Allometric Expression of Sesame Cultivars in Response to Various Environments and Nutrition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experimentation and Treatments
2.2. Soil and Weather Data
2.3. Crop Data
2.4. Statistical Analysis
3. Results
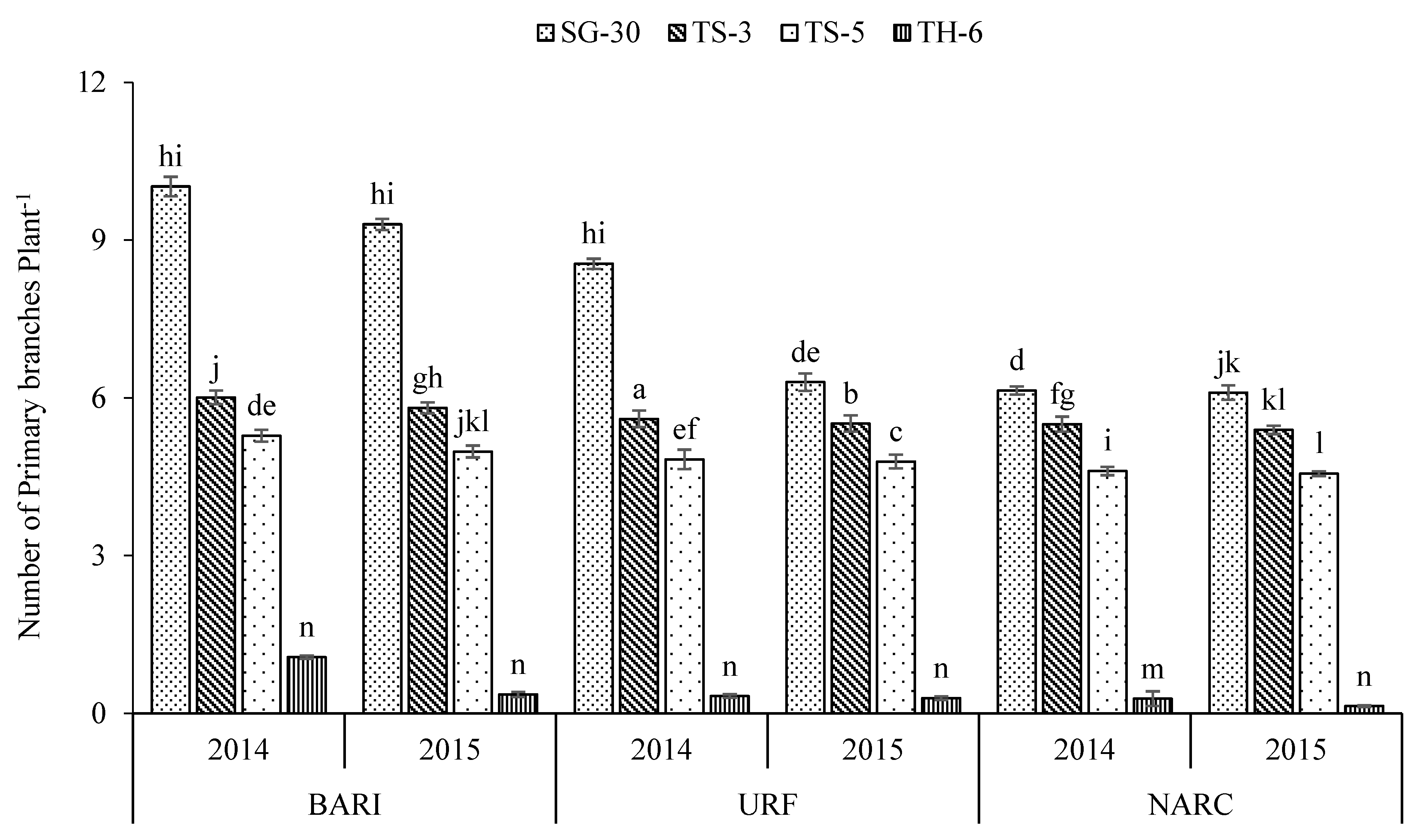
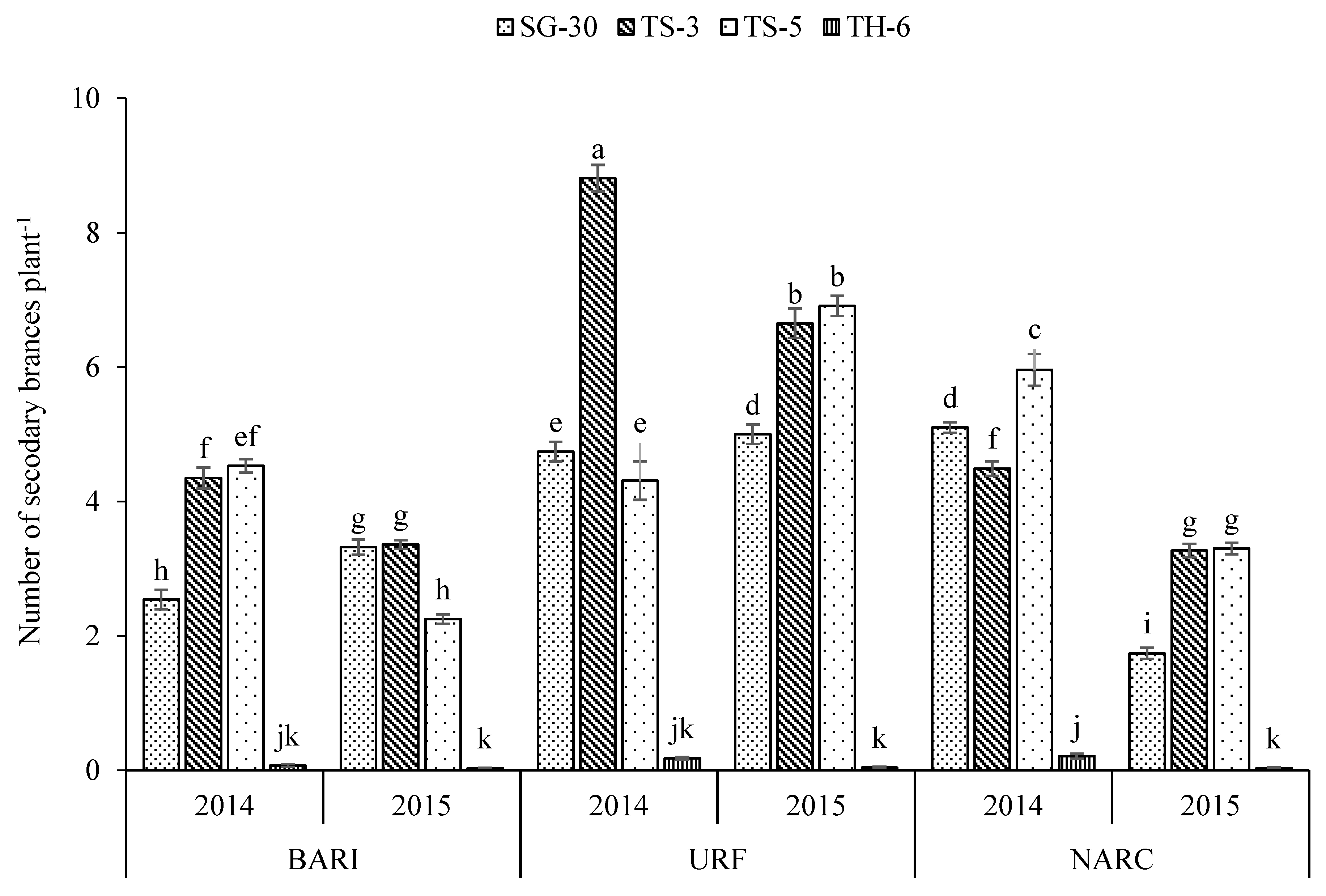
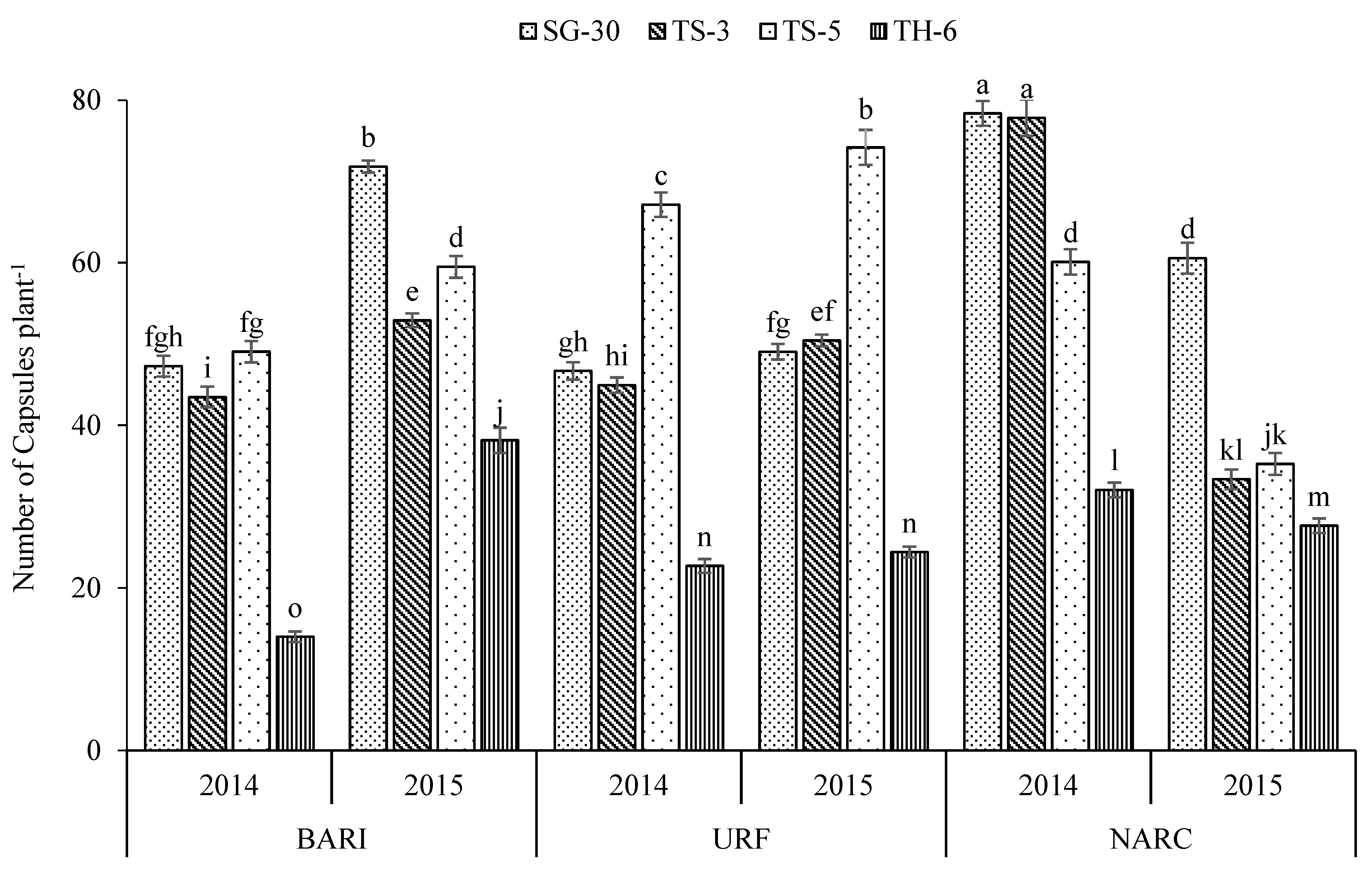
3.1. Cultivars
