Floristic Composition: Dynamic Biodiversity Indicator of Tree Canopy Effect on Dryland and Improved Mediterranean Pastures
Abstract
:1. Introduction
2. Materials and Methods
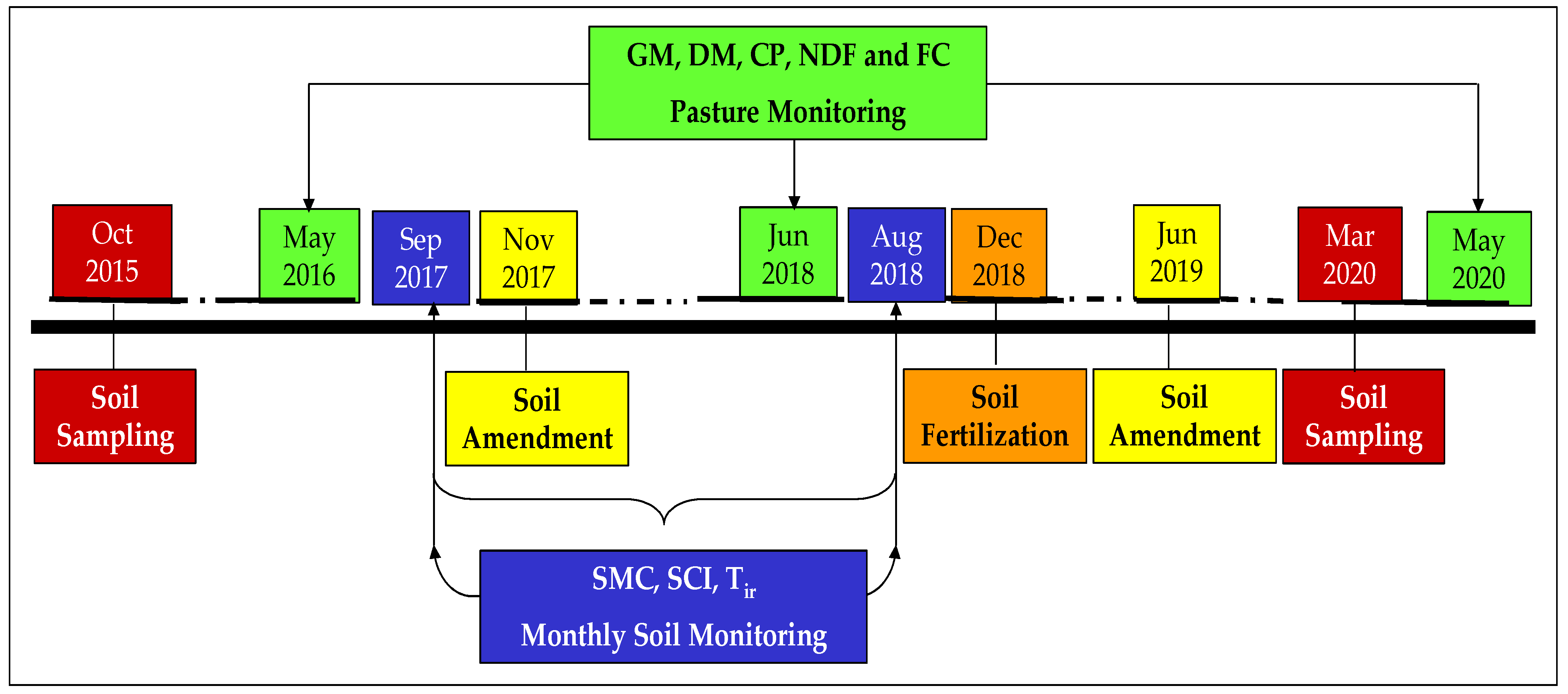
2.1. Chronological Approach
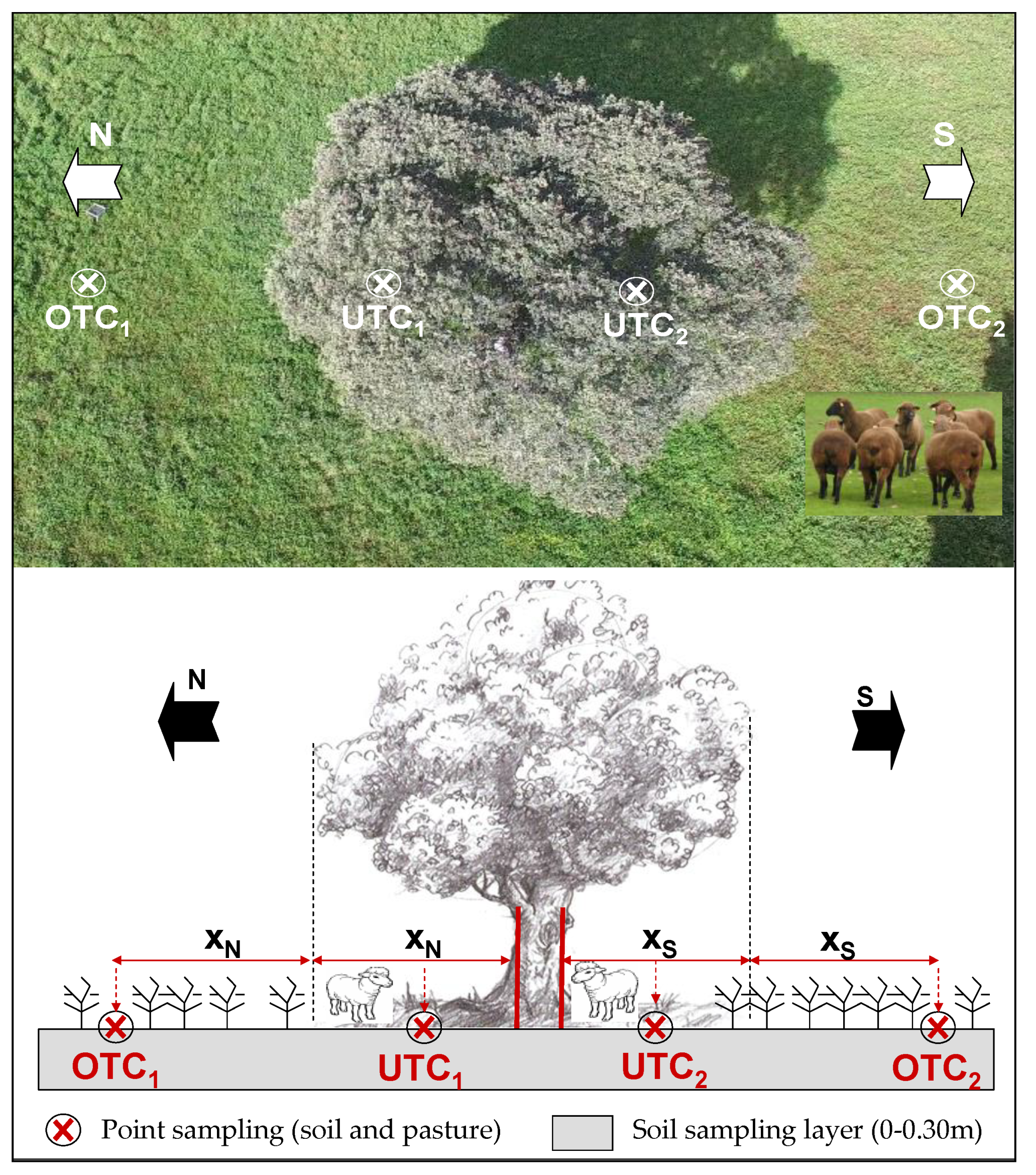
2.2. Experimental Field Characteristics and Sampling Scheme
2.3. Soil Sample Collection and Analysis
2.4. Pasture Samples Collection and Analysis
2.5. Statistical Analysis of the Data
3. Results
3.1. Soil Characteristics UTC and OTC
3.2. Pasture Productivity and Quality UTC and OTC
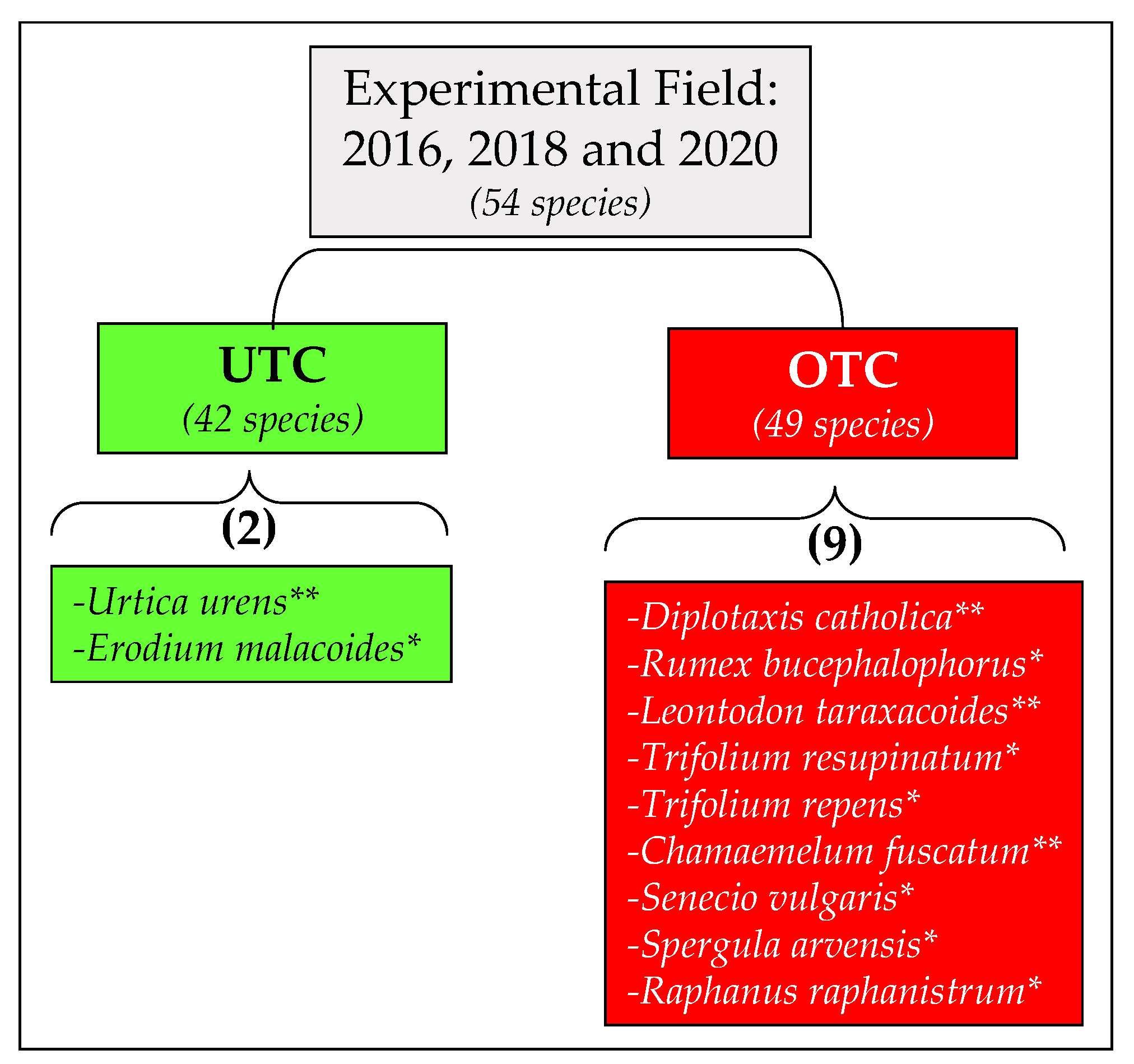
3.3. Pasture Floristic Composition UTC and OTC
4. Discussion
4.1. Soil Variability and Pasture Productivity and Quality UTC and OTC
4.2. Pasture Floristic Composition UTC and OTC: Biodiversity and Indicator Species Analysis
4.3. Perspectives of Application of Grassland Biodiversity Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serrano, J.; Shahidian, S.; Da Silva, J.M.; Paixão, L.; Carreira, E.; Pereira, A.; Carvalho, M. Climate Changes Challenges to the Management of Mediterranean Montado Ecosystem: Perspectives for Use of Precision Agriculture Technologies. Agronomy 2020, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- Punalekar, S.M.; Thomson, A.; Verhoef, A.; Humphries, D.J.; Reynolds, C.K. Assessing Suitability of Sentinel-2 Bands for Monitoring of Nutrient Concentration of Pastures with a Range of Species Compositions. Agronomy 2021, 11, 1661. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Da Silva, J.M.; Sales-Baptista, E.; De Oliveira, I.F.; De Castro, J.L.; Pereira, A.; De Abreu, M.C.; Machado, E.; de Carvalho, M. Tree influence on soil and pasture: Contribution of proximal sensing to pasture productivity and quality estimation in montado ecosystems. Int. J. Remote Sens. 2017, 39, 4801–4829. [Google Scholar] [CrossRef]
- Benavides, R.; Douglas, G.B.; Osoro, K. Silvopastoralism in New Zealand: Review of effects of evergreen and deciduous trees on pasture dynamics. Agrofor. Syst. 2008, 76, 327–350. [Google Scholar] [CrossRef]
- Marcos, G.M.; Obrador, J.J.; Garcia, E.; Cubera, E.; Montero-Parejo, M.J.; Pulido, F.; Dupraz, C. Driving competitive and facilitative interactions in oak dehesas through management practices. Agrofor. Syst. 2007, 70, 25–40. [Google Scholar] [CrossRef]
