Combining Selenium Biofortification with Vermicompost Growing Media in Lamb’s Lettuce (Valerianella locusta L. Laterr)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Growing Media Characteristics
2.2. Experimental Design
2.3. Analysis of the Growing Media
2.3.1. pH Determination
2.3.2. EC
2.3.3. Determination of the Organic Matter and Ash Contents
2.3.4. Determination of the Organic C Content, Total N and C/N Ratio
2.3.5. Determination of the Heavy Metal Concentrations
2.4. Plant Material Analysis
Determination of the Total Se and Total Zn Concentrations in the Plant Tissue
2.5. Statistical Analysis
3. Results
3.1. Physico-Chemical Analysis of the Growing Media
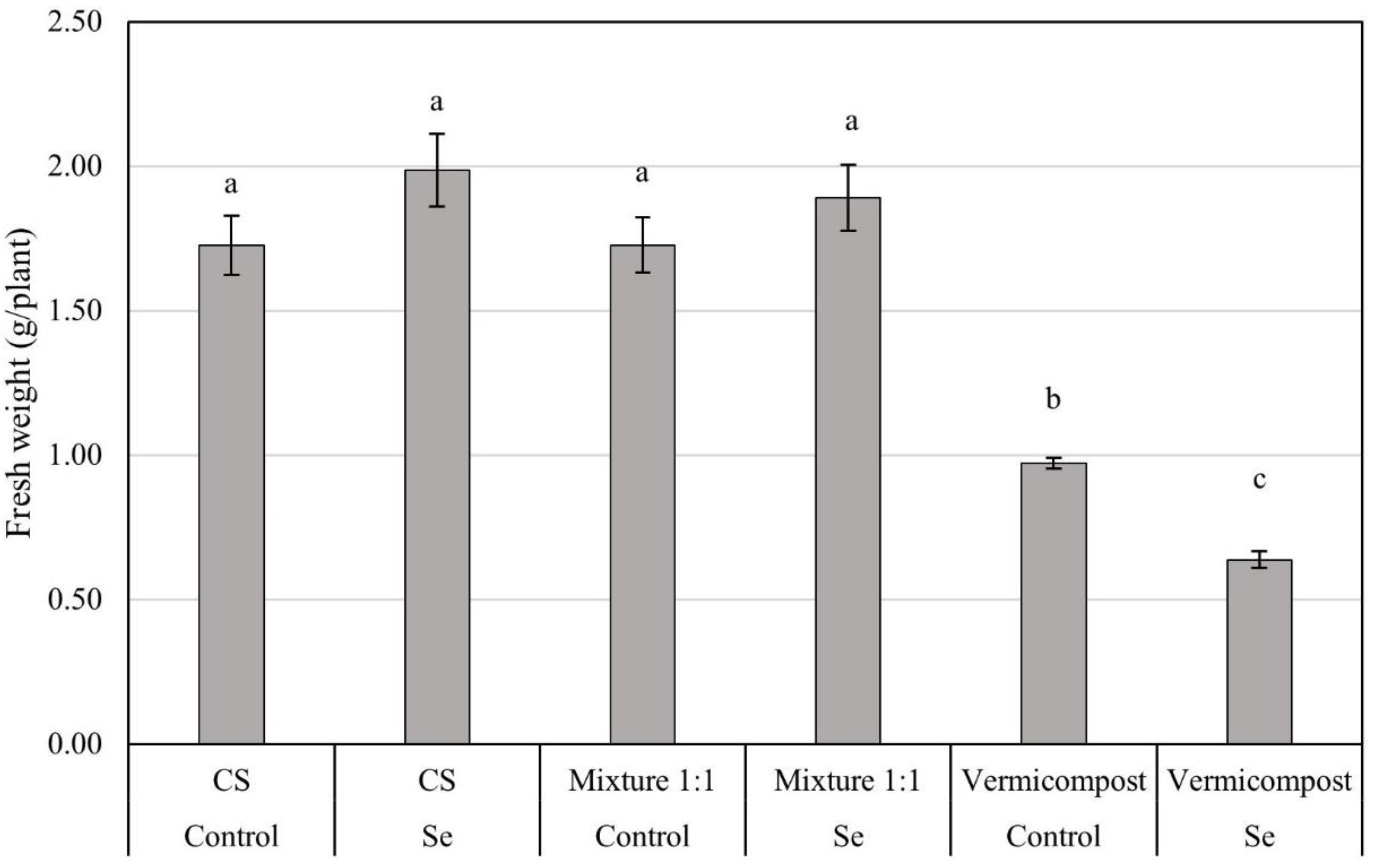
3.2. Analysis of Plant Material
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, U.; Sajid, N.; Khalid, A.; Riaz, L.; Rabbani, M.M.; Syed, J.H.; Malik, R.N. A Review on Vermicomposting of Organic Wastes. Environ. Prog. Sustain. Energy 2015, 34, 1050–1062. [Google Scholar] [CrossRef]
- Bachman, G.R.; Metzger, J.D. Growth of bedding plants in commercial potting substrate amended with vermicompost. Bioresour. Technol. 2008, 99, 3155–3161. [Google Scholar] [CrossRef]
- Gruda, N. Sustainable peat alternative growing media. Acta Hortic. 2012, 927, 973–979. [Google Scholar] [CrossRef]
- Pathma, J.; Sakthivel, N. Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. SpringerPlus 2012, 1, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikary, S. Vermicompost, the story of organic gold: A review. Agric. Sci. 2012, 3, 905–917. [Google Scholar] [CrossRef] [Green Version]
- Demir, Z. Effects of Vermicompost on Soil Physicochemical Properties and Lettuce (Lactuca sativa Var. Crispa) Yield in Greenhouse under Different Soil Water Regimes. Commun. Soil Sci. Plant Anal. 2019, 50, 2151–2168. [Google Scholar] [CrossRef]
- Ramos, S.J.; Faquin, V.; Guilherme, L.R.G.; Castro, E.M.; Ávila, F.W.; Carvalho, G.S.; Bastos, C.; Oliveira, C. Selenium biofortification and antioxidant activity in lettuce plants fed with selenate and selenite. Plant Soil Environ. 2010, 56, 584–588. [Google Scholar] [CrossRef] [Green Version]
- Galić, L.; Vinković, T.; Ravnjak, B.; Lončarić, Z. Agronomic Biofortification of Significant Cereal Crops with Selenium—A Review. Agronomy 2021, 11, 1015. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Commission of European Communities. Commission Directive 2008/100/EC of 28 October 2008 amending Council Directive 90/496/EEC on nutrition labelling for foodstuffs as regards recommended daily allowances, energy conversion factors and definitions. ALINORM 10/33/26. Off. J. Eur. Union 2008, 038, 9–12. [Google Scholar]