3.2. Locations
3.3. Year
3.4. Cultivar × Location × Year Interactions
3.5. Correlations among Agronomic Traits
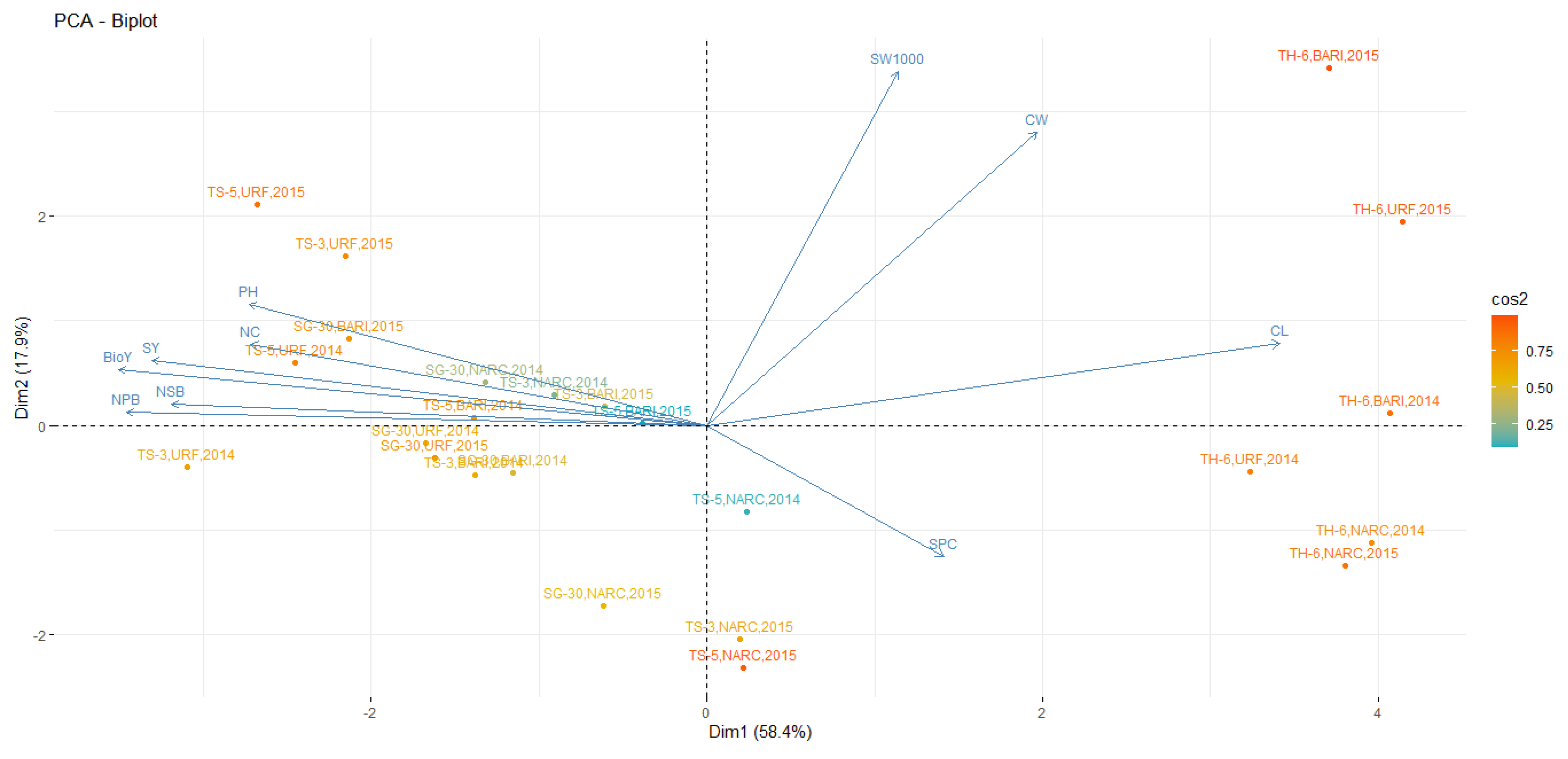
3.6. Principal Component Analysis
3.7. Micronutrients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GoP. Economic Survey of Pakistan 2020–2021; Government of Pakistan, Finance Division: Islamabad, Pakistan, 2021; Chapter-2; pp. 17–43.