- Paço, A.; Da-Silva, J.R.; Torres, D.P.; Glick, B.R.; Brígido, C. Exogenous ACC Deaminase Is Key to Improving the Performance of Pasture Legume-Rhizobial Symbioses in the Presence of a High Manganese Concentration. Plants 2020, 9, 1630. [Google Scholar] [CrossRef] [PubMed]
- Socher, S.A.; Prati, D.; Boch, S.; Müller, J.; Klaus, V.H.; Hölzel, N.; Fischer, M. Direct and productivity-mediated indirect effects of fertilization, mowing and grazing on grassland species richness. J. Ecol. 2012, 100, 1391–1399. [Google Scholar] [CrossRef]
- García-Baquero, G.; Odriozola, I.; Aldezabal, A. Floristic Composition Mediates Change in Forage Nutritive Quality of Atlantic Mountain Grasslands after Experimental Grazing Exclusion. Agronomy 2020, 11, 25. [Google Scholar] [CrossRef]
- Scocco, P.; Piermarteri, K.; Malfatti, A.; Tardella, F.M.; Catorci, A. Short communication: Effects of summer rainfall variations on sheep body state and farming sustainability in sub-Mediterranean pastoral systems. Span. J. Agric. Res. 2016, 14, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Efe Serrano, J. Pastures in Alentejo: Technical Basis for Characterization, Grazing and Improvement; Universidade de Évo-ra—ICAM, Ed.; Gráfica Eborense: Évora, Portugal, 2006; pp. 165–178. (In Portuguese) [Google Scholar]
- Ghajar, S.; Tracy, B. Proximal Sensing in Grasslands and Pastures. Agriculture 2021, 11, 740. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.M.; Cambrolléb, J.; Hidalgo-Galveza, M.D.; Matías, L.; Montero-Ramírez, A.; Santolaya, S.; Godoy, Ó. Phe-nological responses to climate change in communities of plants species with contrasting functional strategies. Environ. Exp. Bot. 2020, 170, 103852. [Google Scholar] [CrossRef]
- Salmerón-Sánchez, E.; Mendoza-Fernández, A.J.; Lorite, J.; Mota, J.F.; Peñas, J. Plant conservation in Mediterranean-type ecosystems. Mediterr. Bot. 2021, 42, e71333. [Google Scholar] [CrossRef]
- Socher, S.A.; Prati, D.; Boch, S.; Müller, J.; Baumbach, H.; Gockel, S.; Hemp, A.; Schöning, I.; Wells, K.; Buscot, F.; et al. Interacting effects of fertilization, mowing and grazing on plant species diversity of 1500 grasslands in Germany differ between regions. Basic Appl. Ecol. 2013, 14, 126–136. [Google Scholar] [CrossRef]
- Schellberg, J.; Hill, M.J.; Gerhards, R.; Rothmund, M.; Braun, M. Precision agriculture on grassland: Applications, perspectives and constraints. Eur. J. Agron. 2008, 29, 59–71. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Da Silva, J.M.; Paixão, L.; Calado, J.; De Carvalho, M. Integration of Soil Electrical Conductivity and Indices Obtained through Satellite Imagery for Differential Management of Pasture Fertilization. AgriEngineering 2019, 1, 567–585. [Google Scholar] [CrossRef] [Green Version]