- Hawrylak-Nowak, B.; Dresler, S.; Rubinowska, K.; Matraszek-Gawron, R.; Woch, W.; Hasanuzzaman, M. Selenium biofortification enhances the growth and alters the physiological response of lamb’s lettuce grown under high temperature stress. Plant Physiol. Biochem. 2018, 127, 446–456. [Google Scholar] [CrossRef]
- Galić, L.; Špoljarević, M.; Jakovac, E.; Ravnjak, B.; Teklić, T.; Lisjak, M.; Perić, K.; Nemet, F.; Lončarić, Z. Selenium biofortification of soybean seeds influences physiological responses of seedlings to osmotic stress. Plants 2021, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [Green Version]
- Shini, S.; Sultan, A.; Bryden, W. Selenium Biochemistry and Bioavailability: Implications for Animal Agriculture. Agriculture 2015, 5, 1277–1288. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Xing, G.; Tang, S.; Pang, Y.; Yi, Q.; Huang, Q.; Huang, X.; Huang, J.; Li, P.; Fu, H. Improving soil selenium availability as a strategy to promote selenium uptake by high-Se rice cultivar. Environ. Exp. Bot. 2019, 163, 45–54. [Google Scholar] [CrossRef]
- Ferrante, A.; Martinetti, L.; Maggiore, T. Biochemical changes in cut vs. intact lamb’s lettuce (Valerianella olitoria) leaves during storage. Int. J. Food Sci. Technol. 2009, 44, 1050–1056. [Google Scholar] [CrossRef]
- Enninghorst, A.; Lippert, F. Postharvest Changes in Carbohydrate Content of Lamb’s Lettuce. Int. Conf. Qual. Chain. 2003, 604, 553–558. [Google Scholar]
- Fabek, S.; Toth, N.; Benkoa, B.; Borošić, J.; Žutić, I.; Novak, B. Lamb’s lettuce growing cycle and yield as affected by abiotic factors. Acta Hortic. 2011, 893, 887–894. [Google Scholar] [CrossRef]
- Ramos-Bueno, R.P.; Rincón-Cervera, M.A.; González-Fernández, M.J.; Guil-Guerrero, J.L. Phytochemical Composition and Antitumor Activities of New Salad Greens: Rucola (Diplotaxis tenuifolia) and Corn Salad (Valerianella locusta). Plant Foods Hum. Nutr. 2016, 71, 197–203. [Google Scholar] [CrossRef]
- EN Soil Improvers and Growing Media—Sample Preparation for Chemical and Physical Tests, Determination of Dry Matter Content, Moisture Content and Laboratory Compacted Bulk Density 13037:2011. 2011. Available online: https://standards.iteh.ai/catalog/standards/cen/f65ff417-8656-4f00-a840-a6ca1846fb6f/en-13040-2007 (accessed on 27 October 2021).
- EN Soil Improvers and Growing Media—Determination of Electrical Conductivity; German Version prEN 13038:2009. 2009. Available online: https://standards.iteh.ai/catalog/standards/cen/121ac421-7003-471a-b75d-73eb4b8d784d/en-13038-2011 (accessed on 27 October 2021).