- Hussain, I.; Cheema, A.M.; Khan, A.A. Small rodents in the crop ecosystem of Pothwar Plateau, Pakistan. Wildl. Res. 2003, 30, 269–274. [Google Scholar] [CrossRef]
- Nizami, M.I.; Shafiq, M.; Rashid, A.; Aslam, A. The Soils and Their Agricultural Development Potential in Pothwar; WRRI & NARC: Islamabad, Pakistan, 2004; pp. 5–7. [Google Scholar]
- Khan, M.A.H.; Sultana, N.A.; Islam, M.N.; Hasanuzzaman, M. Yield and yield contributing characters of sesame as affected by different management practices. Am. Eurasian J. Sci. Res. 2009, 4, 195–197. [Google Scholar]
- Suddhiyan, P.; Suwannaketnikom, S.; Dumkhum, W.; Duandao, N. Fertilizers for organic sesame. Asian J. Food Agrio-Ind. 2009, 197–204. Available online: https://www.thaiscience.info/journals/Article/AFAI/10850208.pdf (accessed on 2 November 2021).
- Uzun, B.; Arslan, I.; Furat, E. Variation in fatty acid compositions, oil content and oil yield in a germ plasm collection of sesame (Sesamum indicum L.). J. Am. Oil Chem. Soc. 2008, 85, 1135–1142. [Google Scholar] [CrossRef]
- Pham, T.D.; Nguyen, T.D.T.; Carlsson, A.S.; Bui, T.M. Morphological evaluation of sesame (Sesamum indicum L.) varieties from different origins. Aust. J. Crop Sci. 2010, 4, 498–504. [Google Scholar]
- Kumaresan, D.; Nadarajan, N. Stability analysis for yield and its components in sesame (Sesamum indicum L.). Ind. J. Agric. Res. 2005, 39, 60–63. [Google Scholar]
- Etukudo, O.O.; Babatola, L.A.; Fagbola, O. Effect of mycorrhiza, organo-mineral and NPK fertilizer on the performance and post-harvest quality of sweet corn. J. Horti. Forest. 2015, 7, 99–103. [Google Scholar] [CrossRef]
- Zainab, M.A.; Vincent, E.C.; Ayodele, O.S.; Lakwannum, G.Y.; Kehinde, O.S. Effects of organic and inorganic fertilizers on the growth of NH-Ae 47-4 variety of okra. J. Appl. Sci. Environ. Manag. 2016, 20, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Wasaya, A.; Shabir, M.S.; Hussain, M.; Ansar, M.; Aziz, A.; Hassan, W.; Ahmad, I. Foliar application of zinc and boron improved the productivity and net returns of maize grown under rainfed conditions of Pothwar Plateau. J. Soil Sci. Plant Nutr. 2017, 17, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Micronutrients in crop production. Adv. Agron. 2002, 77, 185–268. [Google Scholar]
- Prakash, M.; Ganesan, J. Effect of plant growth regulators and micronutrients on certain growth analysis parameters in sesame. Sesame Safflower Newslett. 2000, 15, 48–58. [Google Scholar]
- Manal, F.M.; Thalooth, A.T.; Khalifa, R.K.M. Effect of foliar spraying with uniconazole and micronutrients on yield and nutrients uptake of wheat plants grown under saline condition. J. Am. Sci. 2010, 6, 398–404. [Google Scholar]
- Seervi, D.; Choyal, P.; Seervi, K.S. The effect of micronutrients applied as foliar spray, on yield & yield attributes and oil content of sesame crop (Sesamum indicum L.). J. Pharmacogn. Phytochem. 2018, 7, 1402–1404. [Google Scholar]
- Shirazy, B.J.; Islam, M.M.; Haque, M.A.; Mahbub, M.M.; Somee, T.A. Influence of combined effect of nitrogen and micronutrients on yield and yield contributing characters of sesame (Sesamum indicum L.). Bot. Res. Int. 2015, 8, 73–76. [Google Scholar]
- Singaravel, R.; Imayavaramban, V.; Thanunathan, K.; Shanmughapriya, V. Effect of micronutrients on yield and nutrient uptake of sesame (Sesamum indicum L.) in a vertisol soil. Sesame Safflower Newslett. 2002, 17, 46–48. [Google Scholar]
- Shaheen, A.; Shafiq, M.; Naeem, M.A.; Jilani, G. Soil characteristics and plant nutrient status in the eroded lands of Fatehjang in the Pothwar plateau of Pakistan. Soil Environ. 2008, 27, 208–214. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Bingham, F.T. Boron, methods of soil analysis, part-2: Chemical and microbiological properties. Am. Soc. Agron. 1982, 431–448. Available online: https://acsess.onlinelibrary.wiley.com/doi/pdf/10.2134/agronmonogr9.2.2ed.frontmatter (accessed on 2 November 2021).