- Serrano, J.; Shahidian, S.; Marques da Silva, J. Calibration of GrassMaster II to estimate green and dry matter yield in Medi-terranean pastures: Effect of pasture moisture content. Crop Pasture Sci. 2016, 67, 780–791. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Marques da Silva, J.; Paixão, L.; Carreira, E.; Carmona-Cabezas, R.; Nogales-Bueno, J.; Rato, A.E. Evaluation of near infrared spectroscopy (NIRS) and remote sensing (RS) for estimating pasture quality in Mediterranean Montado ecosystem. Appl. Sci. 2020, 10, 4463. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Costa, F.; Carreira, E.; Pereira, A.; Carvalho, M. Can soil pH correction reduce the animal supple-mentation needs in the critical autumn period in Mediterranean Montado ecosystem? Agronomy 2021, 11, 514. [Google Scholar] [CrossRef]
- Wachendorf, M.; Fricke, T.; Möckel, T. Remote sensing as a tool to assess botanical composition, structure, quantity and quality of temperate grasslands. Grass Forage Sci. 2017, 73, 1–14. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P.; Moretti, M. Improving indicator species analysis by combining groups of sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014; International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. Update 2015; World Soil Resources Report 106; Food and Agriculture Organization (FAO): Rome, Italy, 2015; 188p. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Method of Analysis of AOAC International, 18th ed.; AOAC International: Arlington, VA, USA, 2005. [Google Scholar]
- Egner, H.; Riehm, H.; Domingo, W.R. Utersuchungeniiber die chemische Bodenanalyse als Grudlagefir die Beurteilung des Nahrstof-zunstandes der Boden. II. K. Lantbrhogsk. Annlr 1960, 20, 199–216. (In German) [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie, 3rd ed.; Grundzüge der Vegetationskunde; Springer: Vienna, Austria; New York, NY, USA, 1964. [Google Scholar]
- Serrano, J.; Shahidian, S.; Da Silva, J.M.; Moral, F.; Carvajal-Ramirez, F.; Carreira, E.; Pereira, A.; De Carvalho, M. Evaluation of the Effect of Dolomitic Lime Application on Pastures—Case Study in the Montado Mediterranean Ecosystem. Sustainability 2020, 12, 3758. [Google Scholar] [CrossRef]
