchel, F.G. Table grape packaging Influences ‘flame seedless’ and ‘redglobe’ storage quality. Cent. Vally Postharvest Newsl. 2001, 10, 1–10. [Google Scholar]
- Lo’ay, A.A.; Taha, N.A. Evaluation rachis browning phenomena of ‘Superior Seedless’ vines grafted on different rootstocks during shelf life. Sci. Hortic. 2020, 261, 109040. [Google Scholar] [CrossRef]
- A.O.A.C. Association of Official of Analytical Chemist, 15th ed.; A.O.A.C.: Washington, DC, USA, 1995. [Google Scholar]
- Lo’ay, A.A.; Taha, N.A.; El-Khateeb, Y.A. Storability of ‘Thompson Seedless’ grapes: Using biopolymer coating chitosan and polyvinyl alcohol blending with salicylic acid and antioxidant enzymes activities during cold storage. Sci. Hortic. 2019, 249, 314–321. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for the determination of reducing suga. Anal. Chem. 1959, 31, 426–429. [Google Scholar] [CrossRef]
- Collmer, A.; Reid, J.L.; Mount, M.S. Assay methods for pectic enzymes. In Methods in Enzymology; Wood, W.A., Kellogg, S.T., Eds.; Academic Press: San Diego, CA, USA, 1988; Volume 161, pp. 329–335. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Jiang, Y.; Louce, D.C.; Jayas, W.; Lu, W. Effect of chilling temperatures on ethylene binding by banana fruit. Plant Growth Regul. 2004, 43, 109–115. [Google Scholar] [CrossRef]
- Ke, D.; Salveit, M.E. The effect of calcium and auxin on russet spotting and phenylalanine ammonia lyase activity in iceberg lettuce. HortiScience 1986, 21, 1169–1171. [Google Scholar]
- Hoff, J.F.; Singleton, K.I. A method for determination of tannin in foods by means of immobilized enzymes. J. Food Sci. 1977, 42, 1566–1569. [Google Scholar] [CrossRef]
- Iturbe-Ormaetxe, I.; Escuredo, P.R.; Arrese-Igor, C.; Becana, M. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 1998, 116, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Lo’ay, A.A. Biological indicators to minimize berry shatter during handling of “Thompson seedless” grapevines. World Appl. Sci. J. 2011, 12, 1107–1113. [Google Scholar]
- Rogiers, S.; Hatfield, J.; Jaudzems, V.; White, R.; Keller, M. Grape berry cv. Shiraz epicuticular wax and transpiration during ripening and preharvest weight loss. Am. J. Enol. Vitic. 2004, 55, 121–127. [Google Scholar]
- Ritenour, M.A.; Stover, E.; Boman, B.J.; Bowman, K.D.; Castle, W.S. Effect of rootstock on stem-end rind breakdown and decay of fresh citrus. Horttechnology 2004, 13, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Zhang, Z.; Lv, X.; Cheng, N.; Peng, B.; Cao, W. Effect of 24-epibrassinolide on chilling injury of peach fruit in relation to phenolic and proline metabolisms. Postharvest Biol. Technol. 2016, 111, 390–397. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Gil, M.I.; Castaner, M.; Artes, F.; Saltveit, M.E. Effect of selected browning inhibitors on phenolic metabolism in stem tissue of harvested lettuce. J. Agric. Food Chem. 1997, 45, 583–589. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Dawood, H.D. Minimize browning incidence of banana by postharvest active chitosan/PVA Combines with oxalic acid treatment to during shelf-life. Sci. Hortic. 2017, 226, 208–215. [Google Scholar] [CrossRef]