- Berger, K.C.; Truog, E. Boron tests and determination for soils and plants. Soil Sci. 1944, 57, 25–36. [Google Scholar] [CrossRef]
- Kirch, W. Pearson′s correlation coefficient. Encyclop. Pub. Health 2008, 1090–1091. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Baraki, F.; Berhe, M. Evaluating performance of sesame (Sesamum indicum L.) genotypes in different growing seasons in Northern Ethiopia. Int. J. Agron. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Akter, M.; Khaliq, Q.A.; Islam, M.R.; Ahmed, J.U. Photosynthesis, dry matter partitioning and yield variation in sesame genotypes. Bangladesh Agron. J. 2016, 19, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.; Bibi, T.; Mustafa, H.S.B.; Mahmood, T.; Ali, M. TH-6: A high yielding cultivar of sesame released for general cultivation in Punjab. Adv. Life Sci. 2013, 1, 44–51. [Google Scholar]
- Baraki, F.; Tsehaye, Y.; Abay, F. Analysis of genotype × environment interaction and seed yield stability of sesame in Northern Ethiopia. J. Plant Breed. Crop Sci. 2016, 8, 240–249. [Google Scholar]
- Sarkar, P.K.; Khatu, A.; Singha, A. Effect of duration of water-logging on crop stand and yield of sesame. Int. J. Inno. Appl. Stud. 2016, 14, 1–6. [Google Scholar]
- Ahmed, E.M.; Mahmod, F.A. Effect of irrigation on consumptive use, waterless efficiency and crop coefficient of sesame. J. Agric. Ext. Rural Dev. 2010, 2, 59–63. [Google Scholar]
- Ryu, J.; Baek, I.; Kwak, K.; Han, W.; Bae, J.; Park, J.; Chun, H.C. Excessive soil water stress responses of sesame (Sesamum indicum L.) and perilla (Perilla frutescens L.) cultivated from paddy fields with different topographic features. Korean J. Agric. Sci. 2018, 45, 749–760. [Google Scholar]
- Chun, H.C.; Jung, K.Y.; Choi, Y.D.; Lee, S.H.; Kang, H.W. The growth and yield changes of foxtail millet (Setaria italic L.), proso millet (Panicum miliaceum L.), sorghum (Sorghum bicolor L.), adzuki bean (Vigna angularis L.), and sesame (Sesamum indicum L.) as affected by excessive soil-water. Korean J. Agric. Sci. 2016, 43, 547–559. [Google Scholar]