- Matusiewicz, H.; Sturgeon, R.E.; Berman, S.S. Trace element analysis of biological material following pressure digestion with nitric acid—Hydrogen peroxide and microwave heating. J. Anal. At. Spectrom. 1989, 4, 323–327. [Google Scholar] [CrossRef]
- Smith, J.L.; Doran, J.W. Measurement and use of pH and electrical conductivity for soil quality analysis. Methods Assess. Soil Qual. 2015, 49, 169–185. [Google Scholar] [CrossRef]
- Gong, X.; Li, S.; Sun, X.; Wang, L.; Cai, L.; Zhang, J.; Wei, L. Green waste compost and vermicompost as peat substitutes in growing media for geranium (Pelargonium zonale L.) and calendula (Calendula officinalis L.). Sci. Hortic. 2018, 236, 186–191. [Google Scholar] [CrossRef]
- WHO. WHO Permissible Limits for Heavy Metals in Plant and Soil; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Zhang, Y.; Yang, X.; Zhang, S.; Tian, Y.; Guo, W.; Wang, J. The influence of Humic acids on the accumulation of lead (Pb) and cadmium (Cd) in tabacco leaves grown in different soils. J. Soil Sci. Plant Nutr. 2013, 13, 43–53. [Google Scholar] [CrossRef]
- Acosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A.; Martínez-Martínez, S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef]
- Singh, J.; Kalamdhad, A.S. Chemical Speciation of Heavy Metals in Compost and Compost Amended Soil—A Review. Int. J. Environ. Eng. Res. 2013, 2, 27–37. [Google Scholar]
- Swati, A.; Hait, S. Fate and bioavailability of heavy metals during vermicomposting of various organic wastes—A review. Process Saf. Environ. Prot. 2017, 109, 30–45. [Google Scholar] [CrossRef]
- Huang, L.; Liu, X.; Wang, Z.; Liang, Z.; Wang, M.; Liu, M.; Suarez, D.L. Interactive effects of pH, EC and nitrogen on yields and nutrientabsorption of rice (Oryza sativa L.). Agric. Water Manag. 2017, 194, 48–57. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Saltali, K.; Gezerel, O. Response of strawberry grown at high salinity and alkalinity to supplementary potassium. J. Plant Nutr. 2002, 25, 1415–1427. [Google Scholar] [CrossRef]
- Churilova, E.V.; Midmore, D.J. Vermiliquer (Vermicompost leachate) as a complete liquid fertilizer for hydroponically-grown pak choi (Brassica chinensis L.) in the tropics. Horticulturae 2019, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Haug, A.; Graham, R.D.; Christophersen, O.A.; Lyons, G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007, 19, 209–228. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, K.F.M.; Berton, R.S.; Coscione, A.R. Selenium biofortification of rice and radish: Effect of soil texture and efficiency of two extractants. Plant Soil Environ. 2014, 60, 105–110. [Google Scholar]