- Shore, A. DESeq and Indicator Species Analysis R Script; Figshare, Software: Cambridge, MA, USA, 2020. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Wagner, H. Vegan: Community Ecology Package; R Package Version 2.5-3; The Comprehensive R Archive Network: Wien, Austria, 2018. [Google Scholar]
- Bakker, J.D. Increasing the utility of Indicator Species Analysis. J. Appl. Ecol. 2008, 45, 1829–1835. [Google Scholar] [CrossRef]
- Ara, I.; Harrison, M.T.; Whitehead, J.; Waldner, F.; Bridle, K.; Gilfedder, L.; Da Silva, J.M.; Marques, F.; Rawnsley, R. Modelling seasonal pasture growth and botanical composition at the paddock scale with satellite imagery. Silico Plants 2021, 3, 1–15. [Google Scholar] [CrossRef]
- Serrano, J.M.; Shahidian, S.; Marques da Silva, J.; Carvalho, M. Monitoring of soil organic carbon over 10 years in a Mediter-ranean silvo-pastoral system: Potential evaluation for differential. Precis. Agric. 2016, 17, 274–295. [Google Scholar] [CrossRef]
- Demarchi, L.O.; Scudeller, V.V.; Moura, L.C.; Dias-Terceiro, R.G.; Lopes, A.; Wittmann, F.K.; Piedade, M.T.F. Floristic composition, structure and soil-vegetation relations in three white-sand soil patches in central Amazonia. Acta Amaz. 2018, 48, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Škornik, S.; Vidrih, M.; Kaligarič, M. The effect of grazing pressure on species richness, composition and productivity in North Adriatic Karst pastures. Plant Biosyst. 2010, 144, 355–364. [Google Scholar] [CrossRef]
- Gómez-Rey, M.X.; Garcês, A.; Madeira, M. Soil organic-C accumulation and N availability under improved pastures estab-lished in Mediterranean Oak Woodlands. Soil Use Manag. 2012, 28, 497–507. [Google Scholar] [CrossRef]
- Seddaiu, G.; Porcua, G.; Luigi, L.; Roggero, P.P.; Agnelli, A.; Cortic, G. Soil organic matter content and composition as influ-enced by soil management in a semi-arid Mediterranean agro-silvo-pastoral system. Agric. Ecosyst. Environ. 2013, 167, 1–11. [Google Scholar] [CrossRef]
- Guevara, S.; Meave, J.; Moreno-Casasola, P.; Laborde, J. Floristic composition and structure of vegetation under isolated trees in neotropical pastures. J. Veg. Sci. 1992, 3, 655–664. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Marques da Silva, J. Evaluation of normalized difference water index as a tool for monitoring pasture seasonal and inter-annual variability in a Mediterranean agro-silvo-pastoral system. Water 2019, 11, 62. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, M.; Goss, M.J.; Teixeira, D.M. Manganese toxicity in Portuguese Cambisols derived from granitic rocks: Causes, limitations of soil analyses and possible solutions. Rev. Cienc. Agrar. 2015, 38, 518–527. [Google Scholar] [CrossRef]