- Roul, B.; Mishra, B.K.; Prusty, N. Physiological effect of micronutrient on uptake of major nutrient by plant and oil content of seed of sesame crop for coastal Odisha situation. J. Pharmacog. Phytochem. 2017, 6, 1990–1993. [Google Scholar]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification. Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Mathew, J.; George, S. Sulphur and boron improves yields of oilseed sesame in sandy loam soil of Onattukara. Kerala Better Crops South Asia 2011, 14–15. Available online: http://www.ipni.net/publication/bca.nsf/0/135AF8BE54222DE1852579A4007A2938/$FILE/Page%2014%20BCSA%202011.pdf (accessed on 2 November 2021).
- Shireen, F.; Nawaz, M.A.; Chen, C.; Zhang, Q.; Zheng, Z.; Sohail, H.; Sun, J.; Cao, H.; Huang, Y.; Bie, Z. Boron: Functions and approaches to enhance its availability in plants for sustainable agriculture. Int. J. Mol. Sci. 2018, 19, 1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B., Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Elayaraja, D. Effect of micronutrients and organic manures on sesame. J. Ecobiotech. 2018, 10, 12–15. [Google Scholar] [CrossRef] [Green Version]
- Sahu, G.; Chatterjee, N.; Bera, M.; Ghosh, G.K.; Mondal, S.; Biswas, P.K.; Kundu, M.C. Lateritic soils of West Bengal. Int. J. Plant Anim. Environ. Sci. 2017, 7, 137–146. [Google Scholar]
- Hajiboland, R.; Bahrami-Rad, S.; Bastani, S. Phenolics metabolism in boron-deficient tea (Camellia sinensis (L.) O. Kuntze) plants. Acta Biol. Hung. 2013, 64, 196–206. [Google Scholar] [CrossRef] [PubMed]
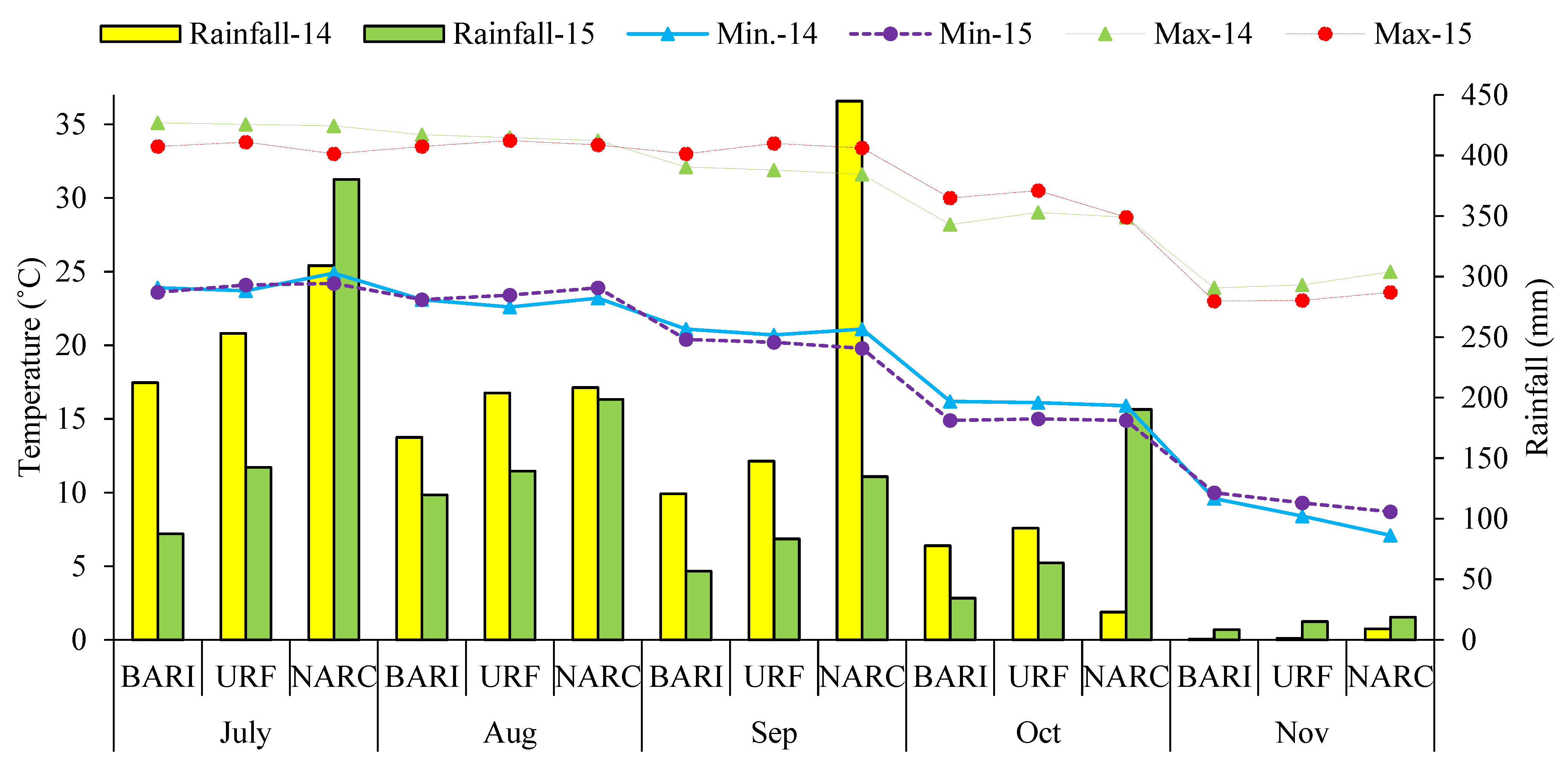


| BARI (Chakwal) | URF (Rawalpindi) | NARC (Islamabad) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | |||||||
| Depth (cm) | 0–15 | 15–30 | 0–15 | 15–30 | 0–15 | 15–30 | 0–15 | 15–30 | 0–15 | 15–30 | 0–15 | 15–30 |
| Soil texture | SL | SL | SL | SL | SCL | SCL | SCL | SCL | SCL | SCL | SCL | SCL |
| pH | 7.78 | 7.77 | 7.75 | 7.73 | 7.84 | 7.81 | 7.91 | 7.82 | 7.61 | 7.59 | 7.69 | 7.65 |
| OM (%) | 0.83 | 0.80 | 0.77 | 0.75 | 0.75 | 0.72 | 0.81 | 0.79 | 1.07 | 1.02 | 1.1 | 0.98 |
| EC (dS m−1) | 0.39 | 0.38 | 0.51 | 0.49 | 0.61 | 0.58 | 0.59 | 0.56 | 0.81 | 0.79 | 0.8 | 0.79 |
| BD (g cm−3) | 1.47 | 1.49 | 1.46 | 1.47 | 1.53 | 1.55 | 1.49 | 1.5 | 1.39 | 1.4 | 1.38 | 1.39 |
| Porosity (%) | 44.5 | 43.8 | 44.9 | 44.5 | 42.3 | 41.5 | 43.8 | 43.4 | 47.5 | 47.2 | 47.9 | 47.5 |
| NO3 N (mg kg−1) | 5.61 | 5.37 | 5.5 | 5.43 | 3.74 | 3.68 | 4.01 | 3.95 | 7.3 | 7.29 | 7.36 | 7.32 |
| P (mg kg−1) | 4.52 | 4.33 | 4.7 | 4.65 | 3.79 | 3.74 | 3.88 | 3.82 | 4.13 | 4.09 | 4.19 | 3.98 |