- De Oliveira, V.C.; Faquin, V.; Guimarães, K.C.; Andrade, F.R.; Pereira, J.; Guilherme, L.R.G. Agronomic biofortification of carrot with selenium. Cienc. Agrotecnol. 2018, 42, 138–147. [Google Scholar] [CrossRef]
- Thavarajah, D.; Ruszkowski, J.; Vandenberg, A. High potential for selenium biofortification of lentils (Lens culinaris L.). J. Agric. Food Chem. 2008, 56, 10747–10753. [Google Scholar] [CrossRef] [PubMed]
- Eich-Greatorex, S.; Sogn, T.A.; Øgaard, A.F.; Aasen, I. Plant availability of inorganic and organic selenium fertiliser as influenced by soil organic matter content and pH. Nutr. Cycl. Agroecosyst. 2007, 79, 221–231. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Z.H.; Bu, Y.; Ren, C.Z.; Li, J.Z.; Han, J.J.; Tao, C.; Zhang, K.; Wang, X.X.; Lu, G.X.; et al. Effects of selenium fertilizer on grain yield, se uptakeand distribution in common buckwheat (Fagopyrum esculentum Moench). Plant Soil Environ. 2015, 61, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Dai, H.; Wei, S.; Twardowska, I. Biofortification of soybean (Glycine max L.) with Se and Zn, and enhancing its physiological functions by spiking these elements to soil during flowering phase. Sci. Total Environ. 2020, 740, 139648. [Google Scholar] [CrossRef]
- Ei, H.H.; Zheng, T.; Farooq, M.U.; Zeng, R.; Su, Y.; Zhang, Y.; Liang, Y.; Tang, Z.; Ye, X.; Jia, X.; et al. Impact of selenium, zinc and their interaction on key enzymes, grain yield, selenium, zinc concentrations, and seedling vigor of biofortified rice. Environ. Sci. Pollut. Res. 2020, 27, 16940–16949. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Wang, M. Effects of Selenium—Zinc Interactions on the Bioavailability of Selenium/Zinc in Soil and Its Mechanism. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Mangueze, A.V.d.J.; Pessoa, M.F.G.; Silva, M.J.; Ndayiragije, A.; Magaia, H.E.; Cossa, V.S.I.; Reboredo, F.H.; Carvalho, M.L.; Santos, J.P.; Guerra, M.; et al. Simultaneous Zinc and selenium biofortification in rice. Accumulation, localization and implications on the overall mineral content of the flour. J. Cereal Sci. 2018, 82, 34–41. [Google Scholar] [CrossRef]
- Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef]
| Growing Media | % Organic Matter | % Ash | pHH2O | EC (mS/m) | Total N (g/kg) | C/N Ratio |
|---|---|---|---|---|---|---|