- Li, G.D.; Conyers, M.K.; Helyar, K.R.; Lisle, C.J.; Poile, G.J.; Cullis, B.R. Long-term surface application of lime ameliorates subsurface soil acidity in the mixed farming zone of south-eastern Australia. Geoderma 2018, 338, 236–246. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; da Silva, J.M.; Carvalho, M. A holistic approach to the evaluation of the Montado ecosystem using proximal sensors. Sensors 2018, 18, 570. [Google Scholar] [CrossRef] [Green Version]
- Guevara-Escobar, A.; Kemp, P.D.; Mackay, A.; Hodgson, J. Pasture production and composition under poplar in a hill environment in New Zealand. Agrofor. Syst. 2007, 69, 199–213. [Google Scholar] [CrossRef]
- Rayburn, E.B.; Griggs, T.C. Light Interception and the Growth of Pastures under Ideal and Stressful Growing Conditions on the Allegheny Plateau. Plants 2020, 9, 734. [Google Scholar] [CrossRef]
- Pullanagari, R.R.; Yule, I.J.; Tuohy, M.P.; Hedley, M.J.; Dynes, R.A.; King, W. Proximal sensing of the seasonal variability of pasture nutritive value using multispectral radiometry. Grass Forage Sci. 2012, 68, 110–119. [Google Scholar] [CrossRef]
- Avdiu, B.; Aliu, S.; Fetahu, S.; Zeka, D.; Rusinovci, I. The floristic composition of the natural pastures in Massive of Novo-berba. Agric. For. 2018, 64, 235–241. [Google Scholar]
- Costa, J.P.; Mesquita, M.L.R. Floristic and phytosociology of weeds in pastures in Maranhão State, Northeast Brazil. Rev. Cien. Agron. 2016, 47, 414–442. [Google Scholar] [CrossRef] [Green Version]
- Irume, M.V.; Morais, M.L.C.S.; Zartman, C.E.; Amaral, I.L. Floristic composition and community structure of epiphytic angiosperms in a terra firme forest in central Amazonia. Acta Bot. Bras. 2013, 27, 378–393. [Google Scholar] [CrossRef] [Green Version]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flflexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- David, T.S.; Pinto, C.A.; Nadezhdina, N.; Kurz-Besson, C.; Henriques, M.O.; Quilhó, T.; Cermak, J.; Chaves, M.M.; Pereira, J.S.; David, J.S. Root functioning, tree water use and hydraulic redistribution in Quercus suber trees: A modeling approach based on root sap flow. For. Ecol. Manag. 2013, 307, 136–146. [Google Scholar] [CrossRef] [Green Version]
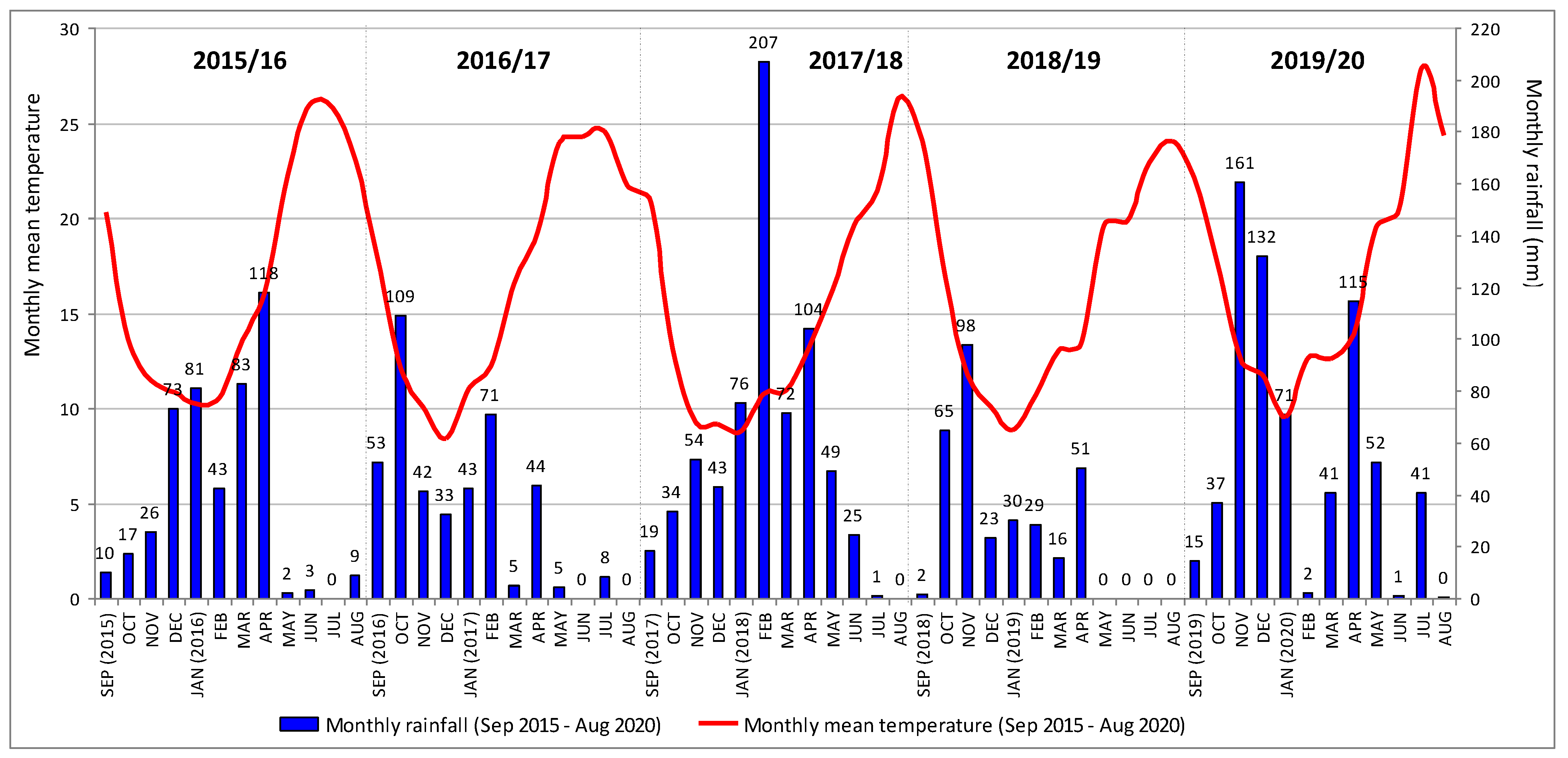