| K (mg kg−1) | 139 | 130 | 143 | 137 | 126 | 120 | 136 | 128 | 118 | 117 | 130 | 124 |
| Zn (mg kg−1) | 0.41 | 0.38 | 0.43 | 0.39 | 0.46 | 0.45 | 0.42 | 0.41 | 0.26 | 0.25 | 0.32 | 0.29 |
| Mn (mg kg−1) | 1.15 | 1.09 | 1.03 | 0.98 | 1.2 | 1.16 | 1.19 | 1.12 | 1.16 | 1.11 | 1.02 | 0.97 |
| B (mg kg−1) | 0.39 | 0.36 | 0.4 | 0.37 | 0.43 | 0.42 | 0.44 | 0.41 | 0.36 | 0.33 | 0.35 | 0.31 |
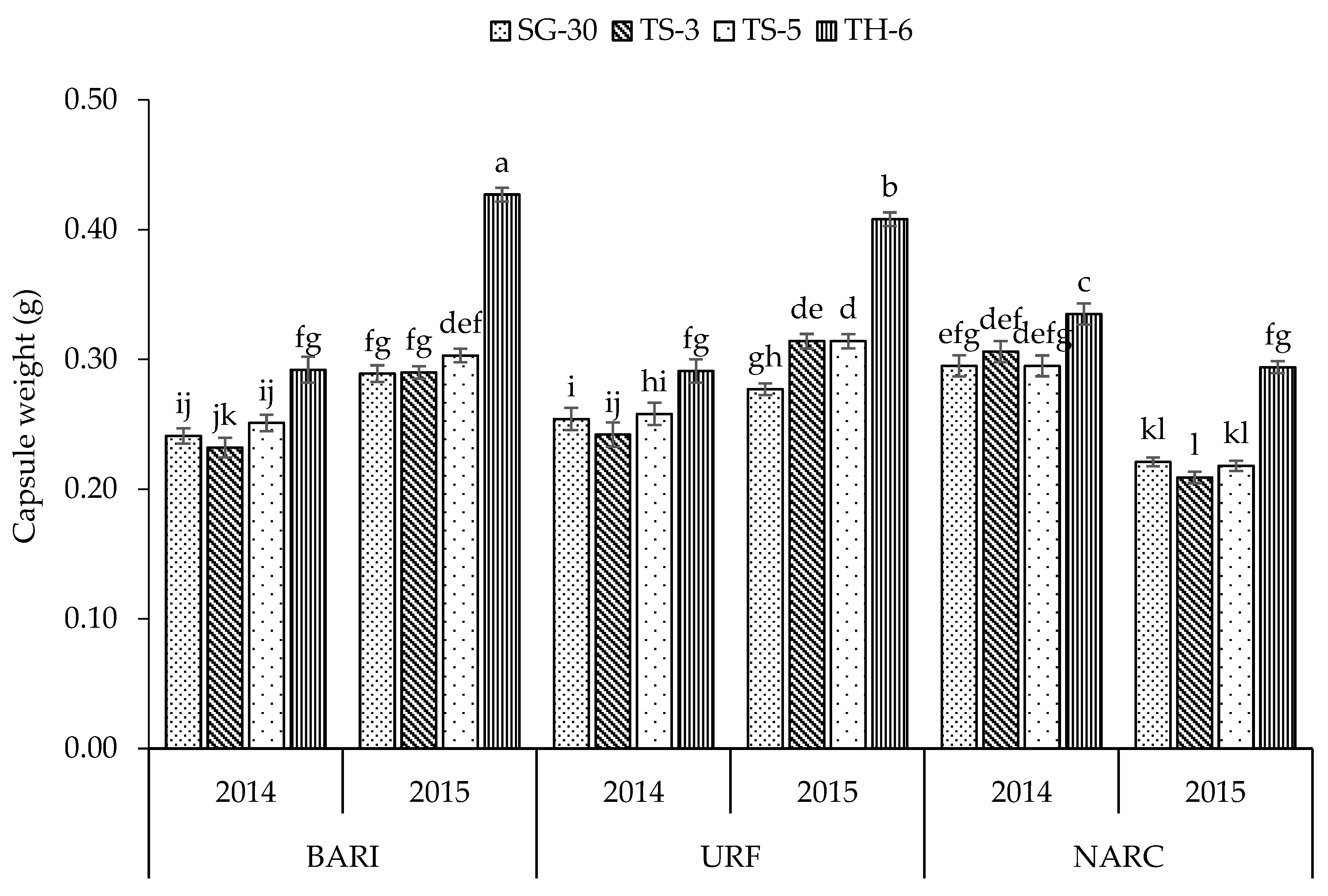
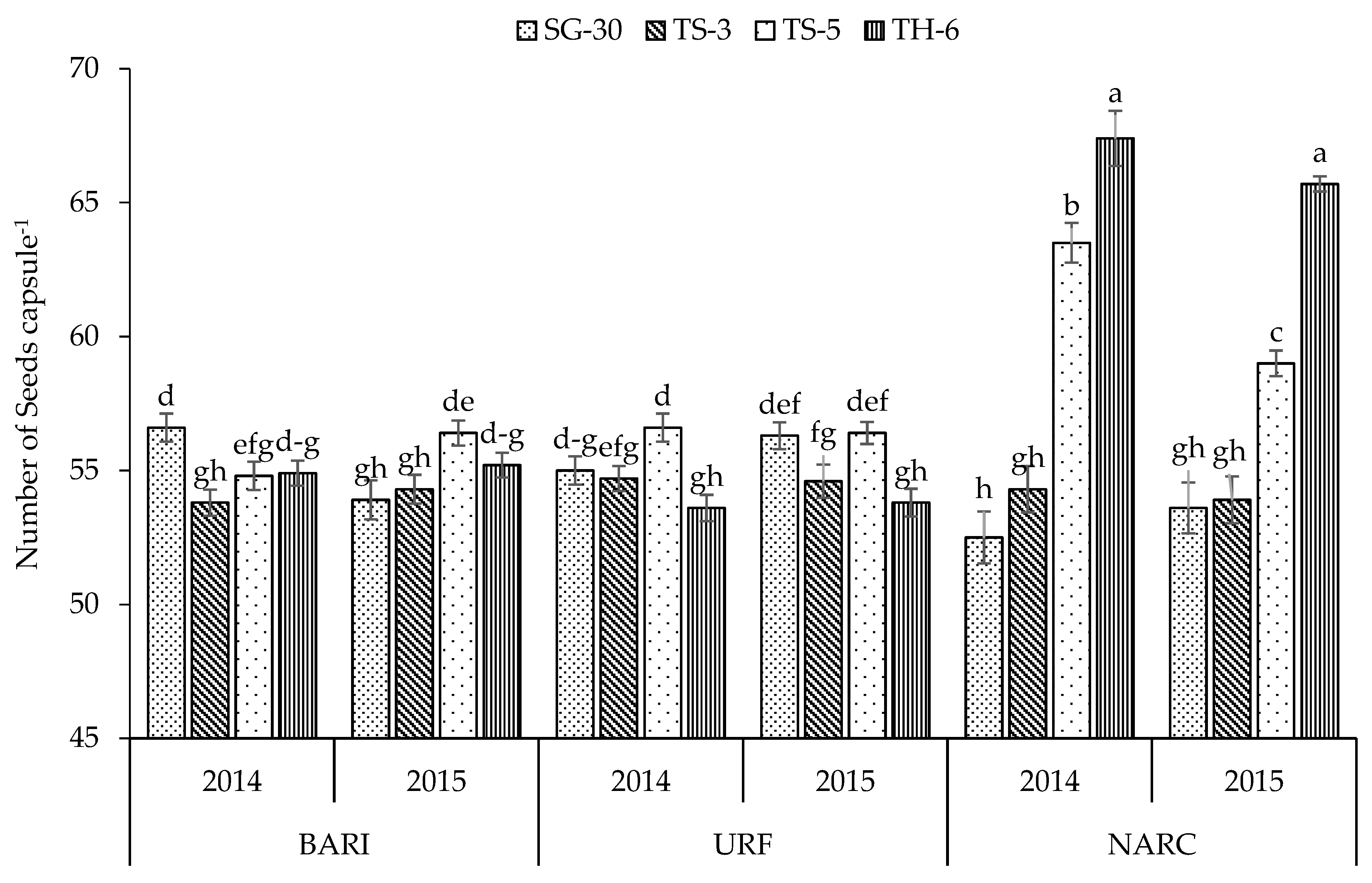
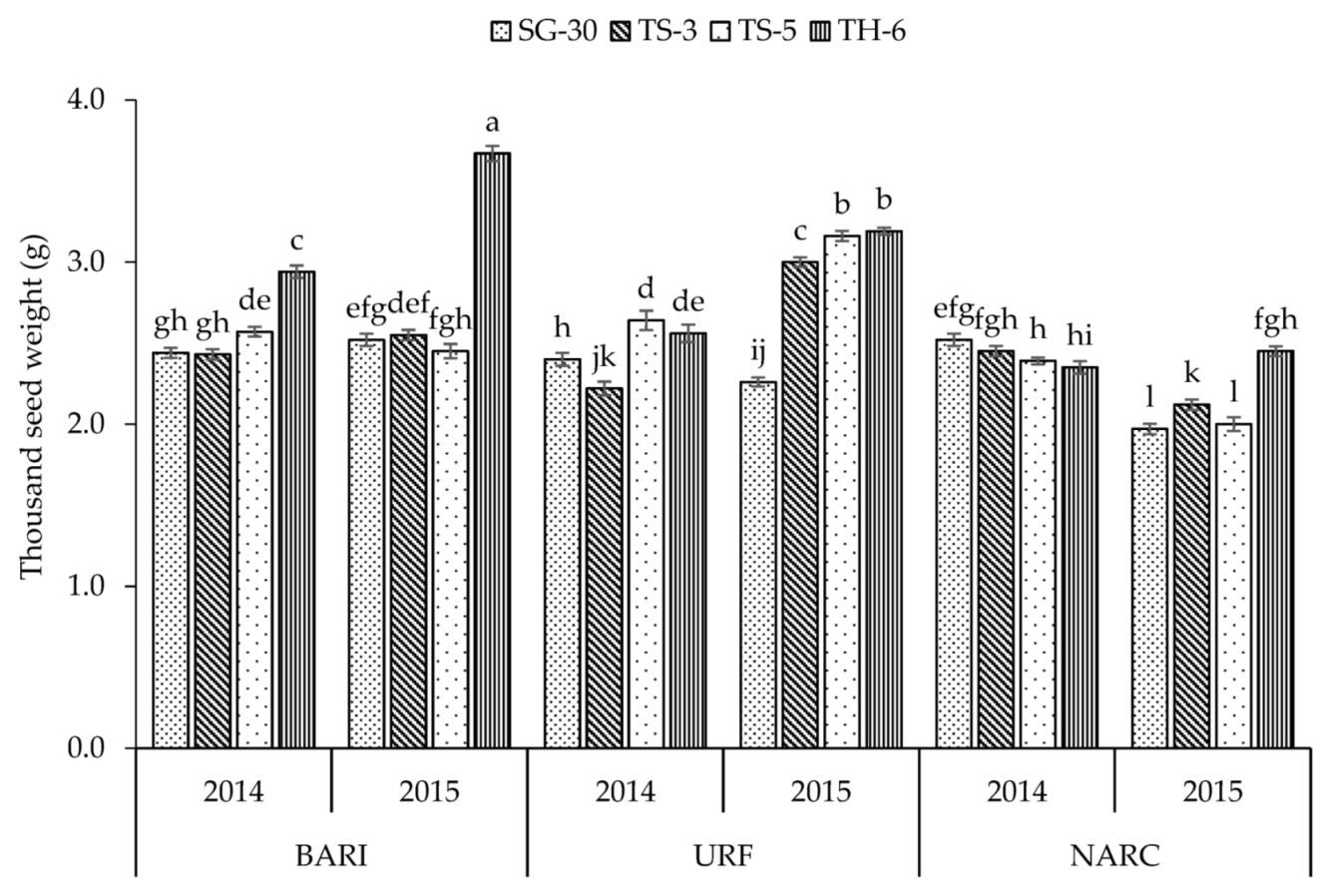
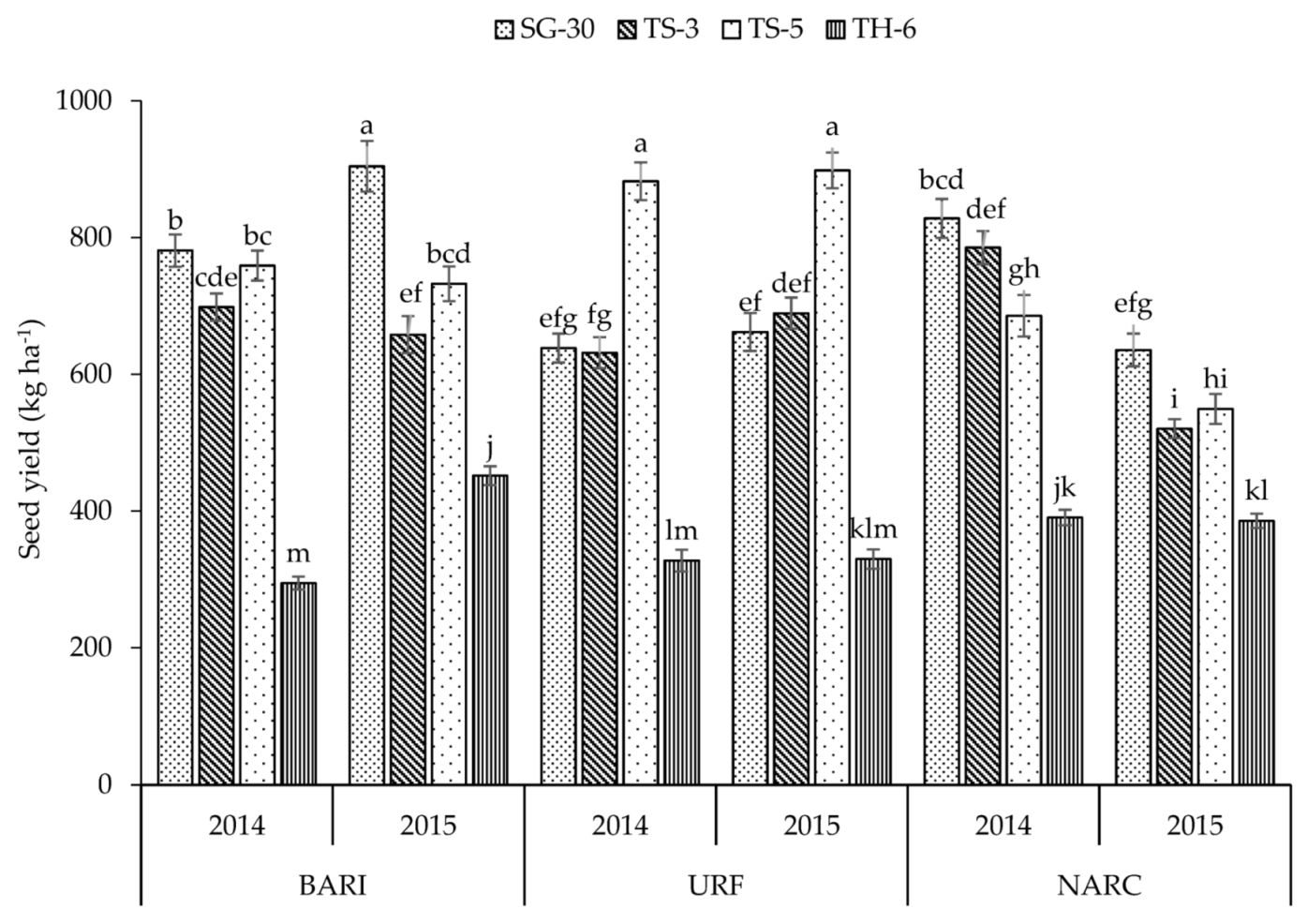
| Plant Height (cm) | No. of Primary Branches | No. of Secondary Branches | Capsules Plant−1 | Capsule Length (cm) | Capsule Weight (g) | Seeds Capsule−1 | 1000 Seed Weight (g) | Biomass Yield (kg ha−1) | Seed Yield (kg ha−1) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar (C) | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| SG-30 | 118.4 a | 5.50 c | 3.74 c | 55.6 a | 2.56 c | 0.263 c | 54.7 b | 2.35 d | 3281 a | 725.0 a |
| TS-3 | 114.4 b | 6.72 a | 5.15 a | 47.2 c | 2.58 c | 0.265 c | 54.2 b | 2.46 c | 3141 b | 647.2 b |
| TS-5 | 114.1 b | 5.87 b | 4.54 b | 54.2 b | 2.64 b | 0.273 b | 57.8 a | 2.54 b | 2894 c | 734.6 a |
| TH-6 | 92.3 c | 0.47 d | 0.09 d | 26.5 d | 3.41 a | 0.341 a | 58.4 a | 2.86 a | 1144 d | 363.4 c |
| SE± | 1.11 | 0.05 | 0.051 | 0.65 | 0.025 | 0.004 | 0.378 | 0.022 | 54.65 | 13.01 |
| Locations (L) | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| BARI | 115.1 b | 4.07 b | 2.55 c | 47.0 b | 2.82 a | 0.291 a | 55.0 b | 2.70 a | 2614 b | 660.0 a |
| URF | 123.0 a | 5.74 a | 4.58 a | 47.4 b | 2.74 b | 0.295 a | 55.1 b | 2.68 a | 3161 a | 632.5 b |
| NARC | 91.3 c | 4.10 b | 3.01 b | 43.1 a | 2.83 a | 0.272 b | 58.7 a | 2.28 b | 2071 c | 560.1 c |
| SE± | 0.96 | 0.043 | 0.044 | 0.57 | 0.021 | 0.0034 | 0.327 | 0.019 | 47.33 | 11.27 |
| Year (Y) | ** | ** | ** | ** | NS | ** | NS | ** | * | NS |
| 2014 | 111.4 a | 4.76 a | 3.77 a | 43.6 | 2.81 | 0.274 b | 56.5 | 2.49 b | 2657 a | 617.0 |
| 2015 | 108.2 b | 4.55 b | 2.99 b | 48.1 | 2.78 | 0.297 a | 56.1 | 2.61 a | 2573 b | 618.1 |
| SE± | 0.78 | 0.035 | 0.036 | 0.48 | 0.018 | 0.0028 | 0.267 | 0.015 | 38.65 | 9.20 |