- Bassetto, E.; Jacomino, A.P.; Pinheiro, A.L.; Kluge, R.A. Delay of ripening of ‘Pedro Sato’ guava with 1-methylcyclopropene. Postharvest Biol. Technol. 2005, 35, 303–308. [Google Scholar] [CrossRef]
- Nunan, K.J.; Sims, I.M.; Bacic, A.; Robinson, S.P.; Fincher, G.B. Changes in Cell Wall Composition during Ripening of Grape Berries. Plant Physiol. 1998, 118, 783–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köse, B.; Karabulut, B.; Ceylan, K. Effect of rootstock on grafted grapevine quality. Eur. J. Hortic. Sci. 2014, 79, 197–202. [Google Scholar]
- EL-Gendy, H.A. The universal associative envelope of the anti-Jordan triple system of n × n matrices. J. Algebra 2013, 383, 1–28. [Google Scholar] [CrossRef]
- Bugaud, C.; Deverge, E.; Daribo, M.-O.; Ribeyre, F.; Fils-Lycaon, B.; Mbéguié-A-Mbéguié, D. Sensory characterisation enabled the first classification of dessert bananas. J. Sci. Food Agric. 2011, 91, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.B.H.; Lim, J.S.K.; Greenfield, H. Composition of Australian foods. 31. Tropical and sub-tropical fruit. Food Technol. Aust. 1986, 38, 118–123. [Google Scholar]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant. Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Hodges, D.M.; Lester, G.E.; Muneo, K.D.; Toivonen, P.M. Oxidative stress: Importance for postharvest quality. HortScience 2004, 39, 924–929. [Google Scholar] [CrossRef] [Green Version]
- McCollum, T.; Bowman, K.; Castle, W. Effects of rootstock on fruit quality and postharvest behavior of ‘Marsh’ grapefruit. Proc. Fla. State Hortic. Soc. 2002, 115, 44–46. [Google Scholar]
- Satisha, J.; Doshi, P.; Adsule, P.G. Influence of rootstocks on changing the pattern of phenolic compounds in Thompson seedless grapes and its relationship to the incidence of powdery mildew. Turk. J. Agric. For. 2008, 32, 1–9. [Google Scholar] [CrossRef]
- Promyou, S.; Ketsa, S.; Van Doorn, W.G. Effect of surface coating on ripening and early peel spotting in ‘sucrier’ banana (Musa acuminata). N. Z. J. Crop Hortic. Sci. 2007, 35, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Barden, C.L.; Bramlage, W.J. Acclimation of antioxidants in apple peel as related to preharvest factors and superficial scald susceptibility of the fruit. J. Am. Soc. Hortic. Sci 1994, 119, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef] [Green Version]
- Lo’ay, A.A.; Dawood, H.D. Chilling injury, fruit color maturity stages, and antioxidant enzyme activities of lemon ‘baladi CV’ fruits under cold storage stress. Sci. Hortic. 2019, 257, 108676. [Google Scholar] [CrossRef]
- Shewfelt, R.L.; Del Rosario, B.A. The role of lipids peroxidation in storage disorders of freash fruits and vegetables. HortScience 2000, 35, 575–579. [Google Scholar] [CrossRef]






| WL% | RB Index | BS% | ho | XYL | CEL | PG | PT | BF | BSF | SSC% | TA% | SSC/TA% | TF | TFv | PPO | PAL | EL% | MDA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W% | * 1.0000 | ||||||||||||||||||