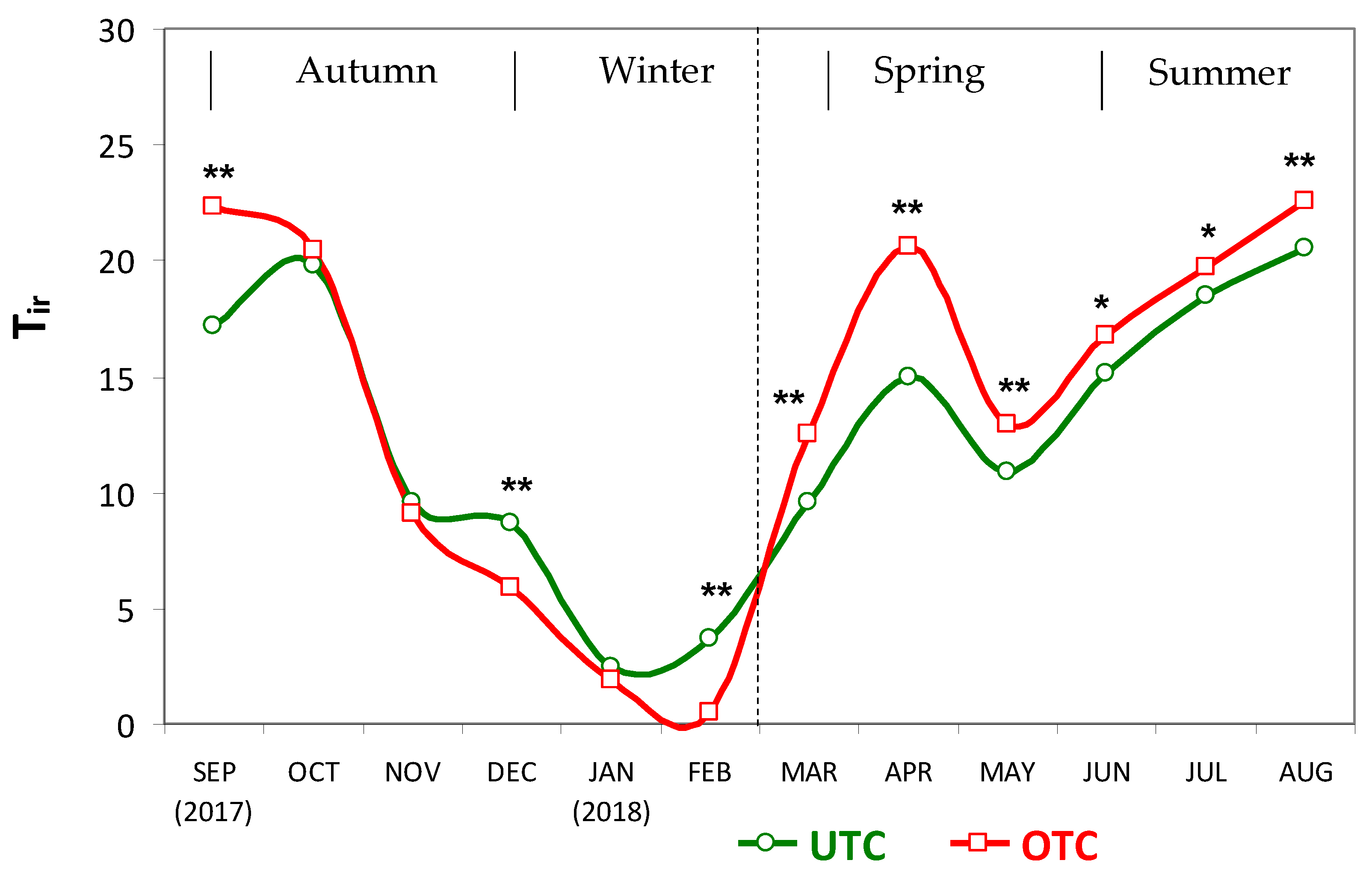

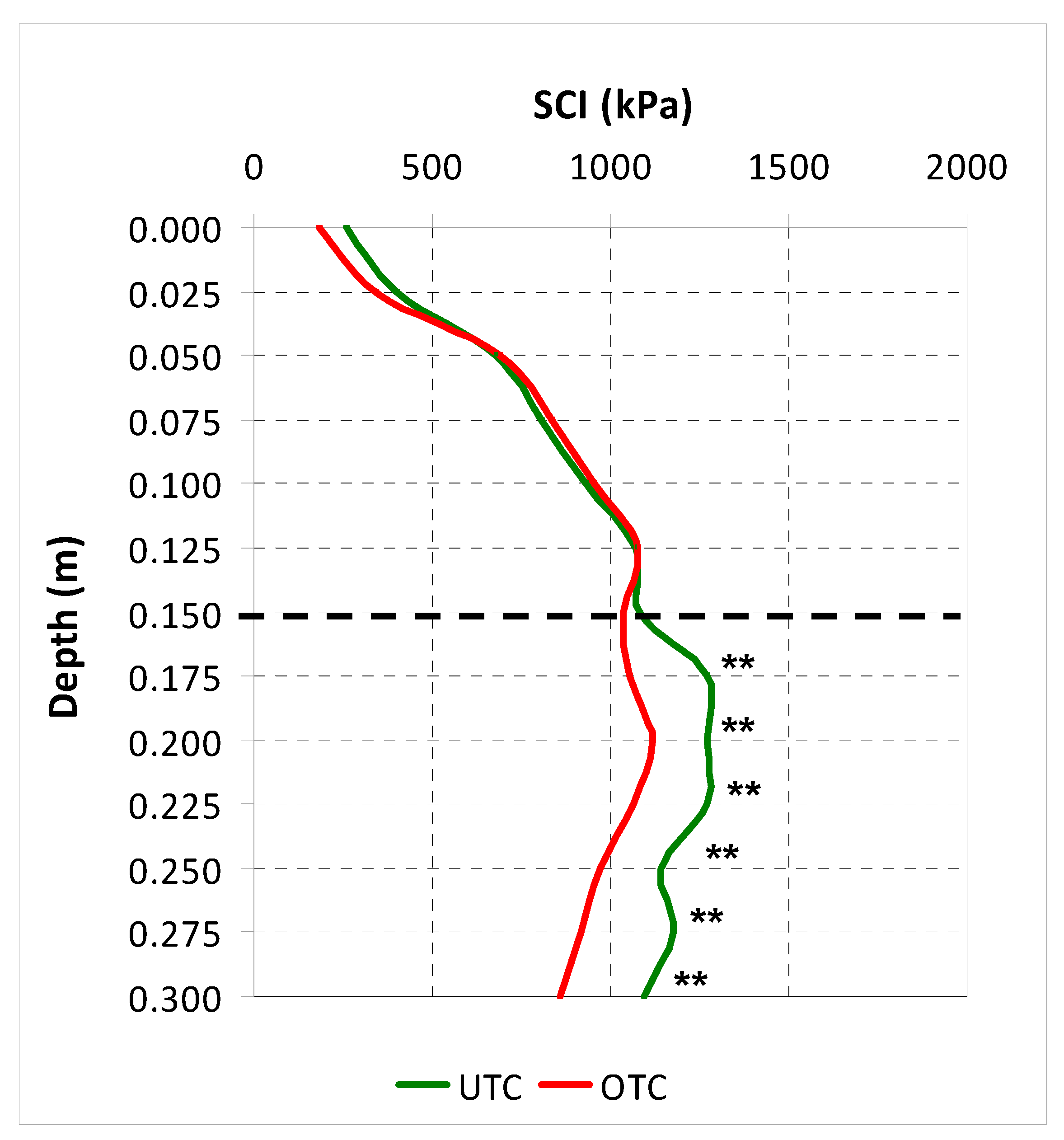




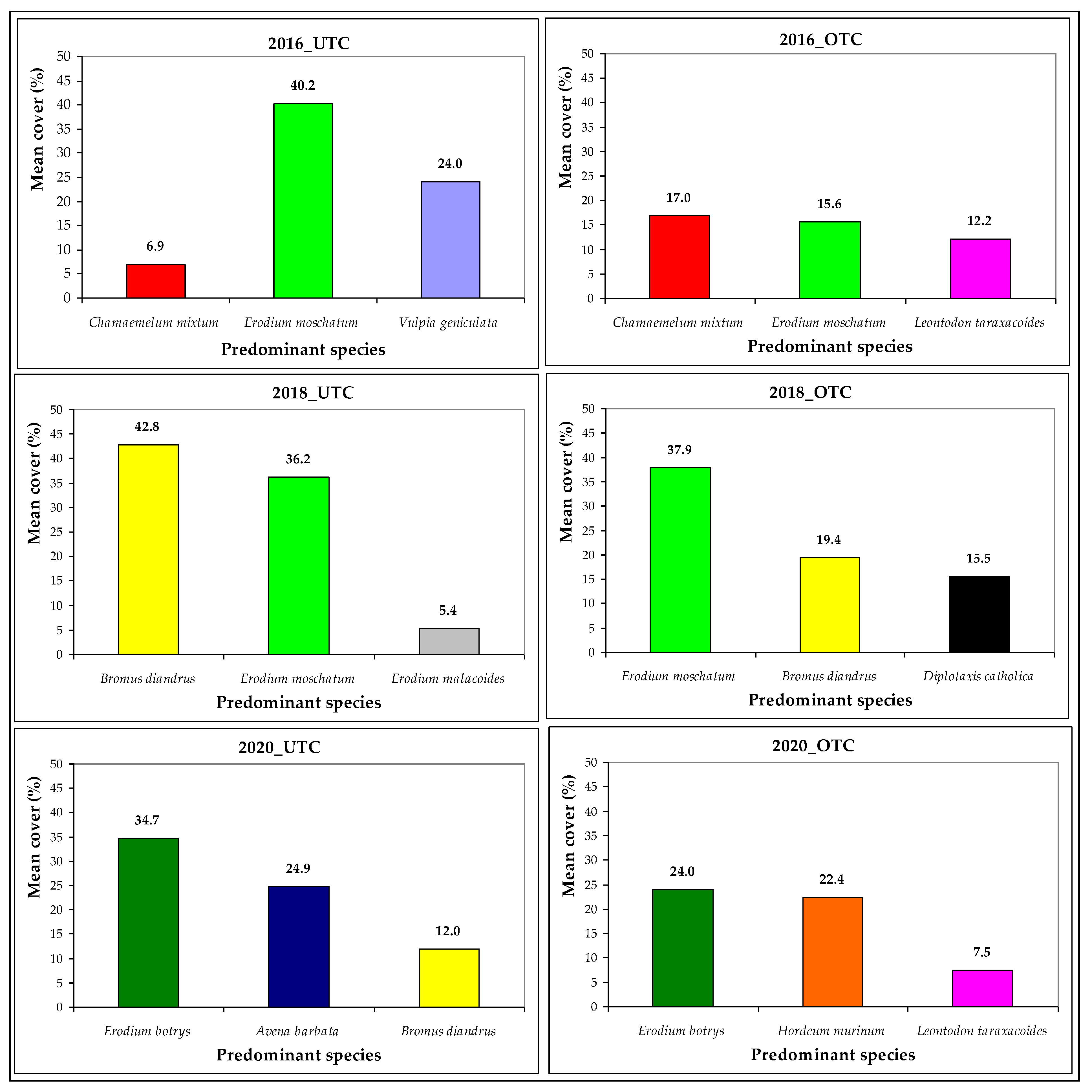
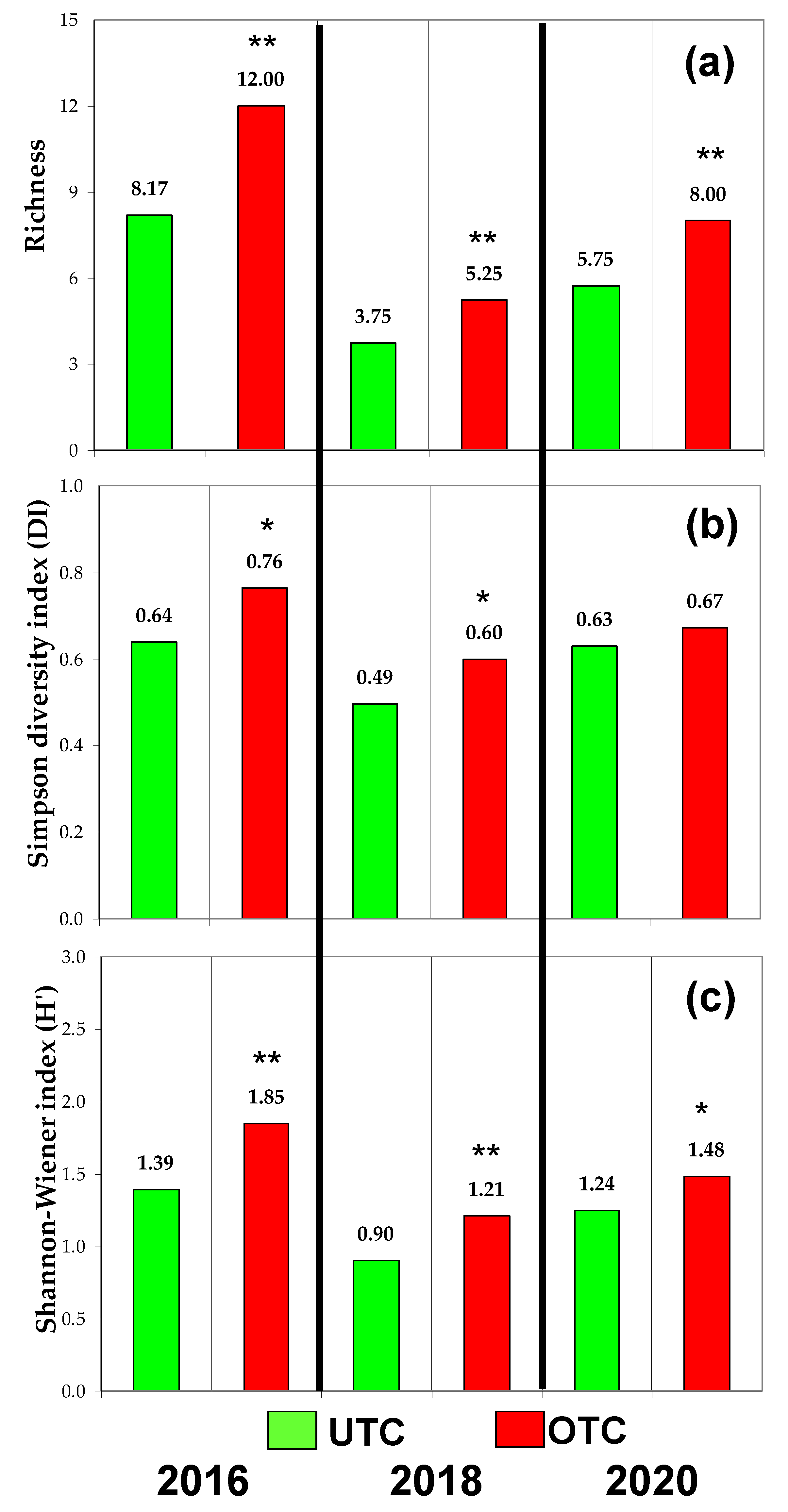


| Agricultural Year (September/August) | Accumulated Rainfall (mm) | Maximum Monthly Rainfall | |||||
|---|---|---|---|---|---|---|---|
| Autumn | Winter | Spring | Summer | Annual | (mm) | (Month) | |
| 2015/2016 | 53 | 197 | 203 | 13 | 466 | 118 | April |
| 2016/2017 | 204 | 146 | 53 | 9 | 412 | 109 | October |
| 2017/2018 | 106 | 326 | 225 | 26 | 683 | 207 | April |
| 2018/2019 | 165 | 82 | 66 | 0 | 313 | 98 | November |
| 2019/2020 | 212 | 205 | 208 | 42 | 668 | 161 | November |
| Period 1981–2010 | 203 | 208 | 145 | 29 | 585 | 95 | December |
| Soil Parameters | UTC | OTC | Probability | CV (%) |
|---|---|---|---|---|
| October 2015 | ||||
| Coarse sand, % | 49.0 | 47.8 | ns | 5.3 |
| Fine sand, % | 31.8 | 32.6 | ns | 6.3 |
| Silt, % | 9.8 | 9.5 | ns | 26.2 |
| Clay, % | 9.4 | 10.1 | ns | 27.8 |
| OM, % | 2.7 | 1.3 | 0.0000 | 17.8 |
| pH | 5.4 | 5.3 | ns | 5.4 |
| Nt, % | 0.16 | 0.09 | 0.0001 | 22.0 |
| P2O5, mg kg−1 | 117.7 | 68.2 | 0.0571 | 63.0 |
| K2O, mg kg−1 | 359.3 | 180.5 | 0.0012 | 39.9 |
| Mg, mg kg−1 | 115.0 | 76.3 | 0.0503 | 46.3 |
| Mn, mg kg−1 | 100.0 | 52.8 | 0.0131 | 53.2 |
| March 2020 | ||||
| pH | 5.8 | 5.6 | 0.0331 | 4.9 |
| Mg, mg kg−1 | 102.4 | 79.8 | 0.0215 | 40.1 |
| Mn, mg kg−1 | 47.6 | 34.3 | 0.0441 | 55.2 |
| Botanical Species | FAMILY | IV_ISA | Spring 2016 | Spring 2018 | Spring 2020 | |||
|---|---|---|---|---|---|---|---|---|
| (Mean Cover, %) | (%) | UTC | OTC | UTC | OTC | UTC | OTC | |
| Anagalis arvensis | PRIMULACEAE | 9 | 1.0 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| Arum italicum | ARACEAE | 12 | 0.0 | 0.0 | 1.1 | 0.0 | 0.5 | 0.0 |
| Avena barbata | POACEAE | 18 | 0.0 | 0.0 | 0.0 | 0.0 | 24.9 | 5.8 |
| Biserula pelecinus | FABACEAE | 4 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| Bromus diandrus | POACEAE | 54 | 1.7 | 0.4 | 42.8 | 19.4 | 12.0 | 6.2 |
| Bromus hordeaceus | POACEAE | 6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.5 |
| Calendula arvensis | ASTERACEAE | 9 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| Cerastium glomeratum | CARYOPHYLLACEAE | 36 | 0.1 | 1.8 | 4.6 | 0.0 | 0.5 | 1.6 |
| Chamaemelum fuscatum | ASTERACEAE | 5 | 0.0 | 0.0 | 0.4 | 8.1 | 0.0 | 0.0 |
| Chamaemelum mixtum | ASTERACEAE | 9 | 6.9 | 17.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Crepis capillaris | ASTERACEAE | 5 | 0.0 | 0.0 | 0.0 | 2.4 | 0.0 | 0.0 |