| Micronutrients (MN) | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| Control | 105.7 c | 4.33 d | 3.08 c | 41.9 d | 2.71 c | 0.271 c | 55.2 c | 2.48 c | 2408 d | 563.1 c |
| Zinc | 111.9 a | 4.89 a | 3.61 a | 47.0 b | 2.84 a | 0.293 a | 56.8 ab | 2.58 a | 2858 a | 642.0 a |
| Boron | 112.5 a | 4.78 b | 3.56 a | 49.2 a | 2.86 a | 0.294 a | 57.0 a | 2.60 a | 2657 b | 657.7 a |
| Manganese | 109.1 b | 4.56 c | 3.27 b | 45.4 c | 2.79 b | 0.284 b | 56.1 b | 2.54 b | 2537 c | 607.4 b |
| SE± | 1.11 | 0.05 | 0.051 | 0.65 | 0.025 | 0.004 | 0.378 | 0.022 | 54.65 | 13.01 |
| Y × L | ** | ** | ** | ** | ** | ** | * | ** | ** | ** |
| Y × C | ** | ** | ** | ** | NS | ** | NS | ** | * | ** |
| Y × MN | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| L × C | ** | ** | ** | ** | ** | NS | ** | ** | ** | ** |
| L × MN | NS | NS | ** | NS | NS | NS | NS | NS | NS | NS |
| C × MN | NS | ** | ** | NS | * | NS | NS | NS | NS | NS |
| Y × L × C | ** | ** | ** | ** | NS | ** | ** | ** | ** | ** |
| Y × L × MN | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Y × C × MN | NS | NS | NS | NS | * | NS | NS | NS | NS | NS |
| L × C × MN | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Y × L × C × MN | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Traits | PH | NPB | NSB | NC | CL | CW | SPC | 1000-SW | BioY |
|---|---|---|---|---|---|---|---|---|---|
| NPB | 0.603 ** | ||||||||
| NSB | 0.561 ** | 0.927 ** | |||||||
| NC | 0.326 ** | 0.652 ** | 0.578 ** | ||||||
| CL | −0.469 ** | −0.736 ** | −0.624 ** | −0.540 ** | |||||
| CW | −0.195 ** | −0.379 ** | −0.311 ** | −0.077 NS | 0.556 ** | ||||
| SPC | −0.300 ** | −0.233 ** | −0.148 ** | −0.153 ** | 0.327 ** | 0.111 * | |||
| 1000 SW | 0.079 NS | −0.221 ** | −0.161 ** | −0.066 NS | 0.422 ** | 0.708 ** | −0.128 * | ||
| BioY | 0.755 ** | 0.869 ** | 0.796 ** | 0.620 ** | −0.667 ** | −0.288 ** | −0.218 ** | −0.142 ** | |
| SY | 0.597 ** | 0.717 ** | 0.610 ** | 0.770 ** | −0.624 ** | −0.229 ** | −0.172 ** | −0.097 NS | 0.848 ** |
| Variable | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 | PC9 | PC10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Plant high (PH) | 0.307 | 0.237 | −0.251 | 0.518 | −0.44 | 0.232 | 0.218 | −0.328 | 0.238 | 0.242 |
| Primary branches (NPB) | 0.389 | 0.025 | 0.118 | 0.118 | 0.403 | −0.043 | −0.166 | 0.462 | 0.453 | 0.458 |