| RB index | 0.8698 | 1.0000 | |||||||||||||||||
| BS% | 0.8762 | 0.8445 | 1.0000 | ||||||||||||||||
| ho | −0.1044 | −0.1207 | 0.0410 | 1.0000 | |||||||||||||||
| XYL | 0.9083 | 0.8714 | 0.9549 | 0.1442 | 1.0000 | ||||||||||||||
| CEL | 0.9027 | 0.8881 | 0.9512 | 0.1103 | 0.9706 | 1.0000 | |||||||||||||
| PG | 0.7758 | 0.6834 | 0.7151 | 0.4199 | 0.8234 | 0.7723 | 1.0000 | ||||||||||||
| PT | 0.7311 | 0.6360 | 0.6839 | 0.4283 | 0.7872 | 0.7381 | 0.9378 | 1.0000 | |||||||||||
| BF | −0.7629 | −0.7595 | −0.7957 | −0.3775 | −0.8831 | −0.8477 | −0.9468 | −0.8882 | 1.0000 | ||||||||||
| BSF | −0.6582 | −0.6335 | −0.6469 | −0.5935 | −0.7652 | −0.7546 | −0.9272 | −0.8915 | 0.9062 | 1.0000 | |||||||||
| SSC% | 0.7044 | 0.7808 | 0.6961 | 0.1403 | 0.7483 | 0.7141 | 0.7468 | 0.6973 | −0.7754 | −0.6941 | 1.0000 | ||||||||
| TA% | −0.4331 | −0.4593 | −0.4346 | −0.7364 | −0.5836 | −0.5505 | −0.8332 | −0.8104 | 0.7968 | 0.9364 | −0.6351 | 1.0000 | |||||||
| SSC/TA% | 0.5601 | 0.6243 | 0.5767 | 0.6088 | 0.6998 | 0.6685 | 0.8686 | 0.8235 | −0.8637 | −0.9341 | 0.8144 | −0.9601 | 1.0000 | ||||||
| TF | −0.7030 | −0.6746 | −0.7206 | −0.5008 | −0.8299 | −0.7781 | −0.9291 | −0.8582 | 0.9427 | 0.9055 | −0.7519 | 0.8448 | −0.8934 | 1.0000 | |||||
| TFv | −0.6400 | −0.5908 | −0.6561 | −0.6006 | −0.7808 | −0.7288 | −0.9508 | −0.9463 | 0.9326 | 0.9465 | −0.6980 | 0.9105 | −0.9109 | 0.9366 | 1.0000 | ||||
| PPO | 0.8259 | 0.7763 | 0.7933 | 0.2844 | 0.8985 | 0.8541 | 0.9365 | 0.8709 | −0.9531 | −0.8658 | 0.7346 | −0.7294 | 0.7943 | −0.9226 | −0.8943 | 1.0000 | |||
| PAL | 0.8654 | 0.7901 | 0.7719 | 0.2859 | 0.8763 | 0.8389 | 0.9497 | 0.8976 | −0.9200 | −0.8852 | 0.7848 | −0.7477 | 0.8255 | −0.9124 | −0.8826 | 0.9382 | 1.0000 | ||
| EL% | 0.9325 | 0.8691 | 0.8605 | 0.0761 | 0.9313 | 0.9253 | 0.8607 | 0.7838 | −0.8839 | −0.7963 | 0.7386 | −0.5845 | 0.6914 | −0.8244 | −0.7608 | 0.9283 | 0.9321 | 1.0000 | |
| MDA | 0.9186 | 0.8902 | 0.8975 | 0.1205 | 0.9658 | 0.9460 | 0.8531 | 0.8176 | −0.8954 | −0.7818 | 0.7845 | −0.6124 | 0.7291 | −0.8365 | −0.8015 | 0.9182 | 0.9099 | 0.9484 | 1.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo’ay, A.A.; Ismail, H.; Kassem, H.S. The Quality of Superior Seedless Bunches during Shelf Life as Determined by Growth on Different Rootstocks. Agriculture 2021, 11, 990. https://doi.org/10.3390/agriculture11100990
Lo’ay AA, Ismail H, Kassem HS. The Quality of Superior Seedless Bunches during Shelf Life as Determined by Growth on Different Rootstocks. Agriculture. 2021; 11(10):990. https://doi.org/10.3390/agriculture11100990
Chicago/Turabian StyleLo’ay, A. A., Hamed Ismail, and Hazem S. Kassem. 2021. "The Quality of Superior Seedless Bunches during Shelf Life as Determined by Growth on Different Rootstocks" Agriculture 11, no. 10: 990. https://doi.org/10.3390/agriculture11100990
APA StyleLo’ay, A. A., Ismail, H., & Kassem, H. S. (2021). The Quality of Superior Seedless Bunches during Shelf Life as Determined by Growth on Different Rootstocks. Agriculture, 11(10), 990. https://doi.org/10.3390/agriculture11100990