| Daucus carota | APIACEAE | 3 | 0.0 | 0.0 | 0.0 | 0.0 | 6.2 | 0.0 |
| Diplotaxis catholica | BRASSICACEAE | 19 | 0.6 | 6.3 | 0.5 | 15.5 | 0.0 | 0.8 |
| Echium plantagineum | BORAGINACEAE | 6 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 3.8 |
| Erodium botrys | GERANIACEAE | 18 | 0.0 | 0.0 | 0.0 | 0.0 | 34.7 | 24.0 |
| Erodium cicutarium | GERANIACEAE | 18 | 0.0 | 0.0 | 0.0 | 0.0 | 1.4 | 3.6 |
| Erodium malacoides | GERANIACEAE | 2 | 0.0 | 0.0 | 5.4 | 0.0 | 0.0 | 0.0 |
| Erodium moschatum | GERANIACEAE | 45 | 40.2 | 15.6 | 36.2 | 37.9 | 0.0 | 0.0 |
| Geranium dissectum | GERANIACEAE | 6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 |
| Geranium molle | GERANIACEAE | 11 | 1.0 | 1.4 | 0.0 | 0.0 | 3.6 | 2.9 |
| Gynandriris sisyrinchium | IRIDACEAE | 31 | 1.4 | 0.0 | 0.0 | 0.2 | 0.0 | 0.4 |
| Holcus lannatus | POACEAE | 18 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 |
| Hordeum murinum | POACEAE | 6 | 0.0 | 0.0 | 5.1 | 0.0 | 3.1 | 22.4 |
| Leontodon taraxacoides | ASTERACEAE | 30 | 6.2 | 12.2 | 0.0 | 2.4 | 0.0 | 7.5 |
| Lolium multiflorum | POACEAE | 3 | 0.0 | 0.0 | 0.0 | 0.0 | 1.2 | 0.3 |
| Lolium rigidum | POACEAE | 18 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.2 |
| Medicago polymorpha | FABACEAE | 3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 |
| Ornithopus isthmocarpus | FABACEAE | 4 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| Plantago coronopus | PLANTAGINACEAE | 9 | 1.8 | 8.4 | 0.0 | 0.0 | 0.0 | 0.0 |
| Plantago lagopus | PLANTAGINACEAE | 5 | 0.0 | 0.0 | 0.0 | 1.4 | 0.0 | 0.0 |
| Plantago lanceolata | PLANTAGINACEAE | 4 | 0.4 | 1.7 | 0.0 | 0.0 | 0.0 | 0.2 |
| Poa annua | POACEAE | 9 | 1.2 | 1.1 | 0.0 | 0.0 | 0.1 | 0.0 |
| Ranunculus muricatus | RANUNCULACEAE | 6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 |
| Raphanus raphanistrum | BRASSICACEAE | 41 | 0.0 | 1.0 | 0.0 | 0.6 | 0.0 | 1.1 |
| Rumex bucephalophorus | POLYGONACEAE | 4 | 0.2 | 5.9 | 0.0 | 2.5 | 0.0 | 0.6 |
| Rumex conglomeratus | POLYGONACEAE | 1 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 |
| Scandix pecten-veneris | APIACEAE | 3 | 0.0 | 0.0 | 0.0 | 0.0 | 4.8 | 5.0 |
| Senecio jacobae | ASTERACEAE | 18 | 0.0 | 0.0 | 0.0 | 0.0 | 1.8 | 5.1 |
| Senecio vulgaris | ASTERACEAE | 5 | 0.0 | 0.4 | 1.7 | 4.5 | 0.0 | 0.0 |
| Sherardia arvensis | RUBIACEAE | 51 | 0.1 | 0.1 | 0.0 | 0.5 | 0.0 | 0.3 |