| Secondary branches (NSB) | 0.359 | 0.042 | 0.164 | 0.253 | 0.597 | −0.125 | 0.264 | −0.404 | −0.034 | −0.415 |
| Number of capsules (NC) | 0.306 | 0.158 | 0.398 | −0.583 | −0.099 | 0.103 | 0.414 | −0.186 | −0.153 | 0.365 |
| Capsule length (CL) | −0.385 | 0.16 | 0.128 | 0.183 | 0.024 | 0.054 | 0.725 | 0.454 | 0.157 | −0.129 |
| Capsule weight (CW) | −0.222 | 0.57 | 0.271 | −0.073 | 0.134 | 0.574 | −0.335 | −0.101 | 0.208 | −0.184 |
| Seeds capsule−1 (SPC) | −0.157 | −0.253 | 0.785 | 0.419 | −0.224 | −0.122 | −0.155 | −0.105 | −0.055 | 0.128 |
| 1000 Seed weight (SW1000) | −0.129 | 0.688 | −0.023 | 0.108 | 0.02 | −0.62 | −0.103 | −0.009 | −0.252 | 0.195 |
| Biomass yield (BioY) | 0.394 | 0.11 | 0.044 | 0.223 | −0.081 | 0.306 | −0.069 | 0.461 | −0.666 | −0.142 |
| Seed yield (SY) | 0.372 | 0.128 | 0.17 | −0.193 | −0.449 | −0.303 | −0.079 | 0.203 | 0.369 | −0.549 |
| Eigenvalue | 5.84 | 1.78 | 0.96 | 0.69 | 0.43 | 0.12 | 0.07 | 0.04 | 0.02 | 0.01 |
| Proportion | 58.4 | 17.8 | 9.6 | 6.9 | 4.4 | 1.2 | 0.8 | 0.5 | 0.2 | 0.1 |
| Cumulative | 58.4 | 76.3 | 85.9 | 92.8 | 97.2 | 98.4 | 99.2 | 99.7 | 99.9 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.M.; Manaf, A.; Hassan, F.u.; Ahmad, M.S.; Qayyum, A.; Shah, Z.H.; Alsamadany, H.; Yang, S.; Chung, G. Allometric Expression of Sesame Cultivars in Response to Various Environments and Nutrition. Agriculture 2021, 11, 1095. https://doi.org/10.3390/agriculture11111095
Khan MM, Manaf A, Hassan Fu, Ahmad MS, Qayyum A, Shah ZH, Alsamadany H, Yang S, Chung G. Allometric Expression of Sesame Cultivars in Response to Various Environments and Nutrition. Agriculture. 2021; 11(11):1095. https://doi.org/10.3390/agriculture11111095
Chicago/Turabian StyleKhan, Meer Muhammad, Abdul Manaf, Fayyaz ul Hassan, Muhammad Sheeraz Ahmad, Abdul Qayyum, Zahid Hussain Shah, Hameed Alsamadany, Seunghwan Yang, and Gyuhwa Chung. 2021. "Allometric Expression of Sesame Cultivars in Response to Various Environments and Nutrition" Agriculture 11, no. 11: 1095. https://doi.org/10.3390/agriculture11111095
APA StyleKhan, M. M., Manaf, A., Hassan, F. u., Ahmad, M. S., Qayyum, A., Shah, Z. H., Alsamadany, H., Yang, S., & Chung, G. (2021). Allometric Expression of Sesame Cultivars in Response to Various Environments and Nutrition. Agriculture, 11(11), 1095. https://doi.org/10.3390/agriculture11111095