| CS | 39.6 | 51.4 | 6.34 | 42.1 | 2.2 | 180:1 |
| Vermicompost | 12.6 | 87.4 | 9.23 | 67.9 | 5.4 | 23.3:1 |
| Mixture 1:1 | 22.4 | 78.6 | 7.55 | 59.5 | 3.1 | 72.3:1 |
| Growing Media | Zn | Cu | Se | Cd | Pb | Mo | Ni | Cr | Hg | As |
|---|---|---|---|---|---|---|---|---|---|---|
| CS | 60.2 | 16.9 | 0.55 | 0.64 | 20.1 | 0.098 | 7.1 | 4.6 | <0.01 | 0.012 |
| Vermicompost | 106.0 | 34.0 | 0.21 | 0.49 | 18.9 | 0.720 | 23.0 | 30.0 | 0.063 | 5.570 |
| Mixture 1:1 | 77.0 | 23.4 | 0.32 | 0.54 | 19.3 | 0.411 | 17.1 | 19.4 | 0.041 | 3.213 |
| Fresh Weight per Plant | Plant Dry Weight | Se mg/kg FW | Se mg/kg DW | |
|---|---|---|---|---|
| Growing media | *** | *** | * | 0.122 |
| Se treatment | 0.6735 | 0.129 | *** | *** |
| Growing media: Se treatment | * | * | * | 0.121 |
| Se FW (mg/kg) | Se DW (mg/kg) | |
|---|---|---|
| Se Treatment | ||
| Control | 0.0065 ± 0.0019 b | 0.0691 ± 0.0131 b |
| Se | 1.1236 ± 0.5914 a | 12.2615 ± 4.3845 a |
| LSD 0.05 | 0.2794 | 2.629 |
| Growing Media | ||
| CS | 0.532 ± 0.584 ab | 6.7207 ± 7.3576 a |
| Vermicompost | 0.848 ± 1.01 a | 7.4656 ± 9.0653 a |
| Mixure 1:1 | 0.314 ± 0.361 b | 4.3096 ± 4.6585 a |
| LSD 0.05 | 0.342 | 3.221 |
| Treatment | Growing Media | Zn FW (mg/kg) | Zn DW (mg/kg) |
|---|---|---|---|
| Control | CS | 14.51 ± 0.910 a | 170.85 ± 10.65 a |
| Se | CS | 11.86 ± 0.064 b | 149.95 ± 1.05 b |
| Control | Mixture 1:1 | 9.49 ± 2.462 cd | 104.55 ± 0.15 c |
| Se | Mixture 1:1 | 6.83 ± 1.396 e | 97.67 ± 5.72 cd |
| Control | Vermicompost | 9.10 ± 0.635 d | 88.245 ± 4.68 d |
| Se | Vermicompost | 11.70 ± 0.379 bc | 101.81 ± 6.39 c |
| LSD 0.05 | 2.226 | 10.52 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galić, L.; Špoljarević, M.; Auriga, A.; Ravnjak, B.; Vinković, T.; Lončarić, Z. Combining Selenium Biofortification with Vermicompost Growing Media in Lamb’s Lettuce (Valerianella locusta L. Laterr). Agriculture 2021, 11, 1072. https://doi.org/10.3390/agriculture11111072
Galić L, Špoljarević M, Auriga A, Ravnjak B, Vinković T, Lončarić Z. Combining Selenium Biofortification with Vermicompost Growing Media in Lamb’s Lettuce (Valerianella locusta L. Laterr). Agriculture. 2021; 11(11):1072. https://doi.org/10.3390/agriculture11111072
Chicago/Turabian StyleGalić, Lucija, Marija Špoljarević, Alicja Auriga, Boris Ravnjak, Tomislav Vinković, and Zdenko Lončarić. 2021. "Combining Selenium Biofortification with Vermicompost Growing Media in Lamb’s Lettuce (Valerianella locusta L. Laterr)" Agriculture 11, no. 11: 1072. https://doi.org/10.3390/agriculture11111072