| Silene gallica | CARYOPHYLLACEAE | 1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Sonchus oleraceus | ASTERACEAE | 9 | 2.7 | 2.1 | 0.0 | 0.0 | 0.0 | 0.1 |
| Spergula arvensis | CARYOPHYLLACEAE | 19 | 0.5 | 1.7 | 0.0 | 2.7 | 0.0 | 0.0 |
| Stachys arvensis | LAMIACEAE | 4 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| Stellaria media | CARYOPHYLLACEAE | 9 | 3.5 | 1.0 | 0.0 | 0.8 | 0.0 | 0.0 |
| Tolpis barbata | ASTERACEAE | 6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.8 |
| Trifolium glomeratum | FABACEAE | 1 | 2.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| Trifolium incarnatum | FABACEAE | 4 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Trifolium repens | FABACEAE | 20 | 1.4 | 4.2 | 0.0 | 0.0 | 0.0 | 2.3 |
| Trifolium resupinatum | FABACEAE | 4 | 0.5 | 9.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| Trifolium subterraneum | FABACEAE | 5 | 0.0 | 0.0 | 0.0 | 1.1 | 0.0 | 0.0 |
| Urtica urens | URTICACEAE | 22 | 1.9 | 0.1 | 2.2 | 0.0 | 2.7 | 0.0 |
| Vicia sativa | FABACEAE | 18 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.1 |
| Vulpia geniculata | POACEAE | 49 | 24.0 | 5.0 | 0.0 | 0.0 | 1.6 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, J.; Shahidian, S.; Machado, E.; Paniagua, L.L.; Carreira, E.; Moral, F.; Pereira, A.; de Carvalho, M. Floristic Composition: Dynamic Biodiversity Indicator of Tree Canopy Effect on Dryland and Improved Mediterranean Pastures. Agriculture 2021, 11, 1128. https://doi.org/10.3390/agriculture11111128
Serrano J, Shahidian S, Machado E, Paniagua LL, Carreira E, Moral F, Pereira A, de Carvalho M. Floristic Composition: Dynamic Biodiversity Indicator of Tree Canopy Effect on Dryland and Improved Mediterranean Pastures. Agriculture. 2021; 11(11):1128. https://doi.org/10.3390/agriculture11111128
Chicago/Turabian StyleSerrano, João, Shakib Shahidian, Eliana Machado, Luís L. Paniagua, Emanuel Carreira, Francisco Moral, Alfredo Pereira, and Mário de Carvalho. 2021. "Floristic Composition: Dynamic Biodiversity Indicator of Tree Canopy Effect on Dryland and Improved Mediterranean Pastures" Agriculture 11, no. 11: 1128. https://doi.org/10.3390/agriculture11111128
APA StyleSerrano, J., Shahidian, S., Machado, E., Paniagua, L. L., Carreira, E., Moral, F., Pereira, A., & de Carvalho, M. (2021). Floristic Composition: Dynamic Biodiversity Indicator of Tree Canopy Effect on Dryland and Improved Mediterranean Pastures. Agriculture, 11(11), 1128. https://doi.org/10.3390/agriculture11111128